NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2012.

Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General.
Show detailsIntroduction
This chapter addresses the adverse health consequences of tobacco use by children and young adults. Although the chapter focuses primarily on childhood through young adulthood, it also briefly considers the pre-natal period and examines the adverse effects of smoking before conception as well, even though that is not a main focus of this report. Previous Surgeon General’s reports on tobacco use have covered the evidence on the increased risk of specific diseases and other adverse effects of active and involuntary smoking, with the most recent updates in the 2004, 2006, and 2010 reports (U.S. Department of Health and Human Services [USDHHS] 2004, 2006, 2010) discussing active smoking, exposure to secondhand smoke, and the biological basis of disease, respectively. Those reports covered the effects of maternal and paternal smoking on nearly all aspects of reproduction and on risk for congenital malformations as well as the increased risks from exposure to secondhand smoke for sudden infant death syndrome (SIDS), increased lower respiratory illnesses and respiratory symptoms, reduced lung growth, and asthma (see Tables 2.1a and 2.1b for the conclusions of the earlier reports).
Table 2.1a
Conclusions from previous Surgeon General’s reports on the adverse effects of tobacco use and exposure to secondhand smoke in children and young adults.
Table 2.1b
Level of certainty of causality reported in the 2004 and 2006 Surgeon General’s reports.
This chapter complements those earlier reports by reviewing the health consequences of active smoking by adolescents and young adults, a topic last covered, in depth, in the 1994 report. That report reached several key conclusions on the adverse effects of smoking on young people related to their respiratory and cardiovascular health and, in regard to addiction, it noted that “among addictive behaviors, cigarette smoking is the one most likely to become established during adolescence. People who begin to smoke at an early age are more likely to develop severe levels of nicotine addiction than those who start at a later age” (USDHHS 1994, p. 41).
This chapter returns to the topic of the health consequences of smoking for young people who smoke, reviewing the substantial new evidence in detail and placing it within a life-course perspective. It also covers new information on the onset of nicotine addiction during adolescence and young adulthood, which includes prospectively collected data on trajectories of addiction from cohort studies. For young people, particularly females, considerations about weight play a role in the decision to start smoking and to continue this behavior; this issue, which is critical for efforts in prevention and cessation, is comprehensively reviewed in the present chapter. Information on the health consequences of smokeless tobacco use are documented in multiple prior publications (National Cancer Institute [NCI] 2012).
Smoking During Adolescence and Young Adulthood: A Critical Period for Health
Since the 1994 report, the basis for concern about smoking during adolescence and young adulthood has expanded beyond the immediate health consequences for the young smoker to a deeper understanding of the implications for health of exposure to tobacco smoke across the life course, including into the next generation. This broadened concern reflects the emergence of a body of evidence linking risk exposures in early life, even in the antenatal period, to risk for chronic disease in adulthood. The general hypothesis that has been constructed from this evidence is often called the “developmental origins of adult disease” hypothesis or the “Barker” hypothesis, in reference to David Barker, who documented associations between early-life nutrition and subsequent risk for cardiovascular disease (Barker 2004; de Boo and Harding 2006).
Research in humans that is relevant to this hypothesis has largely come from epidemiologic studies that have tied nutrition in early life to subsequent risk for hypertension and other cardiovascular diseases (Huxley et al. 2000; Barker et al. 2005; de Boo and Harding 2006). There is also relevant experimental research (Nuyt 2008). The proposed underlying mechanisms emphasize genetic and epigenetic changes that could have lasting implications across the life span (Young 2001; Gicquel et al. 2008).
Even before conception, the sperm and oocytes of future parents who smoke are exposed to the DNA- damaging constituents of tobacco smoke (USDHHS 2004); the fetus of a mother who smokes or who is exposed to secondhand smoke will be exposed to these damaging materials, resulting most often in reduced birth weight (USDHHS 2004, 2006). To date, however, there has been little investigation of the molecular changes as a result of these early-life exposures to tobacco smoke. One recent study, however, has demonstrated epigenetic changes in children with in utero exposure to maternal smoking (Breton et al. 2009), a finding consistent with one proposed mechanism for long-term consequences of early-life exposures. Thus, given the numerous known carcinogens and toxins present in tobacco smoke and the known mechanism by which they cause disease, the developmental origins of adult disease is a critical concept to consider when addressing youth tobacco use.
For many of the chronic diseases caused by smoking, the risks increase with the duration and cumulative amount of this behavior. Consequently, the age of starting to smoke has consequences for the age at which the risks of smoking become manifest. In the United States, the age of starting to smoke regularly became increasingly younger late in the twentieth century (NCI 1997), first for males and then for females, but more recently, it has been stable (Figure 2.1). By the early 1990s, the mean age of first trying a cigarette was about 16 years for those who ever smoked (see Chapter 3, “The Epidemiology of Tobacco Use Among Young People in the United States and Worldwide”). In many other countries, the mean age of uptake is similarly young (see Chapter 3).
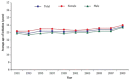
Figure 2.1
Average age when a whole cigarette was smoked for the first time among 9th- to 12th-grade youth; Youth Risk Behavior Survey (YRBS) 1991–2009; United States. Source: 1991–2009 YRBS: Centers for Disease Control and Prevention, Division of (more...)
This earlier age of onset of smoking marks the beginning of exposure to the many harmful components of smoking. This is during an age range when growth is not complete and susceptibility to the damaging effects of tobacco smoke may be enhanced. In addition, an earlier age of initiation extends the potential duration of smoking throughout the lifespan. For the major chronic diseases caused by smoking, the epidemiologic evidence indicates that risk rises progressively with increasing duration of smoking; indeed, for lung cancer, the risk rises more steeply with duration of smoking than with number of cigarettes smoked per day (Doll and Peto 1978; Peto 1986; USDHHS 2004). For chronic obstructive pulmonary disease (COPD), risk varies directly with the total number of cigarettes consumed over a lifetime (USDHHS 2004), which would suggest greater risk for longer duration or higher intensity. There is little direct evidence, however, on whether the age of starting to smoke, by itself, modifies the risk of smoking-related disease later, that is, whether starting to smoke during adolescence versus young adulthood increases the subsequent risk for such disease (International Agency for Research on Cancer 2004).
This chapter has four major sections which correspond to the principal health domains that are related to smoking during adolescence and young adulthood: factors related to initiation and continuation of smoking, including nicotine addiction, smoking and body weight, respiratory symptoms, and cardiovascular effects. Other adverse effects of smoking on adolescents and young adults have been covered in other reports during the last decade, including the effects of smoking on reproduction and on increasing risk for respiratory infections (USDHHS 2004).
This chapter was developed following the approach set out in the 2004 report of the Surgeon General (USDHHS 2004). The authors systematically searched for all relevant evidence that appeared in the scientific literature after earlier reviews on these topics; this evidence, along with the prior findings, was evaluated and classified as described in the 2004 report.
Nicotine Addiction
Introduction
The topic of nicotine and addiction to this substance has been covered in multiple Surgeon General’s reports. The 1988 report concluded that “(1) Cigarettes and other forms of tobacco are addicting. (2) Nicotine is the identified drug in tobacco that causes addiction. (3) The pharmacologic and behavioral processes that determine tobacco addiction are similar to those that determine addiction to drugs such as heroin and cocaine” (USDHHS 1988, p. 78). The 2010 report, which covered the extensive advances in research on nicotine since the 1988 report (USDHHS 2010), reconfirmed nicotine’s key role in causing addiction and concluded that genetic variations in responses to this drug contribute to determining patterns of smoking behavior and cessation.
This report summarizes the research on nicotine dependence among adolescents and young adults but does not address the mechanisms of addiction, which were covered in the 2010 report. It also does not cover the evidence related to maternal smoking during pregnancy and future risk for nicotine addiction; there is a substantial body of relevant experimental evidence as well as more limited observational research on this topic. The experimental studies provide coherent evidence that prenatal exposure to nicotine has lasting effects on the developing brain (Dwyer et al. 2008; Pauly and Slotkin 2008; Poorthuis et al. 2009). However, observational studies on whether maternal smoking during pregnancy increases risk for subsequent addiction of the child have provided mixed evidence (USDHHS 2010).
To meet the clinical diagnosis of nicotine dependence as defined by the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders 4th ed. (text rev.) (DSM-IV-TR) (American Psychiatric Association 2000), an adult must exhibit at least three of the primary symptoms of substance dependence, generally at any time during the same 12-month period. In addition to the two primary characteristics of withdrawal symptoms and unsuccessful quit attempts described below, criteria include tolerance to the aversive effects of nicotine (e.g., nausea and lightheadedness), limiting social or occupational activities because of prohibitions in place against smoking, continued use despite significant health concerns, and greater use than intended (American Psychiatric Association 2000; Fiore et al. 2008). Nicotine dependence among adult smokers is characterized by the emergence of withdrawal symptoms in response to abstinence and by unsuccessful attempts to reduce the use of tobacco or to quit altogether (Fiore et al. 2008). Withdrawal symptoms can occur as early as 4 to 6 hours after the last use of nicotine (USDHHS 1988; Hughes 2007); these early symptoms, which include depressed mood, insomnia, irritability, anxiety, difficulty concentrating, restlessness, increased appetite, and cravings for tobacco/nicotine, are almost immediately alleviated by using tobacco or nicotine. In adults, the severity of nicotine dependence is most commonly measured using the Fagerström Tolerance Questionnaire (FTQ) (Fagerström and Schneider 1989) or a modified version called the Fagerström Test for Nicotine Dependence (FTND) (Heatherton et al. 1991), both of which include inventories of tobacco-specific items.
Baker and colleagues (2009), in an NCI monograph on phenotypes and endophenotypes, characterize the DSM-IV and FTQ as directed at the “distal” phenotype of mature nicotine addiction (Baker et al. 2009). This monograph emphasizes the complexity and multidimensionality of nicotine dependence and its maturation from initial experimentation to addiction.
At present, the defining characteristics of nicotine dependence in adolescent smokers remain a topic of much debate, particularly as the inappropriateness of extending criteria developed for adults to youth smokers has been recognized. Evidence is conflicting as to whether adolescents meet some of the dependence criteria for adults described above, which are generally based on the premise that prolonged use is needed for dependence to be established. Indeed, until about 10 years ago, the dominant concept in the field proposed that adolescents could not be dependent on cigarettes because this population has short and often highly variable patterns of use. However, emerging evidence suggests that key symptoms of physical dependence on nicotine—such as withdrawal and tolerance—can be manifest following even minimal exposure to this substance. For example, DiFranza and colleagues (2000) prospectively followed occasional adolescent smokers and observed that a large proportion experienced at least one symptom of nicotine dependence upon quitting, even in the first 4 weeks after initiating monthly smoking (at least two cigarettes within a 2-month period). This finding, based on an instrument developed specifically for adolescents, suggests that adolescents can become dependent very shortly after initiating smoking. Similarly, a number of retrospective and prospective studies have found that adolescents experience subjective symptoms of withdrawal, such as craving, nervousness, restlessness, irritability, hunger, difficulty concentrating, sadness, and sleep disturbances, after stopping smoking (McNeil et al. 1986; Rojas et al. 1998; Killen et al. 2001; Prokhorov et al. 2005). In addition, Breslau and colleagues (1994) reported that nearly one-half of all young adults who smoked daily were nicotine dependent, a finding based on their having at least three of seven symptoms as ascertained by the National Institute of Mental Health Diagnostic Interview Schedule.
In addition to these reports, more recent preclinical and clinical evidence suggests that the qualitative experience of withdrawal may differ between adolescents and adults. For example, preclinical studies indicate that although adult rats display evidence of withdrawal, adolescent rats do not (O’Dell et al. 2004). Furthermore, in adolescent humans the nicotine patch may not prevent the development of withdrawal symptoms (Killen et al. 2001), and the treatment efficacy of this and other nicotine replacement therapies used in adults has not been established with adolescent smokers. The available studies in this area provide mixed evidence (Smith et al. 1996; Hurt et al. 2000; Hanson et al. 2003; Moolchan et al. 2005), drawing into question the utility of nicotine replacement in this age group. Furthermore, although adolescent smokers report having some withdrawal symptoms, these are generally minimal, with craving tobacco being the predominant symptom experienced during abstinence (Prokhorov et al. 2005; Bagot et al. 2007; Smith et al. 2008a, b). Finally, adolescents’ patterns of tobacco use are likely more highly constrained than those of adults because they are influenced by environmental factors such as rules or regulations enacted by schools or rules in the home (Wiltshire et al. 2005), a difference that should be considered in examining the issue of addiction to nicotine among young people.
Interpretation of the relevant studies is complicated by the lack of adequate, validated measures of dependence for use in adolescent smokers (Colby et al. 2000). A number of measures have been developed to assess nicotine dependence among adolescents, including a modified FTQ (mFTQ) (Prokhorov et al. 1998, 2001). The Nicotine Dependence Syndrome Scale (NDSS) (Substance Abuse and Mental Health Services Administration 2002; Shiffman et al. 2004) measures important components of tobacco use behavior, including drive, priority, tolerance, stereotypy, and continuity. The Hooked on Nicotine Checklist (HONC) (DiFranza et al. 2000; O’Loughlin et al. 2003) measures loss of full autonomy over tobacco use; a DSM-IV checklist measures the physical and psychological consequences of tobacco use as well as tolerance and withdrawal (Kandel et al. 2005). However, most studies have found little if any concordance between results obtained using these scales. Evidence suggests that the DSM-IV scale and the mFTQ may measure different components of dependence (Kandel et al. 2005), that the HONC and mFTQ may be identifying adolescents at different points along the continuum of dependence (MacPherson et al. 2008), and that the NDSS complements information on tobacco use measured with the FTND (Clark et al. 2005). Moreover, classifications by many of the measures of nicotine dependence are strongly related to measures of the quantity/frequency of tobacco use and/or serum cotinine concentrations (Clark et al. 2005; Kandel et al. 2005; Rubinstein et al. 2007). This evidence has led researchers to propose that methods to assess the wide spectrum of use among adolescents, ranging from initiation and progression to maintenance, may be needed to understand nicotine dependence in this population (Strong et al. 2009).
From First Use to Addiction
This section will focus on multiple patterns of use, including experimentation, regular use of tobacco products, and use that is characterized by addiction. It also addresses the roles played by genetic determinants and mental disorders in the risk for addiction and the relationship of tobacco use to the use of other drugs and alcohol. External factors, including the social-environmental and the cultural, are covered in Chapter 4, “Social, Environmental, Cognitive, and Genetic Influences on the Use of Tobacco Among Youth.”
Longitudinal Patterns of Tobacco Use in Adolescents
Mayhew and colleagues (2000) identified several stages of adolescent smoking, from not smoking at all to established smoking, as well as common and distinct predictors of the various stages. In addition, to characterize the course of adolescent smoking and to identify determinants of the trajectories of smoking across adolescence into adulthood, several cohort studies have been carried out that included appropriate statistical modeling. Chassin and colleagues (2000), who applied such models to data from a cohort study of smoking trajectories from adolescence to adulthood, identified four groups with different trajectories: early stable smokers, late stable smokers, experimenters, and quitters. Similarly, White and colleagues (2002) used growth mixture modeling to assess smoking behavior at five time points across 18 years, from early adolescence to adulthood (age 30). They identified three groups with different trajectories: heavy/regular users, occasional users/those maturing out of use, and nonsmokers/experimental smokers.
Colder and colleagues (2001), who used data from an annual assessment of adolescents 12–16 years of age, identified five kinds of smokers: early rapid escalators, late moderate escalators, late slow escalators, stable light smokers, and stable puffers. Similarly, Soldz and Cui (2002) examined the longitudinal patterns of smoking among adolescents, assessed on an annual basis from grades 6 to 12, and identified six clusters: nonsmokers, quitters, experimenters, early escalators, late escalators, and continuous smokers. Audrain-McGovern and colleagues (2004) used evidence from a longitudinal cohort study of 9th to 12th graders to identify four kinds of smokers by trajectory: never smokers, experimenters, earlier/faster smoking adopters, and later/slower smoking adopters. They also examined predictors of smoking behavior and found that early adopters, compared with never smokers, tended to be more novelty seeking, with poorer academic performance, more depressive symptoms, greater exposure to other smokers, and greater use of other substances. In another study, Robinson and colleagues (2004) reported that adolescents who initiated smoking early (before 14 years of age) had slower progression to daily smoking than those who initiated later and that earlier onset of daily smoking was associated with higher FTND scores. In contrast, in follow-ups of two prior studies (Hops et al. 2000; Swan et al. 2003), Lessov-Schlaggar and colleagues (2008) found that while higher levels of nicotine dependence among adolescents were associated with smoking trajectories marked by heavier smoking, there was no relationship between quantity/frequency of cigarette use during adolescence and lifetime levels of nicotine dependence. Thus, various studies point to heterogeneity in the onset and progression of smoking among adolescents (Schepis and Rao 2005).
Several predictors of being on a particular trajectory have been identified. For example, differences by race have been reported: in one study, African American adolescents initiated smoking and also became daily smokers an average of 1 year later than adolescents of other racial/ethnic groups (Robinson et al. 2004). Using similar trajectory analyses, Karp and coworkers (2005) found that among novice smokers (mean age = 13 years), only one-fourth reported rapid escalation toward patterns of heavier use; this escalation was predicted by male gender, poor academic performance, and having more than 50% of their friends smoke. A recent large, population-based cohort study found that the likelihood of being in a trajectory group defined by heavier use was enhanced by having parents who smoked, a greater number of friends who smoked, and a greater perception of the number of adults and adolescents who smoked. Conversely, negative perceptions of the tobacco industry, higher perceived difficulty regarding smoking in public places, and stricter home smoking policies were protective (Bernat et al. 2008). Finally, Riggs and colleagues (2007) evaluated the relationship between adolescent trajectories of tobacco use and nicotine dependence in early adulthood and found that adolescents who demonstrated early stable use of tobacco (two cigarettes per week by 12 years of age) were more likely to have greater nicotine dependence as young adults.
In summary, these results indicate that adolescent smoking patterns follow different trajectories from experimentation to addiction. Approaches using trajectory analyses allow researchers not only to account for variability in tobacco use behaviors, but also to extend the analyses to examine interindividual changes in smoking patterns across time and to assess the predictors of various trajectories. Several predictors of smoking trajectory have been identified through prospective cohort studies, and additional trajectory analyses from national data are shown in Chapter 3.
Genetic Influences
Emerging evidence indicates that addiction to tobacco smoking has a heritable component, with genetic factors contributing to all phases of the smoking trajectory, from initiation to dependence and cessation (for review, see NCI 2009; Bierut 2011). NCI’s Monograph 20 addresses this topic in depth (NCI 2009). In addition, the mechanics of nicotine addiction and the role of genetics in determining addiction were addressed in the 2010 Surgeon General’s report (USDHHS 2010). This is an active area of research, but the emphasis in this chapter is on genetic studies related to initiation and the trajectories of smoking across adolescence (see also Chapter 4). Recently, researchers have identified specific genetic markers as strongly associated with nicotine dependence (Li et al. 2008). Investigations into the specific genes that mediate cigarette smoking are complicated by different definitions of the nicotine dependence phenotype (Ho and Tyndale 2007). In fact, several components of the phenotype of nicotine dependence appear to be heritable, including tolerance, withdrawal, difficulty quitting, time to first cigarette in the morning, and number of cigarettes smoked per day (Lessov et al. 2004; Swan et al. 2009). The need for a broad framework for assessing the role of genetic factors in nicotine dependence is now well recognized (NCI 2009). It is clear that multiple genes may act through various pathways, and environmental factors also need consideration. For adolescents, the age of starting to smoke, trajectory of smoking, and persistence of smoking constitute the appropriate focus for genetic studies.
Reported investigations on the genetics of smoking now include some that have looked at the initiation and progression of smoking in adolescents (Haberstick et al. 2007). Laucht and colleagues (2008) found that among adolescent smokers, initiation was associated with allelic variation in the dopamine receptor D4 (DRD4) gene, and continuation of smoking and dependence were associated with the dopamine receptor D2 (DRD2) gene (Laucht et al. 2008). Another genetic influence on tobacco use and dependence has to do with the relative rate of nicotine metabolism (Malaiyandi et al. 2005); individuals with polymorphisms in genes encoding the enzymes primarily involved in nicotine metabolism (e.g., cytochrome P-450, family 2, subfamily A, polypeptide 6; CYP2D6) tend to smoke fewer cigarettes and are less likely to be current smokers. This finding could be driven by the fact that faster metabolizers smoke more cigarettes (Audrain-McGovern et al. 2007). Adolescents who metabolize nicotine normally have been found to progress to nicotine dependence more quickly than those with gene variants associated with slow metabolism (Audrain-McGovern et al. 2007). More recent evidence from a sample of young adult smokers suggests that polymorphisms in the genes encoding the neuronal cholinergic nicotinic subunit receptors, specifically in the genomic region containing the CHRNA5/A3/B4 gene cluster, is a significant predictor of the age of initiation of cigarette smoking (Schlaepfer et al. 2008). In support, research from three independent samples of long-term smokers suggests that the CHRNA5/A3/B4 gene cluster is associated with severity of nicotine dependence and daily smoking at or before 16 years of age (Weiss et al. 2008). This same gene cluster is associated with the transition from experimental to dependent smoking (Bierut et al. 2007; Saccone et al. 2007) and has been one of the most replicated findings in complex genetic studies; four separate meta-analyses have validated a strong association of this cluster with smoking phenotypes (Liu et al. 2010; Saccone et al. 2010; Thorgeirsson et al. 2010; Tobacco and Genetics Consortium 2010). Other studies show that this same cluster is associated with phenotypes that are known consequences of smoking later in life, such as COPD (Pillai et al. 2009), peripheral artery disease (Thorgeirsson et al. 2008), and lung cancer (Amos et al. 2008; Hung et al. 2008; Liu et al. 2008; Saccone et al. 2010; Thorgeirsson et al. 2008).
Summary
Longitudinal studies show differing trajectories of smoking across adolescence—the critical period of time when addiction begins for many young people. These trajectories reflect a range of rates of progression toward addiction, and they represent important phenotypes for researchers and possibly for prevention initiatives by offering an indication of which new smokers may be at greatest risk for addiction. Limited evidence suggests that these trajectories may differ across racial groups.
The documentation that adolescents follow different trajectories of the onset and progression of smoking has implications that extend beyond research to include prevention and intervention. Clearly, having several kinds of trajectories precludes being able to identify particular adolescents who are moving swiftly toward addiction. In addition, the trajectories are not necessarily linear, and the actual point of addiction is not clearly demarcated. Thus, practitioners cannot readily identify specific at-risk youth, and there is uncertainty as to how to tailor cessation initiatives for smokers at different points on these trajectories.
Identifying the determinants of particular trajectories, however, could help with early identification of high-risk adolescents. Some of the predictors that have been examined include the smoking behaviors and attitudes of parents and peers, the use of tobacco products for regulation of mood and affect, developmental changes in risk-taking behaviors, and genetic factors (see Chapter 4,) for discussion of these topics in greater depth). The newer evidence continues to show that peer influence is strongly associated with initiation and, in one study, with a trajectory of heavier use (Bernat et al. 2008). Several characteristics of adolescents are also relevant for predicting trajectories, including gender, impulsivity and risk taking, and affect. In addition, emerging evidence is suggesting that both risk for initiation and continuing to smoke may have genetic determinants. The findings to date indicate that the genes influencing dopaminergic reward pathways, nicotinic cholinergic receptors, and nicotine metabolism are relevant. However, the evidence on genetic determinants for adolescents and young adults is still too limited to make any suggestions concerning interventions based on genetic make-up.
Mental Health and Risk for Smoking
Introduction
Among adults, tobacco use is highly prevalent among people with psychiatric diagnoses over all and for such specific diagnoses as depression, schizophrenia, attention deficit hyperactivity disorder (ADHD), anxiety disorders, and substance abuse. For example, Lasser and colleagues (2000) found higher rates of tobacco use among those with psychiatric disorders (41%) or substance abuse (67%) than in the general population (21% at that time). In addition, adults with mental illness, broadly defined, were found to consume an estimated 44.3% of the cigarettes smoked in the United States (Upadhyaya et al. 2002), even though such adults constituted a far smaller percentage of the population. Explanations for the links between psychiatric disorders and cigarette use have emphasized the possible shared underlying predispositions for tobacco use and having a psychiatric disorder. There may be a genetic basis for this presumed shared predisposition that relates to neurologic pathways in the brain; individuals with serious mental illness, such as schizophrenia and depression, may be self-medicating and thus using nicotine to modulate symptoms related to their illness by influencing neurologic pathways (Ziedonis et al. 2008).
Adolescents
Although the links between tobacco use and both psychiatric comorbidities and disorders of substance abuse have been investigated in adults, they have not been rigorously examined in adolescents. In one study of youth, Kandel and colleagues (1997) examined the cross-sectional relationship between cigarette use and the use of other substances as well as with psychiatric disorders and found that daily cigarette smoking was associated with a 70% increase in the likelihood of diagnoses of anxiety and of disorders of mood and disruptive behavior. Later, a comprehensive review by Upadhyaya and colleagues (2002) found that psychiatric comorbidity is common in adolescent cigarette smokers, especially among those with disorders involving disruptive behavior (such as oppositional defiant disorder, conduct disorder, and ADHD), major depressive disorders, and drug and alcohol use. They concluded that anxiety disorders are modestly associated with cigarette smoking. They also found that early onset of cigarette smoking (before 13 years of age) and early onset of conduct problems were robust markers of increased psychopathology later in life, including substance abuse. Finally, a more recent case-control study found high rates of cigarette smoking in adolescents with bipolar disorder (Wilens et al. 2008).
A number of cross-sectional studies have found positive associations between depressive symptoms or a diagnosis of depression and tobacco use or nicotine dependence (Covey and Tam 1990; Brown et al. 1996; Nelson and Wittchen 1998; Acierno et al. 2000; Sonntag et al. 2000). Compared with their nondepressed peers, adolescents with depressive disorders have been found to be more likely to initiate experimental smoking, to become regular users (Patton et al. 1998), and to be nicotine dependent (Breslau et al. 1993). Furthermore, the presence of an affective disorder increases the likelihood of nicotine dependence by 10-fold in adolescents (Dierker et al. 2001). Evidence on the temporality of this relationship is somewhat equivocal, however. Some cohort studies have indicated that the presence of affective symptoms or the diagnosis of an affective disorder during adolescence leads to increased initiation and progression of smoking as well as to higher nicotine dependence (Kandel and Davies 1986; Fergusson et al. 1996); another cross-sectional study found a relationship between depressive symptoms and smoking among young adults in college (Kenney and Holahan 2008). In contrast, some cohort studies suggest that current smoking predicts depressive symptoms (Wu and Anthony 1999; Goodman and Capitman 2000) and not the other way around. Evidence from the National Longitudinal Alcohol Epidemiologic Survey indicated that onset of smoking before 13 years of age, when compared with onset after 17 years of age, was associated with earlier onset and more episodes of major depressive disorder (Hanna and Grant 1999). A more recent study conducted by Illomäki and colleagues (2008) examined the temporal nature of the relationship between onset of daily smoking and psychiatric disorders among hospitalized adolescents and found that substance use disorders, as well as psychotic and depressive disorders, follow the initiation of daily smoking, while conduct or oppositional defiant disorders appear to precede daily smoking.
Not surprisingly, evidence on the connection between smoking behavior and anxiety disorders is also equivocal. Adolescents with anxiety disorders have been found to have increased rates of smoking and nicotine dependence (Nelson and Wittchen 1998; Sonntag et al. 2000), and some studies indicate that anxiety predicts the initiation and progression of smoking (Patton et al. 1998).
Evidence for a link between nicotine use and ADHD is also somewhat equivocal. For example, a higher smoking prevalence among adolescents and adults diagnosed with ADHD has been reported (Pomerleau et al. 1995; Riggs et al. 1999; Ribeiro et al. 2008), but other studies have found no increased risk for smoking in association with ADHD (Dierker et al. 2001). One longitudinal study, however, found that an early diagnosis of ADHD was associated with an increased rate of later cigarette smoking (Chilcoat and Breslau 1999). It has been proposed that smokers with ADHD may be using nicotine as a way to improve their attention span by increasing the release of dopamine (Dani and Harris 2005); this self-medication hypothesis is supported by the finding that the nicotine transdermal patch improved performance on cognitive reaction tasks in both adult smokers and adult nonsmokers with ADHD (Conners et al. 1996; Levin et al. 1996). More recent evidence from a cohort study examining the temporal relationship between ADHD and conduct disorder in adolescence and smoking in adulthood suggests that the relationship between ADHD and cigarette smoking may be mediated by conduct disorders (Brook et al. 2008). In another study, Rodriguez and colleagues (2008) suggest that ADHD symptoms of inattention are associated with the progression of nicotine dependence in adolescence, while hyperactivity-impulsivity ADHD symptoms are associated with the progression of nicotine dependence in young adulthood.
Research has found an association between childhood oppositional disorder and subsequent daily smoking behavior. Individuals with conduct disorder were found to have increased rates of nicotine dependence (Donovan et al. 1988), and Dierker and colleagues (2001) found that nicotine dependence significantly increased the risk of oppositional defiant disorder. There may be a gender difference in the nature of this relationship: the time between initiation of smoking and childhood oppositional disorder was found to be shorter among girls than among boys (Illomäki et al. 2008).
It should be noted that more serious mental health problems, such as schizophrenia, have generally been studied among adults, even though the precursors to these problems are evident in adolescents. With the very high prevalence of smoking among those with schizophrenia (70–85%), it seems important to identify these precursors for early intervention with this population, given that the onset of smoking generally occurs before 18 years of age and before the onset of the disorder (Weiser et al. 2004; Ziedonis et al. 2008).
Summary
Evidence is emerging that smoking is associated with various developmental and mental health disorders that affect adolescents and young adults. The available evidence extends to mental health disorders, such as schizophrenia, anxiety, and depression, and to developmental disorders, such as ADHD and conduct disorder. One complication in interpreting the available evidence is the temporality of the associations of smoking with the various disorders; that is, do mental health disorders increase risk for starting to smoke or does smoking increase risk for mental health disorders? There also is the possibility that smoking and a mental health disorder are linked through a common predisposition, possibly genetic or environmental. Cohort studies (i.e., longitudinal studies) are needed to conclusively establish the temporal relationship between mental health and developmental disorders and smoking.
The Use of Tobacco and Risk for Using Other Substances
Introduction
Evidence from a number of studies indicates that cigarette smoking is strongly associated with the use of other substances. For example, adult smokers are twice as likely as nonsmokers to have ever used illicit drugs (Farrell and Marshall 2006). In adults, associations vary with the level of nicotine dependence, with dependent smokers at much greater risk for dependence on alcohol, cocaine, and marijuana than are nonsmokers and nondependent smokers. For example, based on 1989 data from a sample of 21- to 30-year-old members of a Michigan health maintenance organization, nicotine-dependent smokers had 12 times the risk for cocaine dependence as that of nonsmokers, but smokers who were not nicotine dependent had only 6.5 times the risk (Breslau 1995). This study used the DSM-III-R definition of nicotine dependence.
Evidence in Adolescents and Young Adults
Among adolescents, early initiation of tobacco use is associated with the use of other substances (Kandel and Yamaguchi 1993). In a cohort study of adolescents, reports of “ever” and “daily” smoking were associated with increased risks in the future of using marijuana and other illicit drugs as well as disorders involving the use of multiple drugs (Lewinsohn et al. 1999). In addition, early-onset smokers were found to be more likely to have substance use disorders than late-onset smokers or nonsmokers (Hanna and Grant 1999). In a study by Lewinsohn and colleagues (1999), lifetime smoking among older adolescents significantly increased the probability of future use of alcohol, marijuana, hard drugs, or multiple drugs during young adulthood. Having been a former smoker, however, did not reduce the risk of future substance abuse disorders, although having maintained smoking cessation for more than 12 months was associated with significantly lower rates of future alcohol abuse. In another study, early onset of smoking was the strongest predictor of high-risk behaviors among middle school students (DuRant et al. 1999). A Finnish study found that younger onset of daily smoking was significantly related to the subsequent incidence of substance use disorders (Illomäki et al. 2008).
The association of tobacco use with alcohol use is strong. Grant (1998), for example, found that early onset of smoking was associated with early onset of drinking as well as with an increased risk for developing alcohol use disorders. In addition, a cross-sectional study by Koopmans and colleagues (1997) found that adolescent and young adult smokers were more likely to drink than were their nonsmoking counterparts, and this relationship appeared to be mediated more by shared environmental factors than by genetic factors. Other authors have found a positive association between the incidence of alcohol use disorders and nicotine dependence (Nelson and Wittchen 1998; Sonntag et al. 2000). More recently, Weitzman and Chen (2005) found that among young adult college students, 98% of smokers drank alcohol and up to 59% of drinkers smoked tobacco; the risk for co-occurrence was highest among students with the highest alcohol consumption, problems with alcohol, and symptoms of alcohol abuse. However, while a positive relationship has been observed between smoking and drinking, the temporality of this relationship remains unclear (Istvan and Matarazzo 1984; Sutherland and Willner 1998). Still, smokers are more likely to drink alcohol than are nonsmokers, and drinkers are more likely to smoke than are nondrinkers. The evidence also indicates a dose-dependent relationship, with greater use of one substance being related to greater use of the other (Zacny 1990). As adolescents enter young adulthood, the risks for tobacco and alcohol use increase. For example, in one study, 22% of college students reported starting to engage in heavy drinking during their first semester in college (Wechsler et al. 1994), a behavior that also is associated with risk for smoking behaviors.
The comorbidity of alcohol and tobacco use in young adulthood may originate in adolescence, as teens’ vulnerability to the use of other substances appears to be exacerbated by even experimental use of tobacco. For example, adolescent smokers are more likely to be heavier drinkers than are never smokers and have four times the risk of a comorbid alcohol use disorder; in fact, even those teens who only experiment with cigarettes are twice as likely to have an alcohol use disorder as are never smokers (Grucza and Bierut 2006). Studies of twins have implicated shared genetic factors as responsible for joint dependence on nicotine and alcohol (True et al. 1999).
Summary
Cohort studies show that smoking often antedates the use of other drugs in adolescents and is a risk factor for future use of drugs and alcohol (Kandel et al. 1992; Levine et al. 2011). In general, drugs of abuse such as smoking can cause neuroplastic changes in the brain that favor continued use (Benowitz 2010; Hong et al. 2010), and these changes may be more dynamic in the developing (e.g., adolescent) brain (Dwyer et al. 2008). Although smoking might increase risk for subsequent drug use through pharmacologic, environmental, developmental, and genetic factors (McQuown et al. 2007), vulnerability to drug use and future use likely relies on a variety of factors.
Smoking and Body Weight
Introduction
Weight control has been prominent in the marketing of cigarettes to females, influencing their decision making on the issues of starting to smoke and continuing to smoke (Suwarna 1985). This section addresses five key questions on smoking and weight for females and males in this age range:
- Do adolescents and young adults believe that smoking helps control body weight?
- Do adolescents and young adults use smoking in an attempt to control their body weight?
- Do concerns about body weight predict the initiation of smoking?
- Does concern about body weight affect the likelihood of smoking cessation?
- Does smoking actually affect body weight in adolescents and young adults?
The organization of this section is based on the mechanisms and pathways postulated as underlying the relationships between messages from the tobacco industry, other external influences, the perceptions of adolescents, and smoking behavior. First, the section addresses the use by industry of messages indicating that smoking is beneficial for weight control. These messages are hypothesized to have a direct impact on concern about weight gain and on the perceptions that cigarette smoking controls body weight and that initiation of cigarette smoking will reduce body weight. Those beliefs, in turn, may lead to the initiation of smoking, at least in certain susceptible groups (e.g., weight-conscious girls). Initiation can lead to nicotine addiction. This section concludes by addressing whether smoking cessation in young adults leads to weight gain and whether continued smoking has weight-control benefits in young adult smokers. Previous Surgeon General’s reports (summarized below) concluded that there is a relationship between smoking and body weight in adults, but this report focuses more specifically on the relationship between smoking and body weight in adolescents and young adults. The chapter does not address the biological basis of an association of smoking with body weight (see Chiolero et al. 2008 for a review). In this section, the same study may provide information to address one or more of the questions above. Additional epidemiological data relevant to smoking and weight control can be found throughout Chapter 3 of this report, too.
Methods for the Evidence Review
Studies investigating beliefs about smoking and body weight, the use of smoking to control weight, and the impact of weight-related attitudes, beliefs, and concerns on smoking behavior were identified through computerized searches of the PubMed, PsycINFO, PsycARTICLES, and PsycCRITIQUES electronic databases. Search terms included Boolean combinations of “smoking” and “weight control” paired with terms used to identify age-appropriate persons, including “youth,” “adolescent,” and “young adult.” To identify prospective studies examining the association between weight-related issues and changes in smoking behavior, the terms “initiation,” “onset,” and “cessation” were added to the searches. The references of identified articles were subsequently reviewed for additional studies that met inclusion criteria.
To address whether smoking affects body weight in younger people, relevant articles were identified through reviews of previous Surgeon General’s reports, computerized searches in databases such as PubMed, PsycINFO, PsycARTICLES, and Google Scholar, and examination of reference lists in primary research and review articles. The search terms used in these computerized searches were variations of the term “smoking” (e.g., “tobacco use”) paired with weight-related terms such as “body weight,” “body composition,” “BMI” (body mass index), and “weight control.” To focus on adolescent and young adult populations, additional terms such as “adolescent” and “youth” were used. The research articles included were peer-reviewed English-language papers published from 1989 to 2008, and the search was completed in August 2008. Relevant articles that did not provide data on age and weight by smoking status were excluded.
Beliefs of Youth and Young Adults Concerning Smoking and Control of Body Weight
Emphasis on Weight Control in Tobacco Advertising
Numerous examples document how the tobacco companies have employed advertising to indicate a relationship between smoking and body weight. Indeed, messages extolling the weight-controlling “benefits” of smoking have been a common theme in cigarette marketing for many decades. In the 1920s, in an early attempt to capture the previously untapped market of female smokers, the American Tobacco Company launched a groundbreaking advertising campaign for its Lucky Strike cigarette brand. The advertisements, which urged women to “Reach for a Lucky Instead of a Sweet,” promoted smoking as a weight-control strategy. Subsequent advertisements were even more direct in their messages (“To stay slender, reach for a Lucky, a most effective way of retaining a trim figure”; “To keep a slender figure no one can deny, reach for a Lucky instead of a sweet”). Other Lucky Strike advertisements employed scare tactics to prey on fears about weight gain by depicting exaggeratedly obese silhouettes in the form of shadows positioned next to trim female figures and featuring captions such as “Avoid that future shadow” or “Is this you five years from now?” (Amos and Haglund 2000; Ernster et al. 2000; USDHHS 2001). American Tobacco’s strategy helped to firmly establish the link between smoking and weight control in the minds of the consumer, and within the first year, the company saw a sales increase of more than 300%, making Lucky Strike the top-ranked brand in the country and marking one of the most successful tobacco advertising campaigns in history (Howe 1984; Ernster 1985; Pierce and Gilpin 1995; USDHHS 2001). The Lucky Strike campaign, combined with concurrent efforts by the makers of the Chesterfield cigarette to market cigarettes directly to women, contributed significantly to the dramatic increase in cigarette smoking in the late 1920s among adolescent girls and young women (Pierce and Gilpin 1995; USDHHS 2001).
Since the highly successful Lucky Strike campaign, an implied association between smoking and weight control has been used countless times. Tobacco companies have commonly employed slender, attractive young models in an effort to generate an image of female smokers as thin, pretty, and glamorous (Krupka et al. 1990; Brown and Witherspoon 2002). Furthermore, several cigarettes have been specifically designed to strengthen the perceived association between cigarette smoking and a slender physique. For example, cigarettes with brand names containing descriptors such as “thins” and “slims” have been manufactured to be longer and slimmer than traditional cigarettes and to appeal directly to women, helping to reinforce the belief that the smoking of certain brands is an effective weight-control strategy (Davis 1987; Albright et al. 1988; Califano 1995). This notion was further strengthened by the inclusion of slogans emphasizing thinness (e.g., Misty’s “Slim ‘n Sassy” and Silva Thins’ “I’m a thinner. Long and lean, that’s the way I like things. I like my figure slim, my men trim, and my cigarette thin”). In addition, several brands, including Virginia Slims and Capri, have come out with “super slim” versions of their cigarettes that are even more slender in design. The marketing campaigns for these products further emphasized weight control in their captions (e.g., Capri: “There is no slimmer way to smoke”; Virginia Slims Superslims: “Fat smoke is history. It took Virginia Slims to create a great tasting ultra thin cigarette that gives you more than a sleek shape”) and images. Furthermore, print advertisements for Virginia Slims Superslims in the early 1990s used images containing thin, elongated shapes and pictures of female models that appear to have been digitally “altered” to exaggerate their tall and lean appearance. As with Lucky Strike 40 years earlier, the introductory marketing of Virginia Slims in the late 1960s (which, in addition to glamour and thinness, famously emphasized autonomy and liberation through the theme “You’ve come a long way, baby”) was tremendously successful and was associated with a dramatic increase in the initiation of smoking among adolescent girls (Pierce et al. 1994; Pierce and Gilpin 1995; USDHHS 2001).
Given the prohibitions against billboard advertising and restrictions on print advertisements that resulted from the 1998 Master Settlement Agreement and changing media environment, tobacco companies have changed their marketing strategies in an effort to reach their target audience. One approach used increasingly has been the Internet, but to date, relatively little attention has been given to the content and impact of tobacco advertising posted on protobacco, primarily non-tobacco-company, Web sites. In one of the few studies in this area, Hong and Cody (2002) randomly selected more than 300 such Web sites and found that tobacco advertising on the Internet was widespread. Furthermore, they found that many of the themes commonly seen earlier in print advertising were included in Web-based campaigns. These advertisements on the Web often glamorize smoking by using youthful and attractive female models.
Young People’s Beliefs About the Impact of Smoking on Body Weight
Numerous studies, summarized in Table 2.2, have examined beliefs among youth about the utility of cigarette smoking as a weight-control strategy. Because of differences in methodology, sample characteristics, time period, and the methods through which beliefs were assessed, specific findings necessarily varied across studies. Regardless, this body of research indicates that a belief in the ability of cigarette smoking to help control body weight is quite pervasive among youth.
Table 2.2
Studies assessing belief that smoking controls body weight.
Most of the studies on the perceived impact of cigarette smoking on body weight have been conducted with samples of adolescents and young adults. Considering that adolescence and young adulthood are the developmental periods with the highest risk for initiation of smoking, a belief that smoking affects weight may have an especially potent effect in this age group. In an early study to examine perceptions about an association between smoking and body weight, Shor and colleagues (1981) surveyed 307 undergraduate students regarding their beliefs about the factors that motivate people to smoke cigarettes. Fifty-five percent reported the belief that smoking helps smokers avoid weight gain, with levels of agreement similar for smokers (59%) and nonsmokers (53%). Respondents were also asked whether they felt that smoking helped to control the quantity of food they ate, with 43% (smokers = 49%, nonsmokers = 41%) agreeing that this is a common characteristic of smoking.
In another early study, Charlton (1984) surveyed nearly 15,175 British students between the ages of 9 and 19 years regarding their smoking behavior and whether they agreed with the statement “Smoking keeps your weight down.” Twenty-three percent agreed that smoking helps to control weight, with similar levels of endorsement in girls (24%) and boys (22%). Beliefs in the weight-controlling effects of smoking were positively associated with personal smoking history; those who had never smoked were least likely to agree (16.6%), while students who smoked at least six cigarettes per week were the most likely to agree (42.2%) that smoking reduces body weight.
Camp and colleagues (1993), who investigated the relationship between concerns about body weight and cigarette smoking in a sample of 659 high school students, asked participants to indicate their agreement with the statement “Smoking cigarettes can help you control your weight/appetite.” Overall, 40.2% of adolescents agreed, with agreement considerably higher among smokers (67%) than among never smokers (37%). Differences were also noted across racial and gender subgroups. White girls were the most likely to believe that smoking helps to control weight (45.7%), followed by White boys (29.9%) and Black boys (13.5%). Among Black girls, only 10% endorsed this belief.
West and Hargreaves (1995) surveyed 117 female and 29 male nursing students (mean age = 24 years) in the United Kingdom in an effort to identify factors associated with smoking in this group. Overall, 34% of the participants were classified as current smokers. Participants rated their levels of agreement with 11 statements representing various beliefs about smoking, including its association with body weight (“Smoking helps with weight control”). Responses were on a five-point Likert scale ranging from “strongly disagree” to “strongly agree.” Smokers were significantly more likely (38%) than either former smokers (26%) or never smokers (11%) to agree or strongly agree that smoking aids weight control. Even so, beliefs about the effect of smoking on weight were not significantly associated with the desire to quit smoking.
Klesges and colleagues (1997a) examined the associations between concerns about weight and smoking as a function of smoking status, race, and gender among a sample of 6,961 seventh-grade students enrolled in the Memphis Health Project. These adolescents were asked whether they believed that smoking cigarettes helps people control their weight; 39.4% endorsed this belief. Levels of agreement increased with smoking history, with daily and other regular smokers most likely to endorse this belief, followed by experimental smokers and never smokers. A significant race-by-gender interaction was also noted. As in Camp and colleagues (1993), White girls were most likely to endorse this belief, but in contrast to that earlier study, White boys were least likely to believe that smoking controls body weight; Black girls and Black boys fell in the middle.
George and Johnson (2001) investigated weight concerns and weight-loss behaviors among an ethnically diverse group of 1,852 college students, an estimated 57% of whom were Hispanic (the remainder classified themselves as White [18%] or “other” [24%]), and 62% were female. More than 90% of the sample were 17–24 years of age. Participants were recruited from two undergraduate classes and completed a 73-item survey assessing lifestyle behaviors, attitudes toward weight control, height and weight, and the 10-item version of the Dietary Restraint Scale (Herman and Mack 1975). Participants were also asked, “How do you think that smoking affects your weight?” Response options were “keeps it down,” “no effect,” “keeps it up,” and “don’t know.” Overall, 24% of men and 17% of women reported that they smoked. Among current smokers, 22% of women and 16% of men said they thought that smoking helped keep their weight down. Forty-five percent of both male and female smokers responded that smoking had “no effect” on their weight, and 34% of men and 27% of women who smoked were uncertain of the impact of smoking on their weight. Associations of smoking with three weight-loss behaviors (dieting, exercise, and use of diet pills) were also assessed. Male smokers were significantly more likely than their nonsmoking counterparts to report having dieted to lose weight during the past month. Among female students, no overall differences in dieting status were observed between smokers and nonsmokers, but smokers were significantly more likely than nonsmokers to have used diet pills in the past month in an effort to lose weight. Among male students, in contrast, use of diet pills did not differ between smokers and nonsmokers. Exercise for weight loss was not related to smoking status among either men or women.
Boles and Johnson (2001) examined associations between beliefs about weight and cigarette smoking in a sample of 1,200 adolescent boys and girls between the ages of 12 and 17 years. Smokers (n = 140), but not non-smokers, were asked whether they thought that smoking helped them control their weight. Overall, 15% of smokers responded that it did, a rate lower than that observed in other studies reported in this review. Female smokers (22.2%) were significantly more likely to endorse this belief than were male smokers (9.9%). Agreement declined with age among males but increased with age among females.
Honjo and Siegel (2003) also investigated beliefs about the weight-controlling effects of smoking, in this case among adolescent girls 12–15 years of age who reported never smoking or smoking no more than one cigarette in their lifetime. Twenty percent of the girls responded affirmatively to the question “Do you believe that smoking helps people keep their weight down?”
Elsewhere, Vidrine and colleagues (2006) examined gender differences in expectations about the outcomes of smoking in a sample of 350 adolescent girls and 315 adolescent boys attending two same-gender high schools. Students were asked to come up with as many positive and negative expected outcomes from smoking as they could in 60 seconds, and they also completed measures of smoking behavior, susceptibility to smoking, and peer smoking. Overall, boys (6%) were less likely than girls (23%) to report expectations for smoking related to weight control (odds ratio [OR] = 0.22; 95% confidence interval [CI], 0.13–0.36, p <.001). Expectations did not differ significantly by smoking status for either gender.
Finally, few studies have examined whether younger children believe that smoking controls body weight. Kendzor and colleagues (2007), however, surveyed 727 children 7–13 years of age (mean age = 9.2 years) about their weight concerns and smoking history. In all, 38% of the children agreed that “smokers are thinner than non-smokers.” In contrast to the studies with older adolescents summarized above, agreement that smoking is related to weight control was greater in Black (50%) than in White (36.6%) children (p = .016). Endorsement of the belief that smokers are thinner than nonsmokers was highest in Black girls (53.1%), and it was lowest in White girls (35.6%), with Black and White boys in between.
The studies described above all involved elementary-age to college students. In contrast, Li and colleagues (1994) examined factors associated with cigarette smoking among a cohort of 585 Asian women 20–41 years of age who worked on airline cabin crews. The majority (87%) of these women were under 30 years of age, and 26% of the sample were current smokers. Participants were asked to rate the perceived probability of a series of potential positive and negative consequences of smoking, including weight control, on a scale from 0% to 100%. Thirty-seven percent of the total sample agreed that smoking helps to control body weight, with endorsement significantly higher among current smokers (48%) than for former smokers (29%) or never smokers (34%).
A few other studies have examined the association between the belief that smoking helps to control body weight and personal smoking status using items and scales devised to assess the perceived consequences of smoking or abstinence. Many of the studies have conducted comparisons according to smoking status or other characteristics without specifying an exact proportion of respondents who endorsed the belief that smoking promotes weight control. Loken (1982), for example, surveyed 178 college women regarding their beliefs about the health- and non-health-related consequences of cigarette smoking using seven-point bipolar scales ranging from −3 to +3. One of the beliefs examined was that “my smoking cigarettes keeps (would keep) my weight down.” Heavy smokers endorsed significantly stronger beliefs than did either light smokers or nonsmokers. No differences were observed between the three groups, however, on an affective scale assessing the positive or negative impact of keeping one’s weight down.
Brandon and Baker (1991) developed the widely used Smoking Consequences Questionnaire (SCQ) in an effort to assess the subjective expected utility (SEU) of cigarette smoking. Undergraduate college students 16–47 years of age (mean age = 18.7 years) rated the likelihood and desirability of some possible consequences of cigarette smoking listed on the SCQ. The cross-product of the likelihood and desirability ratings for each item was calculated to arrive at an index of subjective expected utility. On a factor of five items assessing the perceived impact of smoking on appetite/weight control, daily smokers scored significantly higher than either occasional smokers or never smokers. In addition, among former smokers, female students reported significantly greater expectations regarding the utility of smoking for helping to control weight and appetite than did males. Furthermore, daily smokers reported stronger expectations regarding the likelihood that smoking would aid weight control than did occasional smokers. Overall, comparisons with other categories of smoking status (former smoker, trier/experimenter, and never smoker) on the perceived likelihood that smoking would affect weight and appetite were not significant.
Cepeda-Benito and Ferrer (2000) developed a Spanish-language version of the SCQ (SCQ-S); as with the original questionnaire, the SCQ-S was designed to assess adults’ positive and negative expectancies of cigarette smoking. A confirmatory factor analysis conducted among 212 Spanish-speaking smokers (65% of them female) who were either college students or university employees (mean age = 22.5 years) supported an 8-factor, 40-item model. Among the eight subscales was a five-item scale to assess expectancies related to the effect of smoking on weight control; overall, women reported significantly greater expectancies than did men. Although scores on the weight-control subscale were positively related to a measure of nicotine dependence (β= .15, p = .033), this effect was not significant after Bonferroni adjustments were made for multiple comparisons.
Copeland and Carney (2003) investigated expectancies regarding the perceived consequences of smoking as potential mediators of the association between (1) dietary restraint and disinhibition and (2) cigarette smoking among a sample of 441 undergraduate women. Outcome expectancies related to smoking were assessed using the appetite/weight-control factor from the SCQ. Smokers reported significantly higher expectancies than did non-smokers relative to the impact of smoking on weight and appetite. In addition, expectancies for appetite and weight control were significantly associated with weekly smoking rate, with those consuming more cigarettes reporting greater expectations about the impact of smoking on weight/appetite.
In an effort to evaluate the subjective expected utility of smoking among children, Copeland and colleagues (2007) developed a revised version of the SCQ designed for children 7–12 years of age (SCQ-Child). The scale incorporated much of the original SCQ but was modified to account for reading level and the relevance of the items to make it more developmentally appropriate for the younger age group. In addition, items were modified from a Likert scale to a true/false format. Participants included 742 students in grades two to six who ranged in age from 7 to 13 years (mean age = 9.2 years). A confirmatory factor analysis was conducted to determine whether a one-, two-, three-, or four-factor solution was the most appropriate. Results indicated that a three-factor model (positive reinforcement, negative consequences/effects, appetite/weight control) comprised of 15 items provided the best fit with the data. The scale that assessed smoking-related expectations for appetite and weight control included two items: “Smokers are thinner than nonsmokers” and “Smokers eat less than nonsmokers.” Overall, 37.9% of the sample agreed that smokers are thinner than nonsmokers, and 52.2% agreed that smokers eat less than nonsmokers. Students with a family member who smoked had significantly lower scores on the Appetite/Weight Control scale; however, these students were less likely to perceive smokers as thinner or that smokers ate less than nonsmokers. Scores on that scale did not differ significantly according to gender, age, peer smoking, perceived availability of cigarettes, whether participants could get cigarettes from friends, or history of ever trying cigarettes.
In the largest study to date to assess the perceived impact of smoking on body weight, Wang and coworkers (1998) investigated attitudes and beliefs about smoking among a representative national sample of high school dropouts between the ages of 15 and 18 years as part of the 1993 Teenage Attitudes and Practices Survey (weighted N = 492,352). Beliefs about the weight-controlling properties of smoking were assessed with the statement “Smoking helps people keep their weight down.” The prevalence of smoking among those who agreed with this statement (69.1%) was significantly higher than among those who disagreed (54.6%).
In a study of young adults’ attitudes and beliefs about the positive and negative consequences of smoking, Budd and Preston (2001) surveyed 172 undergraduate students 19–51 years of age (mean age = 21.5 years). Using a scale that measured the perceived impact of smoking on body image, a scale that included items reflecting the degree to which respondents believed that smoking prevents weight gain and helps to keep a person thin, smokers scored significantly higher than did nonsmokers. Thus, smokers were more likely than nonsmokers to believe that smoking helps enhance body image through weight control.
Zucker and colleagues (2001) investigated factors associated with cigarette smoking among 188 female undergraduate college students between the ages of 17 and 25 years (mean age = 19.0 years). Students were surveyed regarding their smoking status, attitudes toward thinness, exposure to media depicting thinness, level of skepticism toward tobacco advertisements, and degree of feminist consciousness. In addition, they were questioned on their beliefs about smoking and body weight using their response to the statement “Smoking helps people control their weight.” Responses were on a seven-point Likert scale ranging from 1 (do not agree at all) to 7 (definitely agree). The belief that smoking helps to control body weight was positively correlated with measures of awareness of the societal emphasis on thinness as well as the degree to which respondents had internalized and accepted societal appearance standards. In addition, smokers endorsed significantly stronger beliefs than did nonsmokers regarding the weight-controlling effects of smoking. In a multivariate logistic regression model, those who considered that smoking is an effective strategy for weight control were significantly more likely to be current smokers.
Cachelin and coworkers (2003) examined the associations between dieting, smoking behaviors and attitudes, acculturation, and family environment in an ethnically diverse sample of 211 adolescent boys and girls (mean age = 16.3 years) recruited from junior and senior high schools. Fifty-seven percent of the youth were Asian, 16% Hispanic, and 27% White. Participants completed a survey assessing smoking behaviors, beliefs and attitudes toward smoking, family functioning, and acculturation. Smoking-related questions included two items from the Smoking Beliefs and Attitudes Questionnaire (Pederson and Lefcoe 1985) assessing beliefs about the impact of smoking on body weight: “Smoking keeps you from eating” and “Smoking helps you control your weight.” In addition, the students were classified as dieters or nondieters depending on their responses to the 10-item Restraint Scale (Herman 1978). Overall, female dieters were more likely than nondieters to be current smokers; female dieters were also more likely to endorse the belief that smoking keeps one from eating. Dieting status was not, however, significantly related to the belief that smoking controls body weight. In addition, compared with nonsmokers, female smokers had significantly higher dietary restraint scores. No significant relationships were observed among male students between dieting and any of the smoking-related items.
In one of the few international studies located in the various searches described above that investigated young people’s beliefs about the impact of smoking on body weight, Facchini and colleagues (2005) surveyed 144 female students in Argentina between the ages of 18 and 27 years (mean age = 20 years) who were attending a state-run school for nurses and preschool teachers. Participants completed items assessing smoking history and beliefs about smoking. With regard to beliefs, participants were asked to indicate their level of agreement with the statement “Smoking helps to control weight” on a five-point scale. In all, 47% of the students were cigarette smokers. Smokers expressed higher endorsement than did non-smokers of the belief that smoking helps to control weight (mean score = 2.6 [1.16] vs. 1.9 [0.99], p <0.01). In addition, in multiple logistic regression analyses, beliefs about the weight-controlling effects of smoking were a significant independent predictor of smoking status.
Cavallo and coworkers (2006) examined the extent to which adolescent smokers believed smoking helped to control their weight. Participants, who were 103 daily smokers between the ages of 14 and 18 years, were asked to respond to the question “How much do cigarettes help you control your weight?” using a Likert scale ranging from 1 (not at all) to 5 (very much). Females endorsed stronger beliefs than did males. The belief that smoking helps to control weight was positively associated with daily smoking rate and negatively associated with number of years of smoking. In addition, a significant interaction between gender and BMI was noted. For males, the belief that smoking controls body weight was positively associated with BMI (p <0.1), but among females there was a nonsignificant inverse relationship between BMI and the perceived weight-controlling effects of smoking.
Recently, Bean and colleagues (2008) investigated attitudes toward smoking and weight control in a sample of 730 rural high school students 12–20 years of age (mean age = 15.7 years). In addition to being asked about smoking history and body weight, participants were questioned about the perceived consequences of abstaining from tobacco (e.g., weight gain) as well as their personal attitudes about the association between smoking and body weight. For the latter, a composite score was derived from students’ levels of agreement with three items asking about weight-related reasons that people might smoke (“it helps them lose weight,” “it helps them stay thin,” and “it makes them less hungry”). Overall, girls scored significantly higher on the belief that people smoke to control weight (i.e., their composite score was significantly higher). Boys, for their part, endorsed stronger beliefs that remaining or becoming tobacco free would lead to weight gain. Interestingly, current smokers were significantly less likely than either experimental smokers or nonsmokers to believe that people smoke to control their weight. However, current smokers were more likely than both experimental smokers and nonsmokers to believe they would gain weight by being tobacco free. In stratified analyses by gender, however, this relationship remained significant only among girls.
Finally, McKee and associates (2006) investigated the associations between dietary restraint, primed visuals of body images, and expectations that smoking can control body weight among 40 undergraduate female smokers (mean age = 20.0 years). Participants were randomly assigned to view one of two sets of images representing either pictures of thin, attractive fashion models or landscape scenes. The former were intended to serve as primes for body image, and the latter were included as neutral control stimuli. Restrained eaters exposed to the body image primes scored significantly higher than those viewing the neutral images on the appetite/weight-control scale of the SCQ. They also scored higher than nonrestrained eaters exposed to either of the two types of primes. These findings suggest that beliefs about the impact of smoking on body weight among smokers may be modified by weight-related attitudes and behaviors as well as by media messages associated with body image.
Summary
These studies show that the belief that smoking helps to control body weight is not unusual among youth and young adults. Adding strength to this conclusion is the fact that the studies were carried out over several decades in diverse populations using varied methodologic approaches. Overall, belief in the weight-controlling effects of smoking tends to increase with smoking experience: current smokers and those having more extensive smoking histories typically endorse stronger beliefs than do nonsmokers. Studies that investigated gender differences regarding beliefs about the effect of smoking on body weight generally found greater endorsement among females, with some exceptions noted. Few studies compared beliefs about smoking and body weight by race or ethnicity (Camp et al. 1993; Klesges et al. 1997a; Kendzor et al. 2007).
Use of Smoking by Children and Young Adults to Control Weight
School and Population Surveys
The fact that many adolescents and young adults believe that cigarette smoking helps to control body weight does not necessarily mean that this belief actually influences smoking behavior. In several studies, however, youth have been questioned about the methods they use to control their weight and the reasons that they smoke in an effort to determine whether young people do, in fact, smoke cigarettes as a weight-control strategy. This section reviews the evidence that some adolescents and young adults smoke specifically for purposes of weight control (Table 2.3).
Table 2.3
Studies assessing use of smoking to control body weight (school and population surveys).
In an early study, Klesges and colleagues (1987) surveyed 204 male and female college students regarding the strategies they had used during the past 6 months to help them control their weight. In addition to reporting commonly used methods of restricting energy intake such as skipping meals, eating less, and controlling portions, a number of respondents indicated that they used cigarettes or caffeine as a weight-control strategy. Because smoking cigarettes and using caffeine were combined to make a single survey item, the authors could not determine the proportion of respondents who used each method. Overall, females (21%) were significantly more likely than males (4%) to endorse this combined item. Use of smoking/caffeine for purposes of weight control was also positively associated with body weight, with overweight males and females most likely to use this method (22%), followed by those who were normal weight (13%) and underweight (2%). Results were not reported by current smoking status.
In a follow-up study, Klesges and Klesges (1988) surveyed a sample of 1,076 university faculty, staff, and students 16–72 years of age (mean age = 21.7 years) about their use of smoking as a weight-control strategy. The prevalence of smoking among the sample was similar for males (21%) and females (18%). Overall, 32.5% of smokers reported using smoking as a weight-loss strategy. Although common in both genders, this practice was reported more frequently by female (39%) than by male (25%) smokers. The proportion of smokers using smoking to control weight did not differ significantly between overweight (34%) and normal-weight smokers (29%). Age appeared to make a difference, however, as smokers under the age of 25 years were significantly more likely than older smokers to use smoking as a weight-control strategy (38.0% vs. 23.4%). Ten percent of male smokers and 5% of female smokers reported that they started smoking specifically to help them lose weight or to maintain their weight. Although there were no main effects of gender or weight status on the proportion of respondents who initiated smoking for weight loss, a significant gender-by-body-weight interaction was found, with overweight women (20%) much more likely than other groups to report starting to smoke for this purpose.
Worsley and coworkers (1990) examined the weight-control practices of 809 15-year-old New Zealand youth, questioning participants about their current weight, perceptions of their ideal weight, monitoring of their body weight, intentions regarding weight control, and reasons for attempting weight loss. The youth were also surveyed about the weight-loss techniques they had used over the past year, including both healthy and unhealthy dietary practices and exercise. Significantly more girls (5%) than boys (2%) reported they had smoked cigarettes to control their weight.
Frank and colleagues (1991) investigated weight loss and disordered eating behaviors among 364 undergraduate female college freshmen (mean age = 18 years). Students completed a questionnaire that assessed use of purgatives (self-induced vomiting, laxatives, diuretics) and diet pills as well as other health behaviors such as cigarette smoking and use of alcohol and other psychoactive substances. Fourteen percent of participants reported being current smokers. Among those who smoked, 37% reported that one of the reasons they did so was to control their weight. Those in the study who reported currently engaging in some form of purging behavior for weight control were four times as likely to smoke as those who did not engage in purging behaviors (44% vs. 11%).
In their study described earlier of the association between smoking and concerns about body weight among high school students, Camp and colleagues (1993) also investigated the use of smoking to control weight. Fifteen percent of the students were classified as regular smokers, defined here as smoking one or more times per week. Thirty-nine percent of all female regular smokers reported using smoking to control their weight versus 12% of male regular smokers. Notably, among regular smokers, 61% of White females and 12% of White males reported smoking for weight control, but no Black regular smoker endorsed smoking for this reason. Multivariate logistic regression analyses indicated that female gender, increasing age, and dietary restraint were all positively associated with smoking for weight control.
In the previously described Memphis Health Project, Klesges and colleagues (1997a) also questioned the 240 seventh graders with a history of active smoking about whether they had ever smoked to control their weight or to lose weight. Twelve percent of smokers reported this practice. As in other studies, among smokers, girls were more likely than boys to report smoking in an effort to control their weight (18% vs. 8% in this study). Differences between Black (9%) and White (15%) smokers were not significant. Consistent with findings of Camp and coworkers (1993), White female smokers (27%) were by far the most likely to report smoking for weight control. Eleven percent of Black females reported smoking to control their weight; rates were lower but generally similar for White (8%) and Black (7%) males.
In a subsequent set of analyses from the same data set (Memphis Health Project), Robinson and colleagues (1997) examined predictors of risk for different stages of smoking. The authors performed multivariate logistic regression analyses to identify demographic, social, environmental, proximal, and distal factors as well as weight-related variables that distinguished between different levels of smoking. Three groups were defined: (1) never smoker, (2) experimental smoker (<1 cigarette per week), and (3) regular smoker (=1 cigarette per week). Use of smoking to control weight emerged as the single best predictor of regular versus experimental smoking. Specifically, students who reported smoking for weight control were 3.34 (95% CI; 1.60–6.95) times as likely to be regular smokers as those who did not report smoking for this reason. These findings suggest that smoking for weight control may be not only a factor in initial decisions to smoke but also a tool for distinguishing those who are more likely to progress to a heavier stage of smoking.
Ryan and colleagues (1998) investigated weight-loss strategies used by 420 female students 14–17 years of age (mean age = 15 years) in Dublin, Ireland; participants indicated whether they had used various weight-loss strategies including exercise, avoiding sugary foods, and several forms of dieting. Also included as strategies were unhealthy practices such as skipping meals, self-induced vomiting, taking laxatives, fasting, using diet pills or formula diets, and smoking. Overall, 13% of the participants reported smoking to control their weight. Among the 286 students who reported they had tried to lose weight in the past, 19% indicated they had smoked for this reason.
In a study of the associations between cigarette smoking and body weight, Crisp and associates (1998) surveyed 2,768 schoolgirls 10–19 years of age in Ottawa, Canada (N = 832), and London, England (N = 1,936). The questionnaire assessed current weight, history of weight change, dietary patterns, weight concerns, reasons for smoking, expected consequences of giving up cigarette smoking, and self-induced vomiting. Overall, 15% of the Ottawa students and 19% of the London students reported cigarette smoking (either occasional or regular, definitions not given). In both locations, girls who smoked were significantly more likely to report weight concerns, self-induced vomiting, and a “proneness for overeating.” Regarding reasons for smoking, 33% of Ottawa students and 21% of students from London reported they smoked “instead of eating.” The proportion of students in Ottawa and London who endorsed smoking because it “makes (them) less hungry” were 36% and 19%, respectively. Thirty-four percent of Ottawa students expected to eat more if they gave up smoking, and 33% anticipated gaining weight. Among London students, the proportions who anticipated these consequences of quitting smoking were 30% and 31%, respectively.
As noted earlier, George and Johnson (2001) investigated the association between weight concerns and lifestyle behaviors among 1,852 male and female college students; as part of the survey, participants were asked to identify their primary reason for smoking. Options included “control weight,” “habit,” “taste-feeling,” and “friends.” The most commonly endorsed reasons were habit (46% of men, 45% of women) and taste-feeling (43% of men, 37% of women). Weight control was cited the least, with just 4% of female smokers and 1% of male smokers identifying this as their primary motivation to smoke.
Crocker and colleagues (2001) examined associations between smoking, dietary restraint, and physical characteristics and self-perceptions in a sample of 702 ninth-grade girls 14–15 years of age. Participants completed a survey assessing physical characteristics, physical self-perceptions, dietary restraint, and smoking behavior, and they completed the Smoking Situations Questionnaire (SSQ; Weekley et al. 1992), a six-item scale designed to assess the use of smoking for purposes of weight control. In all, 19% of the students were classified as weight-control smokers on the basis of a score of less than 2 (out of 6) on the SSQ. BMI did not differ between those who reported and those who did not report smoking to control their weight. However, weight-control smokers demonstrated significantly higher levels of dietary restraint as well as lower scores on measures of global self-esteem, perceived body attractiveness, and physical condition.
Granner and coworkers (2001) investigated the associations between race, risk for eating disorders, use of alcohol, smoking, and motivations for alcohol and tobacco use in a sample of 206 Black and White undergraduate college students (mean age = 20.6 years). Participants were administered a survey that assessed smoking status, alcohol consumption, and reasons for smoking and drinking. In addition, participants completed the Eating Disorder Inventory-2 (EDI-2; Garner 1991) and the Weight Control Smoking Scale (WCSS; Pomerleau et al. 1993). In all, 34.0% of Whites and 8.7% of Blacks in the sample reported being current smokers (no specific definition provided). Twenty percent of White smokers and 11.1% of Black smokers were categorized as smokers for weight control on the basis of a score of =6 on the WCSS (χ2 = 0.38, p = 0.54). Overall, 56% of Black smokers and 60% of White smokers endorsed at least one item regarding the use of smoking to control weight, appetite, or hunger. Smokers scored significantly higher than nonsmokers on several subscales of the EDI-2, including Body Dissatisfaction, Drive for Thinness, Ineffectiveness, and Social Insecurity. Finally, students classified as being at increased risk for an eating disorder on the basis of elevated scores on the Body Dissatisfaction and Drive for Thinness subscales of the EDI-2 were significantly more likely to smoke and scored significantly higher on the WCSS than those not identified as at risk.
Neumark-Sztainer and associates (2002) examined racial and ethnic differences in weight-related concerns and behaviors in a population-based sample of 4,746 adolescent boys and girls in grades 7–12 (mean age = 14.9 years). Participants were surveyed on their current and perceived weight status, weight concerns, and level of body satisfaction as well as on their use of healthy and unhealthy weight-control behaviors, including “smoked more cigarettes.” Overall, 9.2% of girls and 4.7% of boys reported using cigarette smoking as a weight-management strategy. Among all females, Native Americans were most likely to report smoking for weight control (23.3%), followed by Whites (10.5%), Hispanics (9.3%), Asian Americans (7.1%), and African Americans (6.1%). Among all males, Native Americans were also the most likely to report smoking for weight control (8.7%); Hispanic (6.7%) and Asian American boys (6.5%) reported similar levels of smoking to manage their weight, followed by Whites (4.1%). Again, African Americans were least likely to report smoking for weight control (2.8%). These racial/ethnic group differences were statistically significant.
The Minnesota Student Survey, which is administered to middle and high school students in that state, is the largest study to date to examine smoking for weight control among adolescents (Croll et al. 2002; Fulkerson and French 2003). The 1998 survey, which included items to assess disordered eating behavior, was administered to 81,247 9th- and 12th-grade students. Students were asked to identify methods they had used to lose or control their weight during the past 12 months, with options including fasting or skipping meals, using diet pills or speed (methamphetamines), self-induced vomiting after eating, using laxatives, and cigarette smoking. Overall, among all students, 18.2% of girls and 9.8% of boys reported smoking for weight control, with this practice most common among Native Americans (females = 29.4%, males = 20.5%), followed by those identifying themselves as multiracial (females = 26.5%, males = 13.7%). Hispanic (females = 18.4%, males = 15.3%) and White (females = 18.2%, males = 9.8%) youth generally had intermediate rates (data not shown in Table 2.3). Among Asian Americans, the rates were 11.7% for girls and 10.7% for boys; they were lowest for Blacks: 6.6% for girls and 7.4% for boys. The authors did not formally test for heterogeneity by racial/ethnic group.
The 1998 survey also assessed smoking for weight control among students who reported smoking within the past 30 days. Rates of smoking to control weight among smokers (by gender) were as follows (females listed first): multiracial (55.0% and 31.3%), Asian American (50.0% and 35.0%), Native American (49.4% and 38.2%), White (48.6% and 26.5%), and Black (32.6% and 27.8%). Compared with White female smokers, adolescent girls who were multiracial were significantly more likely to smoke to control their weight (OR = 1.25; 95% CI, 1.07–1.48), and Black females were significantly less likely to do so (OR = 0.50; 95% CI, 0.35–0.70). Relative to White male smokers, Native American (OR = 1.62; 95% CI, 1.19–2.22) and Asian American (OR = 1.44; 95% CI, 1.15–1.80) boys were more likely to smoke for weight control. Weight concerns, perceiving oneself as overweight, and higher smoking rates were significantly associated with smoking for weight control, with the strength of these relationships varying across gender and racial/ethnic subgroups.
Forman and Morello (2003) investigated the relationships between weight concerns, smoking, and perceived difficulty in quitting among 2,524 Argentinean adolescents in the 8th and 11th grades. Smoking for weight control was determined by three separate items designed to identify those who (1) initially tried smoking to keep their weight down, (2) smoked to avoid eating when hungry, and (3) continued smoking to maintain their weight. Girls were more likely than boys to report each of these behaviors: tried smoking to keep weight down, 11.3% versus 4.0%; smoked to avoid eating, 22.3% versus 12.9%; and continued to smoke to keep weight down, 16.0% versus 7.0%. In addition, boys and girls who smoked and who reported that they smoked to avoid eating and continued to smoke to keep their weight down were significantly more likely to perceive difficulty in quitting than were those who did not report smoking for these reasons. Having initially tried smoking in an effort to manage weight was not associated with perceived difficulty in quitting for either boys or girls.
Dowdell and Santucci (2004) investigated the prevalence of health-risk behaviors related to nutrition, weight, physical activity, alcohol, and smoking in a seventh-grade class of 54 students in a parochial school, in a low-income neighborhood, by using items from the Youth Risk Behavior Surveillance System questionnaire. Overall, 70% of the students reported trying cigarettes during their lifetime, and 55% reported current daily smoking. Among those who smoked cigarettes, 62% reported that the main reason was to control their weight. The authors indicated that girls were more likely than boys to report smoking as their primary means of weight control, but data by gender were not reported.
Nichter and colleagues (2004) conducted a mixed-methods study that combined ethnographic interviews and quantitative surveys to examine the use of smoking as a weight-control strategy among adolescent girls and young women. The participants were students taking part in a longitudinal study of the relationships between body image, dieting, smoking, and advertising. The students took part in a semistructured interview and completed a questionnaire annually for 3 years, starting in the eighth or ninth grade. In the third year of the study, 205 students provided data on smoking for purposes of weight control. Five years later, 178 students were recontacted for a follow-up interview.
During the study’s third year, when the participants were in the 10th or 11th grade (mean age = 16.02 and 16.99 years, respectively), 30% of the respondents were current smokers (either occasional or regular smokers). Eleven percent of current smokers responded affirmatively to the question “Did you start smoking as a way to control your weight?” An estimated 25% of current smokers endorsed the statement, “I sometimes smoke so I’ll be less hungry,” while 21% of regular smokers indicated they smoked instead of snacking “a lot of the time” and 33% reported they did so “sometimes.” Overall, an estimated 20% of students (i.e., nonsmokers, occasional smokers, plus regular smokers) agreed with the statement, “In general, I think people who smoke cigarettes are thinner than people who don’t smoke.” No differences in the proportion of students who were dieting were observed between smokers and nonsmokers.
At the 5-year follow-up interview (mean age = 21.67 years), 30% of the sample was classified as current smokers and 5% were former smokers. Eight percent of this subgroup of current and former smokers indicated they had initially started smoking to control their weight, while 15% reported smoking at some point to control their weight. Twenty percent of current and former smokers indicated they had sometimes smoked so they would be less hungry, and 3% reported they sometimes smoked at the end of a meal so they would not continue eating. When asked about concerns related to gaining weight if they quit smoking, 48% indicated they were “somewhat concerned,” and 50% reported they were “not at all concerned.”
Facchini and colleagues (2005), in their study of smoking and weight-control beliefs and behaviors among female Argentinean students described earlier, asked participants to indicate their motivations for initiating smoking, reasons they currently smoked, anticipated consequences of quitting smoking, and reasons for not quitting smoking. Included among the response options were reasons related to hunger, eating, and the perceived weight-related effects of smoking. In addition, participants were classified as restrained or unrestrained eaters based on their responses to the 10-item restrained eating subscale from the Dutch Eating Behavior Questionnaire (van Strien et al. 1986). Among the reasons chosen for initially starting smoking were “to avoid eating” (9%), “because it makes them less hungry” (7%), and to “control weight” (4%). Issues related to weight control were also commonly reported as reasons for continuing to smoke. For example, 27% reported “because it makes them less hungry,” 24% “instead of snacking when bored,” 19% “at the end of a meal so won’t eat too much,” and 16% “to avoid eating.” In terms of consequences, nearly one-half (48%) expected to eat more if they quit smoking, and 34% believed they would gain weight if they stopped. Regarding reasons for not quitting, 37% reported concerns about eating more, and 34% identified fears of gaining weight. The researchers also found that smokers classified as restrained eaters scored higher on the restrained eating scale than did nonsmoking restrained eaters. Finally, those who reported smoking for weight control scored higher in dietary restraint than did smokers who did not smoke to control weight.
Malinauskas and colleagues (2006) compared the dieting practices of 113 normal-weight, 35 overweight, and 21 obese female college students between the ages of 18 and 24 years who completed a survey assessing perceptions about weight, perceived sources of pressure to control their weight, and level of physical activity. In addition, these students were asked to identify which of 15 different weight-management practices they currently followed. Such practices included both healthy behaviors (eating low-fat foods, exercise, self-monitoring of energy and kilocalories) and unhealthy behaviors (skipping meals, self-induced vomiting, use of laxatives, and cigarette smoking). Nine percent of the respondents reported that they smoked cigarettes to lose or control weight. This practice was reported most frequently by overweight students (14%), followed by those who were normal weight (8%) and students who were obese (5%).
Two studies (Plummer et al. 2001; Park et al. 2003) addressed associations between stage of change and temptations to smoke to control weight rather than actual smoking behavior. In the first study (Plummer et al. 2001), participants were 2,808 ninth-grade students enrolled in a 4-year study examining behaviors related to smoking, sun protection, and intake of dietary fat. Students completed measures of the stage of cessation (for current smokers) and onset (for nonsmokers) and a measure developed by Ding and colleagues (1994) of temptations to smoke (all participants); this last item assessed the degree to which respondents would feel tempted to smoke in various situations. Included in the measure of temptations were two items that assessed being tempted to smoke for purposes of weight control (“when I am afraid I might gain weight,” “when I want to get thinner”). Among smokers, there was a linear relationship between stage of change and temptations to smoke to control weight, with those in the pre-contemplation stage reporting the highest temptation to smoke for this reason and those in the maintenance stage reporting the least. A similar linear trend was observed for nonsmokers. In that group, those in the acquisition-preparation phase reported significantly higher temptations to smoke for weight control than those in the acquisition-contemplation stage, who, in turn, expressed greater temptations to smoke that were related to weight control than did those in the acquisition-precontemplation stage.
In the second study, Park and colleagues (2003) investigated factors associated with stage of change among 297 male and female high school students in Korea who were current (n = 186) or former (n = 111) smokers. The students completed a survey assessing their smoking history, stage of change, processes of change, and decisional balance (a concept in which pros and cons combine to form a decisional balance sheet of comparative potential gains and losses). In addition, participants completed the measure of being tempted to smoke developed by Ding and coworkers (1994), which included the two items described above on temptation to smoke for weight control. Similar to the results reported by Plummer and colleagues (2001), overall temptations to smoke for purposes of weight control differed significantly as a function of stage of change. Although weight-related temptations to smoke generally decreased across the stages from precontemplation to maintenance, none of the post hoc comparisons between individual groups was statistically significant.
The studies summarized above investigated the prevalence of smoking for weight control among various groups; some other studies did not assess the proportion of the sample engaged in this practice but instead made comparisons between different groups of smokers and nonsmokers on measures of smoking for weight control in an effort to learn more about the mechanisms involved in this behavior. For example, Jarry and colleagues (1998) examined the associations between dieting, smoking status, weight gain, and smoking for purposes of weight control among 220 female undergraduate students. Never smokers (46.8% of the sample) were asked to indicate whether they had ever considered starting to smoke to avoid gaining or to lose weight. Current and former smokers (36.4% and 16.8% of the sample, respectively) were asked the extent to which they agreed with the statements “I started smoking to avoid gaining weight or to lose weight” and “I smoke(d) to avoid gaining weight or to lose weight.” Dieting status was determined from scores on the Revised Restraint Scale (Polivy et al. 1988). Among never smokers, dieters were marginally more likely to agree that they had considered starting smoking to avoid gaining or to lose weight (p = .08). Among current and former smokers, dieters were significantly more likely to report they had started smoking to control their weight and that they continued to smoke for this reason. In addition, current smokers were significantly more likely than former smokers to report that they started to smoke and continued to smoke for purposes of weight control.
In a study described earlier, Zucker and colleagues (2001) also assessed the use of smoking for purposes of weight control among 75 female undergraduate students who reported cigarette smoking on a daily basis; smoking for weight control was assessed using the three-item WCSS (Pomerleau et al. 1993). In a multivariate logistic regression analysis to identify significant predictors of smoking for weight control, the belief that smoking helps people control their weight was associated with smoking for this purpose. Internalization of societal standards for thinness was also positively associated with smoking for purposes of weight control, and scores on a measure of feminist consciousness were negatively related to smoking for that purpose.
In a laboratory study, Jenks and Higgs (2007) examined the associations between dieting and smoking-related behaviors in 30 female smokers (mean age = 20 years), one-half of whom were currently dieting to lose weight. Participants completed a revised version of the WCSS (Pomerleau et al. 1993). Two items were included to assess the extent to which weight concerns influenced decisions to initiate smoking (“I started smoking to control my weight”) and cessation (“I am concerned about weight gain upon smoking cessation”), both of which were scored on a visual analog scale ranging from “totally disagree” to “totally agree.” In addition, participants attended two laboratory sessions; food cues (cookies) were present during one of the sessions but not at the other. Ratings of heart rate, expired carbon monoxide, and mood were obtained both before and after smoking a cigarette. Dieters were more likely than nondieters to report having initiated smoking to control their weight and expressed greater concerns about weight gain upon cessation. In addition, on the WCSS, dieters reported stronger motivation to smoke for purposes of weight control. Finally, dieters (but not nondieters) reported significantly greater urges to smoke during the session in which food cues were present.
Smoking for Weight Control in Clinical Studies
Several studies have demonstrated elevated rates of cigarette smoking among patients with eating disorders, particularly those with bulimia and/or other diagnostic categories containing binge/purge subtypes (Bulik et al. 1992; Anzengruber et al. 2006; Krug et al. 2008), as well as evidence of the use of cigarette smoking for purposes of weight control among patients with eating disorders. These studies are summarized below and presented in Table 2.4.
Table 2.4
Studies assessing use of smoking to control body weight (clinical samples).
Welch and Fairburn (1998) investigated smoking rates and weight-related reasons for smoking and relapse among 102 female patients with bulimia nervosa (mean age = 23.7 years), a control group of 102 patients with anxiety or mood disorders who were matched for age and socioeconomic status (SES), and 204 age- and SES-matched healthy controls. Rates of current smoking were significantly higher among patients with bulimia (57%) than in psychiatric controls (29%) and healthy controls (24%). In addition, patients with bulimia reported substantially higher rates of smoking to avoid eating or to control their weight (73%) than did either psychiatric (19%) or healthy (13%) controls. Among current smokers who had ever achieved at least 6 months of abstinence from smoking, 28% of patients with bulimia indicated they had resumed smoking because of concerns about their weight or their shape. Corresponding rates for psychiatric and nonpsychiatric controls were 4% and 2%, respectively.
Crisp and colleagues (1999) investigated the associations between tobacco use, concerns about body weight, reasons for smoking, and anticipated consequences of giving up smoking in a sample of 879 females from the United Kingdom who were 17–40 years of age and either currently or formerly had an eating disorder. Participants were recruited from a nationwide support organization for eating disorders and were asked to complete a postal questionnaire addressing issues related to smoking and weight control along with the EDI (Garner and Olmsted 1984). Twenty-eight percent of the women were characterized as smokers. Overall, cigarette smokers scored significantly higher on the Bulimia, Interoceptive Awareness, and Maturity Fears subscales of the EDI (Garner et al. 1983) and were more likely to report self-induced vomiting. No differences between smokers and nonsmokers were observed on any of the other five subscales of the EDI, including Drive for Thinness. When questioned regarding their reasons for smoking, participants reported high levels of smoking for weight/appetite control purposes, including “instead of eating” (70%), “makes me less hungry” (52%), “when I feel like bingeing” (50%), and “to control my weight” (48%). In addition, 40% of smokers indicated they expected to experience weight gain as a consequence of giving up smoking.
More recently, Krug and coworkers (2008) compared current and lifetime substance use between patients with eating disorders and healthy controls as well as the use of smoking to influence appetite or weight. Participants included 879 patients with eating disorders (anorexia—restrictive subtype, anorexia—bulimic and/or purging subtype, bulimia, or eating disorder not otherwise specified [ED-NOS]; mean age = 27.2 years, 96.6% female) and 785 healthy controls (mean age = 24.3 years, 91.2% female) who were taking part in the Fifth European Framework Programme on Healthy Eating. Rates of both lifetime smoking (47.5% vs. 35.1%) and current smoking (34.8% vs. 24.2%) were significantly higher among patients with eating disorders than among healthy controls. Lifetime and current rates of smoking instead of eating to control appetite and weight were also significantly higher among patients with eating disorders than in healthy controls (lifetime: 34.1% vs. 9.2%; current: 26.8% vs. 9.1%). Within various subtypes of eating disorders, rates of overall smoking and smoking for weight control tended to be highest for patients with bulimia and anorexia—bulimic and/or purging subtype, followed by those with an ED-NOS and anorexia—restrictive subtype.
Summary
The findings reviewed above and summarized in Tables 2.2 and 2.3 indicate that a notable proportion of youth believe that smoking helps control body weight and that for some young smokers, this belief is an important factor in their decision to use tobacco. The data on use of smoking for weight control, however, are limited by being largely cross-sectional. Consequently, the direction of the associations between smoking and its use for weight control are uncertain. There are few longitudinal studies that examine the association of use of smoking to control body weight over time, particularly as body weight changes during adolescence and young adulthood.
Concerns About Body Weight and Risk for Smoking Initiation
Prior Reviews and Studies
Two earlier systematic reviews summarized the literature on the relationship between weight concerns and smoking in youth (French and Jeffery 1995; Potter et al. 2004); this section summarizes the primary findings from prospective studies included in the more recent review (Potter et al. 2004) of the association between concerns about weight and onset of smoking. It also updates research findings based on longitudinal studies published after the review by Potter et al. (2004) as a way of investigating the relationship between concerns about weight and smoking initiation.
In the first of the seven prospective studies of interest reviewed by Potter and coworkers (2004), French and colleagues (1994) examined the associations between concerns about weight, dieting, and initiation of smoking in a sample of 1,705 adolescents in grades 7–10. The students completed a questionnaire assessing smoking behavior and measures of concerns about weight, dietary restraint, symptoms of eating disorders, and dieting behavior at baseline and 1 year later. Girls with two or more symptoms of eating disorders, those who had tried to lose weight in the past year, and those who experienced constant thoughts about weight were all estimated to be twice as likely to start smoking within the subsequent year as girls not in these classifications. Dietary restraint, concerns about weight gain, and the desire to be thin were not associated with initiation of smoking. Among boys, none of the measures of weight concern and dieting behavior were related to the onset of smoking.
Killen and colleagues (1997) investigated risk factors for initiation of smoking among two cohorts of adolescents (N = 1,901) who were surveyed in the ninth grade and again 3 or 4 years later. A variety of potential predictors of smoking were assessed, including peer influences, alcohol use, temperament, BMI, and depressive symptoms. In addition, female participants completed the Drive for Thinness subscale from the EDI, which assesses level of preoccupation with body weight, concerns with dieting, and pursuit of thinness. Among girls who reported no history of smoking at baseline, levels of concern about weight, as measured by the Drive for Thinness subscale, were not related to initiation of smoking over time.
Patton and associates (1998) examined predictors of smoking initiation over a 3-year period among 2,032 14- and 15-year-old students in Australia. Participants reported their smoking history and cigarette consumption during the past 7 days. Dieting status was assessed using the Adolescent Dieting Scale (Patton et al. 1997), which was employed to place students in one of three categories (nondieter, intermediate dieter, severe dieter). At baseline, severe dieting was associated with reduced odds of any current smoking, with nondieters as the referent (OR = 0.4; 95% CI, 0.2–0.9), but it was not significantly related to current daily smoking. In prospective analyses, dieting status was not predictive of the progression to any current smoking or to daily smoking.
Austin and Gortmaker (2001) prospectively investigated the associations between dieting frequency and smoking initiation among 1,295 sixth- and seventh-grade girls and boys participating in an intervention study involving nutrition and physical activity. Students completed baseline measures of their smoking history and dieting frequency during the past month, and smoking status was assessed 2 years later. Initiation of smoking was defined as having reported no smoking at baseline but smoking within the past 30 days at follow-up. Among baseline nonsmokers, the frequency of dieting was a significant predictor of initiation; relative to those who reported no dieting at baseline and with the use of a multivariate logistic regression model, girls who dieted once a week or less were found to be 1.98 (95% CI, 1.12–3.50) times as likely to initiate smoking. For those who reported dieting more than once per week, the odds of initiating smoking were 3.9 (95% CI, 1.46–10.38) times as great as those for nondieters. Dieting frequency was not associated with the likelihood of smoking initiation among boys.
Field and colleagues (2002) investigated the temporal relationships between smoking initiation, beginning to binge eat and/or purge, and getting drunk for the first time in a sample of 11,358 boys and girls between the ages of 10 and 15 years. Students completed a survey assessing smoking history, alcohol use, binge eating, purging behaviors (use of laxatives, self-induced vomiting), and concerns about weight. Smoking was defined as having smoked during the previous 30 days. Assessments were conducted at baseline and 1 year later. During the follow-up period, 4.3% of girls and 3.6% of boys started smoking. Among girls who were nonsmokers at baseline, those who expressed high levels of concern about weight were significantly more likely to initiate smoking over the subsequent year (OR = 2.2; 95% CI, 1.5–3.2) than were those with lower levels of concern. The relationship between concerns about weight and initiation of smoking was somewhat weaker and only marginally significant among boys (OR = 1.7; 95% CI, 1.0–3.1). Neither binge eating nor purging was associated with starting to smoke for either girls or boys.
Voorhees and colleagues (2002) prospectively investigated predictors of initiating daily smoking among 1,213 Black and 1,116 White girls participating in the National Heart, Lung, and Blood Institute Growth and Health Study. Participants were assessed annually for 10 years. A variety of behavioral/personal, developmental, family/social environmental, and weight-related domains were assessed at baseline, when participants were 9 or 10 years old, and again 2 years later. These variables were used to predict smoking status during the 10th annual visit, at which time participants were 18 or 19 years old. For purposes of analysis, never smokers were compared with those who reported smoking on a daily basis during the past 30 days. Weight-related variables included percent overweight, currently trying to lose weight, ever trying to lose weight, level of body dissatisfaction, feelings of competence and acceptance related to physical appearance, and the Drive for Thinness subscale from EDI (Garner et al. 1983). Among Black girls, drive for thinness at 11 or 12 years of age (OR = 1.11; 95% CI, 1.05–1.17) and currently trying to lose weight at those ages (OR = 2.39; 95% CI, 1.25–4.75) were associated with initiation of daily smoking by 18 or 19 years of age in multivariate logistic regression models. For White girls, currently trying to lose weight at 11 or 12 years of age was significantly predictive of daily smoking by 18 or 19 years of age (OR = 1.51; 95% CI, 1.03–2.21). Drive for thinness also predicted later daily smoking among White girls, but only when trying to lose weight was removed from the model.
Lastly, Stice and Shaw (2003) prospectively examined the relationships between both body image and eating/affective disturbances and subsequent initiation of smoking among adolescent girls; participants included 496 girls 11–15 years of age (modal age = 13 years) upon entry into the study. Assessments were conducted at baseline (time 1) and 1 year later (time 2). Participants reported the frequency of cigarette use during the past year on a scale from 0 (never) to 6 (five to seven times per week). Those who reported never smoking during the previous year were classified as nonsmokers. Occasional (but nondaily) smokers were coded as experimenters, and those who reported smoking on a daily basis were considered regular smokers. Level of satisfaction with nine separate body parts was assessed using a modified version of the Satisfaction and Dissatisfaction with Body Parts Scale (Berscheid et al. 1973). Eating pathology was measured with the Eating Disorder Examination (Fairburn and Cooper 1993). Because of high correlation between these last two independent variables, they were collapsed to create a single body dissatisfaction-eating pathology composite score. In the time between baseline and 1-year follow-up, 6% of time 1 (baseline) nonsmokers became experimental smokers, and 5% became daily smokers. In a multivariate logistic regression model that controlled for negative effects, those with high levels of body dissatisfaction-eating pathology were more than four times as likely to initiate smoking (OR = 4.33; 95% CI, 1.71–10.95) as those who did not have high levels.
Most but not all evidence supports an association between concerns about weight and subsequent initiation of smoking. Notably, the three studies that included samples entirely of females found a significant relationship between concerns about weight and taking up smoking (French et al. 1994; Voorhees et al. 2002; Stice and Shaw 2003). Of the four studies that included both males and females, two failed to find a significant relationship between weight concerns and initiation of smoking in either girls or boys (Killen et al. 1997; Patton et al. 1998), and one (Austin and Gortmaker 2001) found dieting to be a significant predictor of starting to smoke for girls only. The remaining study (Field et al. 2002) found that weight concerns were significantly related to beginning to smoke in girls and marginally related in boys.
More Recent Evidence
Subsequent to the publication of the last of the prospective studies reviewed by Potter and colleagues (2004), eight papers have been published (representing seven different studies) on the topic of weight concerns and smoking. Two papers from the Memphis Health Project investigated the association between weight concerns and the onset and escalation of smoking (Blitstein et al. 2003; Robinson et al. 2006); as described above, the Memphis Health Project was designed to prospectively assess predictors of the onset of smoking in a large cohort of students surveyed annually from 7th to 12th grade. Potential risk factors for smoking initiation included a wide range of psychosocial variables: family and peer influences, the perceived functional utility of smoking, rebelliousness, social success, environmental factors, reactions to initial smoking experiences, and weight concerns. For the last item, students indicated the extent to which they believed smoking helps to reduce body weight and whether they had ever smoked to lose weight or control their weight. In addition, participants completed the six items comprising the “concern for dieting” factor from the Restraint Scale (Herman and Polivy 1980), which measures level of preoccupation with dietary control.
The paper by Blitstein and coworkers (2003) examined factors associated with the speed of transition through the stages of smoking among adolescents who were nonsmokers at the start of the study. Students who progressed from nonsmokers to regular smokers (at least weekly) over the course of 1 year (n = 98) were categorized as rapid progressors, and those who went from being nonsmokers to experimental smokers (less than weekly, n = 555) during this period were considered slow progressors. The belief that smoking controls body weight was not related to speed of progression for either boys or girls. However, girls who reported greater concerns with dieting were significantly more likely to progress rapidly from nonsmoking to regular smoking. Relative to those scoring at the median level on this scale, girls at the 75th and 90th percentiles were 1.90 (95% CI, 1.26–2.86) and 2.91 (95% CI, 1.47–5.75) times as likely, respectively, to be rapid progressors. Among boys, no association was observed between concerns with dieting and smoking progression.
In the paper by Robinson and associates (2006), the authors used data from the Memphis Health Project cohort to investigate racial differences in the potential risk factors (including weight concerns/behaviors) for onset and escalation of smoking. Multivariate regression models were used to identify predictors of several different levels of smoking (monthly smoking, weekly smoking, and daily smoking) in the 12th grade among Black and White adolescents who were never smokers at baseline (7th grade). None of the three measures of weight concerns or behaviors (the belief that smoking controls body weight, the use of smoking as a weight-control strategy, concern with dieting) was associated with onset of smoking.
Honjo and Siegel (2003) investigated associations (Table 2.2) between several measures of weight concerns or dieting behavior and initiation of smoking over a 3-year period among 273 girls between the ages of 12 and 15 years who reported having smoked no more than one cigarette in their lifetime at baseline. The belief that smoking controls weight was assessed by asking “Do you believe that smoking helps people keep their weight down?” Participants were also asked whether they considered themselves to be underweight, just about right, or overweight. The participants also indicated whether they were currently dieting. Finally, drive for thinness was assessed by having the girls rate the importance they gave to being slim or thin on an 11-point scale ranging from 0 (not at all important) to 10 (extremely important). Ratings of 0–4, 5–7, and 8–10 were classified as low, medium, and high concern, respectively.
Relative to those who gave a low rating to being thin, adolescents who gave a rating of medium (OR = 3.34; 95% CI, 1.04–10.94) or high (OR = 4.46; 95% CI, 1.40–16.69) were significantly more likely to progress to established smoking 3 years later, defined as having smoked 100 or more cigarettes in their lifetime by the follow-up assessment. Those who believed that smoking helps to control weight were slightly more likely to become established smokers (26.4%) than those who did not endorse this belief (23.1%), but these differences were not statistically significant. Onset of established smoking was slightly more common among those who considered their weight to be just about right (25.1%) than in those who reported being underweight or overweight (20% for both underweight and overweight groups; all differences between groups were not significant). Finally, those who had engaged in dieting and those who had not had nearly identical rates of smoking initiation over time (23.8% vs. 23.3%).
Using data from the 1997 cohort of the National Longitudinal Survey of Youth, Cawley and associates (2004) examined the relationship between self-perceived weight, attempting to lose weight, and smoking initiation over a 3-year period among 9,022 youth 12–16 years of age. Participants were given five options for describing their weight: very underweight, slightly underweight, about the right weight, slightly overweight, and very overweight. Responses were recoded into three categories: (1) overweight (slightly overweight or very overweight), (2) underweight (slightly underweight or very underweight), and (3) about the right weight. Two measures of smoking initiation were used: in the first, which used a more stringent definition, never smokers at baseline who indicated during one of the three follow-up interviews that they had smoked even a single cigarette were classified as smokers. The second definition required respondents to have smoked on at least 15 of the previous 30 days.
In analyses that included boys and girls together and boys and girls separately, perceiving oneself as underweight was associated with a reduced likelihood of smoking initiation according to the less stringent definition when “about the right weight” was the referent. When the more stringent criterion and the same referent were used, only girls who perceived themselves as underweight were significantly less likely to smoke. Girls who perceived themselves as overweight were significantly more likely than those in the “about the right weight” group to have smoked on the basis of the less stringent definition only. Perceptions of being overweight were not associated with initiation of smoking among boys when either definition was used. Attempting to lose weight was significantly associated with adoption of smoking on the basis of the less stringent definition when both genders were considered together and when girls were assessed separately. With the more stringent definition of smoking initiation, the association between attempted weight loss and initiation was significant only among girls in gender-stratified analyses.
Saules and colleagues (2004) investigated factors associated with the onset of smoking during college among 490 female undergraduate students. Smoking status was assessed during freshman orientation, after 9 months (end of the freshman year), and nearly 4 years after baseline (during the senior year). Disordered eating patterns/dieting concerns were measured using the Dieting and Bingeing Severity Scale (Krahn et al. 1992; Drewnowski et al. 1994). Among students who were non-smokers at baseline, elevated concerns about dieting were a significant predictor of the onset of smoking during their college years.
Chesley and associates (2004) investigated the associations between intended behaviors about one’s weight and the initiation and maintenance of smoking among 3,621 participants in the National Longitudinal Study of Adolescent Health. Participants were asked whether they were attempting to modify their weight (trying to lose weight, trying to gain weight, trying to maintain their weight, not trying to do anything about weight); smoking status was assessed during an initial interview and 1 year later. Among students who reported at baseline that they had never tried a cigarette, those who indicated they were attempting to lose weight were 1.8 (95% CI, 1.1–2.9) times as likely to initiate smoking during the following year as were those not trying to do anything with their weight. For those classified as smokers at baseline and who continued smoking during follow-up, the desire to maintain weight (but not the desire to lose or gain weight) was associated with a greater increase in the number of days smoked in the past month.
Wahl and colleagues (2005) investigated associations between expectancies for outcomes related to smoking and escalation of smoking in a sample of 8th and 10th graders enrolled in a prospective study of the natural progression of cigarette smoking. Participants included 273 students (54% female) who were classified as early experimenters because they had smoked between 2 and 100 cigarettes in their lifetimes. The majority of the sample (74%) was White, with the remainder identifying themselves as Latino (16%), Black (3%), or other/biracial (6%). Expectancies related to smoking were assessed using a revised, 13-item version of the SCQ (Brandon and Baker 1991); the expectancy measure included three items related to weight control: “Smoking keeps my weight down,” “Cigarettes keep me from eating more than I should,” and “Smoking helps me control my weight.” Responses were on a 4-point scale ranging from 1 (disagree) to 4 (agree). Assessments were conducted at baseline and at 6 months. Participants were placed in one of five groups (trier, escalator, rapid escalator, smoker, and quitter) according to their smoking behavior during the follow-up period. Girls had higher baseline expectancies related to weight control than did boys, but no differences in expectancies were noted by race or ethnicity. Significant differences in baseline smoking expectancies related to weight were noted by smoking behavior group. Specifically, escalators reported lower expectancies regarding the impact of smoking on weight and appetite control than did students who were smoking more regularly at baseline and continued as regular smokers. None of the other comparisons by group were significant.
Finally, in Ontario, Canada, Leatherdale and coworkers (2008) examined the association between self-perception of weight and susceptibility to smoking (susceptibility to smoking has been shown to be a reliable predictor of the future onset of smoking [Pierce et al. 1996, 2005; Choi et al. 2001]). Participants included 25,060 students in grades 9–12. In all, of the 14,795 participants who had never smoked cigarettes, 3,809 (25.8%) were classified as susceptible and 10,986 (74.2%) were categorized as non-susceptible to future smoking from their responses to Pierce’s Susceptibility Questionnaire (Pierce et al. 1996). Perception of body weight was assessed by asking students whether they considered themselves very underweight, slightly underweight, about the right weight, slightly overweight, or very overweight. Relative to those who thought they were at about the right weight, those who considered themselves either slightly overweight (OR = 1.21; 95% CI, 1.08–1.35) or slightly underweight (OR = 1.18; 95% CI, 1.05–1.33) were significantly more likely to be susceptible to future smoking. In contrast, self-perception as very overweight or very underweight was not associated with increased susceptibility. Relationships between perceptions of weight and susceptibility to smoking did not differ by gender.
Summary
The eight publications described above, which were based on seven studies published after the review by Potter and colleagues (2004), provide mixed findings regarding the association between concerns about weight and initiation of smoking. With the exception of one study, which did not find a significant relationship between concerns about weight and the onset and escalation of smoking among adolescents (Robinson et al. 2006), each of the studies found at least one association between weight concerns and initiation of smoking. However, methods of these studies differed according to the weight-related constructs assessed and the measures used. Associations between weight concerns and initiation were also frequently modified by gender, with relationships tending to be stronger among females than among males.
Because the associations between initiation of smoking and concerns about weight tend to differ according to how the concerns are conceptualized and assessed, the results are summarized below from all published studies, including those summarized in the 2004 review by Potter and colleagues, according to different dimensions of weight concerns. These include general weight concerns, perceived weight, dieting behaviors, and dispositional weight concerns/symptoms and attitudes relative to disordered eating. These categories were also used in two previous reviews (French and Jeffery 1995; Potter et al. 2004) as well.
General Weight Concerns
Five studies were identified that prospectively investigated the association between general weight concerns and initiation of smoking (French et al. 1994; Field et al. 2002; Honjo and Siegel 2003; Wahl et al. 2005; Robinson et al. 2006). Two of these studies investigated the use of smoking as a weight-control strategy, but neither demonstrated a significant relationship with the onset of smoking (Honjo and Siegel 2003; Robinson et al. 2006). However, Field and colleagues (2002) found that general weight concerns, as measured by the McKnight Risk Factor Survey (Shisslak et al. 1999), were a significant predictor of smoking initiation over 1 year among girls and a marginally significant predictor for boys. In another of the five studies, expectancies regarding the weight-controlling effects of smoking were a significant predictor of smoking trajectories over time (Wahl et al. 2005), with adolescents who increased their smoking over time reporting lower expectancies than those who were initially smoking more regularly and continued as regular smokers. In the remaining study (French et al. 1994), constant thoughts about weight, but not fears about weight gain, predicted smoking initiation during a 1-year period in girls. Neither measure was associated with initiation of smoking among boys.
Thus, general concerns about weight appear to be a modest predictor of the initiation of smoking in prospective studies. The limited evidence on gender differences suggests that this relationship is stronger among girls than boys. The small number of cohort studies and considerable variability in the ways in which weight concerns were conceptualized and measured, however, limit the conclusions that can be made about the nature and strength of this relationship.
Perceived Weight
Two studies were identified that used longitudinal designs to examine the relationship between self-perceived body weight and initiation of smoking (Honjo and Siegel 2003; Cawley et al. 2004), and one cross-sectional study was found that used susceptibility to smoking as a proxy for future initiation of smoking (Leatherdale et al. 2008). In one of the two longitudinal studies, perceptions about body weight were not significantly associated with starting to smoke among adolescent girls (Honjo and Siegel 2003), but in the second one (Cawley et al. 2004), self-perception of being underweight was associated with a reduced likelihood of initiation for both boys and girls on the basis of a liberal definition of smoking (any amount of smoking). When a definition of more regular use was used (smoking on ≥15 of the last 30 days), however, the relationship remained significant only among girls. Relative to those who considered their weight to be “just about right,” adolescent girls who perceived themselves as overweight were significantly more likely to initiate smoking only by the definition of “any” use. Perceiving oneself as overweight did not predict the onset of smoking among boys when either definition was used. In the third study (Leatherdale et al. 2008), perceiving oneself as being slightly underweight or slightly overweight was associated with greater susceptibility to smoking in a sample of male and female adolescents. Those who perceived themselves as being very underweight or very overweight, however, were neither more nor less susceptible to smoking. The fact that these three studies used different designs and definitions of smoking may have contributed to the apparent discrepancies in their findings.
Dieting Behaviors
Seven studies (French et al. 1994; Patton et al. 1998; Austin and Gortmaker 2001; Voorhees et al. 2002; Honjo and Siegel 2003; Cawley et al. 2004; Chesley et al. 2004) prospectively investigated the association between dieting and the initiation of smoking among youth. The majority of findings supported a relatively strong association between dieting and the onset of smoking, particularly among females. In three studies, attempts to lose weight were predictive of smoking initiation among girls but not among boys (French et al. 1994; Austin and Gortmaker 2001; Cawley et al. 2004). In two of the other studies, which examined the association between dieting and onset of smoking in combined samples of males and females and did not stratify the analyses by gender, attempting to lose weight was a significant predictor of starting to smoke in one (Chesley et al. 2004) but not in the other (Patton et al. 1998). In the two remaining studies, both using exclusively female samples, trying to lose weight was a significant risk factor for initiation of smoking in one (Voorhees et al. 2002) but not the other (Honjo and Siegel 2003).
Dispositional Weight Concerns/Symptoms and Attitudes Relative to Disordered Eating
The term “dispositional weight concerns/symptoms” has been previously used in studies to mean individual differences in the tendency toward restrained eating and other extreme dieting behaviors. In total, eight studies have prospectively evaluated the associations between dispositional weight concerns or symptoms of/attitudes about disordered eating and initiation of smoking among adolescents and young adults (French et al. 1994; Killen et al. 1997; Voorhees et al. 2002; Blitstein et al. 2003; Honjo and Siegel 2003; Stice and Shaw 2003; Saules et al. 2004; Robinson et al. 2006). Similar to the results described above involving dieting behaviors, studies that included measures of dispositional weight concerns/disordered eating symptoms and attitudes have demonstrated a fairly consistent association with initiation of smoking, particularly among females. All four studies that included only females found responses to measures of dispositional weight concerns/symptoms and attitudes about disordered eating to be significant predictors of starting to smoke (Voorhees et al. 2002; Honjo and Siegel 2003; Stice and Shaw 2003; Saules et al. 2004). Although Killen and colleagues (1997) included both boys and girls, the Drive for Thinness subscale of the EDI (Garner et al. 1983) was administered only to the girls in the sample, for whom it was not a significant predictor of the onset of smoking. In a sixth study (French et al. 1994), having two or more symptoms of eating disorders predicted the uptake of smoking over 1 year among girls but not boys. Similarly, concern with dieting was a significant predictor of rapid progression from nonsmoking to regular cigarette smoking among girls but not for boys enrolled in the Memphis Health Study (Blitstein et al. 2003). However, in a subsequent set of analyses from the same cohort that examined predictors of the onset and escalation of smoking (Robinson et al. 2006), concern with dieting was not associated with initiation or progression of smoking in either gender.
Weight Concerns and Smoking Cessation in Adolescents and Young Adults
Review of the Evidence
This section examines the limited evidence available on the association between weight concerns and smoking cessation in youth. General concerns about weight and, more specifically, concerns about the weight gain that frequently accompanies smoking cessation have long been recognized as a potential barrier to cessation among adults. However, in contrast to the literature on adults, which includes several relevant studies (Klesges and Klesges 1988; French et al. 1992, 1995; Jeffery et al. 1997, 2000; Meyers et al. 1997), only two prospective studies were identified that investigated this issue in young smokers. In the first, Glasgow and colleagues (1999) focused on 506 female smokers (mean age = 24 years) attending Planned Parenthood clinics who were participating in a randomized clinical trial involving low-intensity interventions for quitting smoking. Participants completed the SSQ which, as noted earlier, is designed to assess the use of smoking for weight control (Weekley et al. 1992). Scores on the SSQ were not a significant predictor of successful cessation, attempts to quit smoking, changes in cigarette consumption, or changes in self-efficacy for quitting smoking.
The second prospective study (Wahl et al. 2005) examined the association between smoking-related outcome expectancies and cessation among 349 high school students enrolled in a cessation program (54% were female). The majority (75%) of the sample was White; 13% were Black; 5%, Latino; and 7% identified themselves as biracial/other. Participants ranged in age from 14 to 19 years (mean age = 16.4 years, SD = 1.1). Expectancies regarding the effect of smoking on weight control were assessed using a 13-item modified version of the SCQ (Brandon and Baker 1991). Participants were surveyed at baseline, end of treatment, and 6 months after baseline. Relative to males, female students reported greater expectancies about the impact of smoking on body weight. Furthermore, baseline expectancies about weight control related to smoking were significantly associated with the likelihood of being abstinent at the 6-month follow-up. Contrary to expectations, students who reported greater expectancies that smoking helps control weight were significantly more likely to successfully quit smoking (OR = 1.54; 95% CI, 1.05–2.24).
Summary
The relevant research is quite limited in scope. In the one study that prospectively investigated the relationship between weight concerns and smoking cessation in young smokers, use of smoking for weight control was not associated with any cessation-related outcome. A second study found that expectancies regarding the effect of smoking on body weight were associated with the likelihood of quitting smoking, but not in the predicted direction. Results from the literature on smoking among adults have been mixed regarding the issue of whether concerns about weight are inversely associated with quitting smoking. Although two studies (Klesges and Klesges 1988; Meyers et al. 1997) found that those with greater concerns about post-cessation weight gain were less likely to quit smoking, several others did not find this to be the case (French et al. 1992, 1995; Jeffery et al. 1997). One other study (Jeffery et al. 2000) found that elevated concerns about weight were associated with a reduced likelihood of quitting smoking in the bivariate analyses but not in multivariate models that controlled for demographics, nicotine dependence, and social factors. Thus, additional prospective studies are needed to clarify the impact of weight concerns on the likelihood of successful smoking cessation in adolescents and young adults.
Smoking and Reduction of Body Weight in Children and Young Adults
Overview and Methods
Two previous Surgeon General’s reports (USDHHS 1988, 1990) evaluated the relationship between smoking and body weight. The 1988 report, which examined nicotine addiction as a health consequence of smoking, concluded from a review of 28 cross-sectional studies that, on average, smokers weighed 3.2 kilograms (kg) less than nonsmokers. In addition, from a review of 43 prospective studies, the report concluded that quitting smoking resulted in a weight gain of 2.8 kg. Similarly, in the 1990 report on the health benefits of smoking cessation, in which 15 prospective studies were reviewed, the average weight gain following cessation was 2.3 kg.
To evaluate the relationship between smoking and body weight in youth and young adults, all studies reporting a relationship between smoking and body weight subsequent to the 1990 Surgeon General’s report were evaluated for the present report. To be included in the review, studies had to include smoking status, body weight or BMI, and sample size. Given the interest in the effects on younger smokers, body weight and smoking status needed to be specified by age group. Some studies reported extremely large age ranges and did not stratify by age (e.g., 18–70 years; Chiriboga et al. 2008; Fogarty et al. 2008) and thus were excluded because the impact of smoking on the body weights of younger versus older smokers could not be determined.
The inconsistent categorization of smoking status poses a potential limitation to interpreting this body of literature. Some studies differed in their definitions of cessation and of active smoking status (Townsend et al. 1991; Cooper et al. 2003; Stice and Martinez 2005; Carroll et al. 2006; Fidler et al. 2007; O’Loughlin et al. 2008), and others did not provide a definition of smoking status at all (Barrett-Connor and Khaw 1989; Freedman et al. 1997; Fulton and Shekelle 1997; Akbartabartoori et al. 2005; Jitnarin et al. 2006; Stavropoulos-Kalinoglou et al. 2008). Clearly, the duration and quantity of smoking status can markedly affect the amount of weight gain attributed to cessation. For example, Klesges and colleagues (1997a) evaluated the weight gain associated with cessation by using both point-prevalent (currently not smoking) and continuous abstinence (for 1 year) criteria for defining cessation. In a sample of 196 participants in a cessation program, the continuously abstinent participants gained 5.90 kg during 1 year, significantly more than those who were abstinent at a specific point (3.04 kg) or those who continuously smoked (1.09 kg).
The age of participants also affects the interpretation of findings, as definitions and categories of smokers typically vary between adolescents and adults. Most of the studies in adults define a smoker as someone who smokes every day (Marti et al. 1989; Shimokata et al. 1989; Molarius et al. 1997; Al-Riyami and Afifi 2003; Bamia et al. 2004; Sneve and Jorde 2008), but most studies of youth (e.g., aged <18 years) define a regular smoker as someone who smokes once a month or once a week (e.g., Townsend et al. 1991; Crawley and While 1995; Cooper et al. 2003). Given the potential difficulty of interpreting the overall findings, the few studies that define smoking among youth as daily smoking (e.g., Klesges et al. 1998a; Stice and Martinez 2005) will be discussed in more detail because these youth are likely to continue to smoke and with greater intensity.
After coding, studies were categorized by whether they addressed the major research questions, the first being whether there is a relationship between smoking and body weight in young people. Most of the studies addressing this issue were cross-sectional, but some cohort studies that had a report on the cross-sectional findings were also included. The second question was whether quitting smoking leads to a significant weight gain. Studies included here were longitudinal studies with participants who were smokers at one time point and had quit smoking at another time point. The final question was whether initiation of smoking is associated with weight loss in youth and young adults. The studies included here were longitudinal studies in which participants were nonsmokers at one time point and smokers at another time point.
Relationship Between Smoking and Body Weight in Youth and Young Adults
As concluded in previous Surgeon General’s reports (USDHHS 1988, 1990), cross-sectional studies have shown a clear relationship between smoking and body weight. However, the majority of these investigations have involved adult samples. To evaluate the relationships between smoking and body weight in both younger and older smokers, studies were placed in one of three age groups: less than 25 years, 25 years and older, or 35 years and older. The results of these 25 studies are presented in Table 2.5.
Table 2.5
Studies assessing association between smoking and body weight.
On the basis of weighted means, the results indicated that among older persons the average BMI was lower for smokers than for nonsmokers. For example, in a large Greek cohort of more than 22,000 adults, the average BMI for smokers 45 years of age and older was 2.1 units (measured as kg of weight/square meters of height) lower than that of nonsmokers (Bamia et al. 2004). Similar results were reported for this age group in a Scottish cohort of more than 9,000 adults (Akbartabartoori et al. 2005). In contrast, in a study of 32,144 U.S. Air Force trainees (mean age = 19.8 years, SD = 2.1), daily smoking was not associated with body weight (p >0.05) in females and was associated with only about a 1-kg difference in body weight in men (Klesges et al. 1998c). Moreover, in a study of 6,751 seventh graders, daily smokers had a significantly higher BMI than their nonsmoking peers (Klesges et al. 1998a).
Average BMI for smokers and nonsmokers in studies reported in Table 2.5 was weighted, averaged, and plotted for the same three age groups described above: less than 25 years, 25 years and older, and 35 years and older (Figure 2.2). Because reported age ranges varied a great deal, these three age groups were selected because most results of the relevant articles could be sorted into these categories. Individual study means that were not explicitly provided were calculated when data on weight and age by smoking status were provided. Study means were then weighted by sample size and averaged across studies.

Figure 2.2
Body mass index (BMI) differences among smokers and nonsmokers by age group. Source: Data from studies in Table 2.5: Barrett-Connor and Khaw 1989; Marti et al. 1989; Shimokata et al. 1989; and Townsend et al. 1991.
BMI dramatically increased with age in both smokers and nonsmokers, but there was a discernible weight difference between smokers and nonsmokers among those 35 years of age and older. This difference was explained by the relatively lower gain in weight for smokers over time. The average BMI for smokers under 18 years of age appeared to be the same, if not slightly higher, than the average BMI for nonsmokers. Thus, these studies do not show a relationship between smoking and body weight in children and young adults.
Quitting Smoking and Weight Gain in Youth and Young Adults
Among smokers in general, cessation leads to weight gain (USDHHS 1988, 1990). Again, however, most of the investigations have reported this relationship in largely adult populations. To evaluate the relationships between cessation and weight change in both younger and older smokers, studies were examined by the age of the sample. Ages ranged from 11 to 15 years in one sample (Stice and Martinez 2005) to 46 years or older in another study (Janzon et al. 2004). The results of these 12 longitudinal studies, which extended from 6 weeks to 9 years, are summarized in Table 2.6.
Table 2.6
Studies assessing change in weight following smoking cessation.
Post-cessation weight gain appears to occur among young people and older adults alike. In one study, Klesges and colleagues (1998b) evaluated the relationship between cessation and weight change from baseline to a 7-year follow-up in a large biracial cohort, the Coronary Artery Risk Development in Young Adults (CARDIA) study; participants were 5,115 young adults 18–30 years of age at baseline. Over 7 years, all groups (smokers, nonsmokers, and former smokers) gained weight, but gains were the greatest among those who quit smoking during the study. Average weight gain attributable to cessation was 4.2 kg for Whites and 6.6 kg for African Americans. Similar findings were reported for 496 adolescent girls in the United Kingdom (Stice and Martinez 2005); in this 3-year prospective study, girls who quit smoking gained an average of 3.4 kg versus gains of 1.4 kg for smokers and 2.9 kg for nonsmokers. Finally, using the weighted means from six studies (Table 2.6) whose participants were adults 25 years of age or older, an average gain of 7.3 kg following cessation can be calculated (Klesges et al. 1997b; O’Hara et al. 1998; Nicklas et al. 1999; Janzon et al. 2004; Hutter et al. 2006; Pisinger and Jorgensen 2007). Thus, limited data suggest that quitting smoking among adolescents and young adults, just as for adults, appears to be associated with weight gain.
Initiation of Smoking and Weight Loss in Youth and Young Adults
Several previous reviews of the literature (USDHHS 1988, 1990; Klesges et al. 1989) concluded that, overall, people who start smoking lose weight. However, these reviews were based on adults and included a very small number of studies. To evaluate the relationship between initiation of smoking and changes in body weight in both younger and older smokers, available studies were coded by age of the sample. Ages ranged from 11 to 15 years (Stice and Martinez 2005) to 38 years of age and older (Lissner et al. 1992); the results of these studies are highlighted in Table 2.7.
Table 2.7
Studies assessing change in weight following smoking initiation.
Although nearly 20 years have passed since the last review in a Surgeon General’s report, even now only a few studies have evaluated the relationship between initiation of smoking and body weight (Table 2.7). Overall, among older people who have participated in these studies, initiation of smoking has been associated with a smaller increase in weight than for nonsmokers (Sneve and Jorde 2008), including for women (Lissner et al. 1992). In the CARDIA study (Klesges et al. 1998b), those who were non-smokers at baseline (age range of 18–30 years) and who reported smoking 7 years later were compared with other smoking groups (e.g., never smokers, former smokers, quitters, initiators, and intermittent smokers); all of the groups gained weight. Relative to the experience of never smokers and continuous smokers, initiation of smoking had no impact on body weight among Whites and only a small impact among African Americans (where weight gain was attenuated by 0.7 to 3.3 kg depending on the comparison group).
Among adolescent samples, initiation of smoking does not appear to have been associated with weight loss. Although some studies found a small attenuation of weight gain in adolescents (Stice and Martinez 2005; Fidler et al. 2007), one prospective study (Cooper et al. 2003) found an absolute weight gain for up to 3 years following initiation. The authors of this last study suggested that these smokers may have been relaxing their other weight-management strategies once they initiated smoking.
Summary
Overall, there is consistent evidence among youth that a substantial minority believe that smoking controls body weight. Moreover, using smoking as a weight-control strategy is not unusual in both youth and young adults. However, the evidence that concerns about body weight predicts either the onset or cessation of smoking is inconclusive. Overall, the results appear more consistently significant in females than in males, but this may in part be due to a greater proportion of females who are concerned about their body weight. Different definitions of concern about body weight and the heterogeneous populations studied may contribute to these conclusions. Finally, there is little evidence that smoking actually controls body weight in youth and young adults. There is evidence for a lowered weight among smokers than among nonsmokers after 35 years of age, but there is no relationship in smokers under 35 years of age. Some have speculated that (Klesges et al. 1998b) the weight-control effects of smoking appear to be very small and may take decades to accrue. The available evidence on the relationship between initiation of smoking and weight loss is mixed, but it suggests minimal, if any, effect of smoking initiation on weight loss in youth and young adults. However, youth and young adults who quit smoking also appear to gain weight. The evidence reviewed in this report, along with the reviews in prior reports, indicates a complicated relationship between initiation of smoking, continued smoking, and cessation over time. Interpretation of the evidence is further complicated by the concurrent secular trend of rising obesity.
Pulmonary Function and Respiratory Symptoms and Diseases
Introduction
This section addresses the consequences for respiratory health of active smoking during childhood, adolescence, and young adulthood. When the effects of active smoking were first investigated in adults, the early studies, in addition to examining the problem of lung cancer, assessed indicators of respiratory health. Questionnaires were used to measure the presence of symptoms, and spirometry, a test of ventilatory lung function, was used to measure damage to the lungs. These studies found strong associations between cigarette smoking and respiratory morbidity, including cough, production of phlegm, shortness of breath, and reduced lung function (U.S. Department of Health, Education, and Welfare [USDHEW] 1964). When these same methods were applied to adolescents and young adults who smoked, the findings were similar, indicating that respiratory morbidity was also increased in young smokers (Peters and Ferris 1967a,b; USDHHS 1994). In one of the first investigations of smoking in young adults, Peters and Ferris (1967b) surveyed male and female college students with a questionnaire on respiratory symptoms as well as a spirometry test; the smokers had more respiratory symptoms and lower lung function than did nonsmokers.
This section covers the principal respiratory consequences of active smoking in childhood, adolescence, and early adulthood: adverse effects on both the expected increase in lung function and its eventual decline as well as increased risk for chronic respiratory symptoms and disease. These topics were last covered specifically for children in the 1994 Surgeon General’s report (USDHHS 1994). At that time, the evidence was characterized as limited and insufficient to support conclusions that active smoking was a cause of adverse respiratory consequences in this age group (USDHHS 1994). Subsequently, the body of relevant evidence enlarged substantially, particularly as follow-up has been extended in key cohort studies and results from more populations have become available. In addition, there is even more epidemiologic evidence on the effects of active smoking on adults (USDHHS 2004) and on the mechanisms by which smoking injures the respiratory tract (USDHHS 2010).
The 2004 Surgeon General’s report (USDHHS 2004) comprehensively covered active smoking and respiratory health (Tables 2.1a and 2.1b). The evidence was found to be sufficient to infer that active smoking causes respiratory symptoms in childhood and adolescence. For this update of the 2004 report, the review on asthma is particularly comprehensive because evidence was limited at the time of the earlier review.
Methods for the Evidence Review
A systematic strategy was used to identify the evidence considered in this comprehensive literature review on the effects of smoking on lung function and on respiratory symptoms and asthma in children, adolescents, and young adults. In addition to reviewing prior Surgeon General’s reports, a systematic search of the literature was conducted through PubMed with the following combinations of key words: cigarette smoking-adolescence-pulmonary function; adolescence-cigarette smoking-lung function growth; age of onset-cigarette smoking-lung function; smoking-allergy; adolescents-active cigarette smoking-allergy development; adolescents-active cigarette smoking; adolescence-cigarette smoking-asthma; adolescence-cigarette smoking-wheeze; and age of onset-cigarette smoking.
Lung Growth in Childhood, Adolescence, and Early Adulthood
Epidemiologic Evidence
Evidence reviewed in the 1994 and 2004 Surgeon General’s reports (USDHHS 1994, 2004) demonstrated that active cigarette smoking during childhood and adolescence has the potential to slow the rate of lung growth and reduce the level of maximum lung function attained, thus increasing risk for development of chronic obstructive pulmonary disease (COPD) in adulthood. Results from six cohort studies of lung function in children and adolescents published from 1982 to 1992 were reviewed in the 1994 Surgeon General’s report (USDHHS 1994), and two additional investigations were reviewed in the 2004 report (Sherrill et al. 1991; Gold et al. 1996). Two representative studies from the previous Surgeon General’s reports are summarized here (see also Table 2.8) along with new evidence regarding (1) the effect of active smoking on growth of lung function and the maximum attained level of such function in females and males; (2) the effect of smoking on the early decline of lung function in adulthood; (3) the benefits of smoking cessation for limiting the early decline of lung function in young adults; and (4) the groups of children who may be particularly vulnerable to the effects of smoking on pulmonary function.
Table 2.8
Longitudinal studies on the association between smoking and maximum attained level of forced expiratory volume in one second (FEV1), rates of growth, age of plateau in lung function, and age of onset of decline in lung function.
Evaluating smoking’s effects on the growth of lung function in growing children and young adults requires an understanding of normative gender differences in growth patterns and in the age at which maximal lung function is attained. Attainment of maximum lung function follows the attainment of maximum height and occurs later for males than for females (Gold et al. 1996). Although females normally achieve peak lung function before 20 years of age, for males, peak height and subsequent peak lung function are reached several years later. Thus, while the effects of smoking on maximal obtained lung function can be studied in girls with follow-up to about 20 years of age, studies of males need to be extended to after 20 years of age to fully capture the effect of smoking on lung growth (Sherrill et al. 1992; Robbins et al. 1995). Because of the range of ages at which males and females reach the peak level of lung function, multiple repeated measures of lung function are needed to characterize whether smoking influences the age at which the peak lung function is reached and the length of the plateau phase after this peak. In the East Boston study, Tager and colleagues (1988) reported that asymptomatic nonsmoking male participants reached peak levels of forced expiratory volume in 1 second (FEV1) at approximately 23–35 years of age, with a plateau phase that extended to age 45. Similarly, in their study of a Tucson, Arizona, population of young asymptomatic male and female nonsmokers, Sherrill and coworkers found that the age of reaching the peak FEV1 level ranged between 17.4 and 25.9 years; the duration of the subsequent plateau phase was somewhat shorter, however, than for the East Boston cohort (Sherrill et al. 1992; Robbins et al. 1995). Both studies found that, on average, the plateau phase began earlier for females and lasted longer than for males. Because growth of lung function is not complete for males until after 20 years of age, this chapter considers reports of investigations that have tracked the effect of smoking in young adulthood as well as in adolescence.
As summarized in the 2004 Surgeon General’s report, in a cohort study of 669 children and adolescents 5–19 years of age in East Boston, Massachusetts, Tager and colleagues (1985) found that among adolescents who started to smoke at 15 years of age and continued to smoke, the percentage of predicted FEV1 level at 20 years of age was only 92% of the expected FEV1 level for non-smokers. Subsequently, Tager and associates (1988) analyzed follow-up data on 974 females and 913 males 5 years of age or older. For females, a linear increase in FEV1 level was estimated to end 1 year earlier for current smokers (at 17 years of age, asymptomatic and symptomatic) than for nonsmokers without respiratory symptoms; the average maximal FEV1 values were 2.9 liters (L) and 3.1 L, respectively. Female current smokers had a more rapid rate of early decline in FEV1 than did nonsmoking females. For males, the estimated maximal FEV1 was attained at an earlier age for current smokers (at 18 or 19 years of age) than for asymptomatic nonsmokers (20–34 years of age) or symptomatic nonsmokers (21 years of age). Also for males, smoothed estimates suggested similar maximum FEV1 levels (4.1 L) for asymptomatic nonsmokers, symptomatic nonsmokers, and current smokers, but estimates suggested that the maximal FEV1 level was slightly lower for smokers. In addition, while asymptomatic nonsmokers had a plateau phase over which lung function remained stable, smokers did not. Finally, in male smokers, FEV1 began to decline almost 15 years earlier than in male non-smokers.
In a cohort of 4,902 girls and 5,158 boys followed from 10 to 18 years of age and evaluated annually with spirometry, Gold and colleagues (1996) examined the effects of cigarette smoking on the level of lung function attained and the rate of growth in lung function (Figures 2.3 and 2.4). Among girls smoking five or more cigarettes per day, the rate of increase in FEV1 level was slower by 31 milliliters (mL) per year (95% CI, 16–46 mL/year) than among girls who had never smoked. Although smoking five or more cigarettes per day slowed the rate of increase in FEV1 level in boys, the magnitude of the effect (slower by 9 mL per year; 95% CI, −6.0 to 24.0 mL per year) was less than estimated in girls.
Figure 2.3
Gender-specific effects of smoking on level of pulmonary function in youth 10–18 years of age. Source: Gold et al. 1996. Reprinted with permission from the Massachusetts Medical Society, ©1996. Note: Percentage differences and 95% confidence (more...)

Figure 2.4
Mean rates of pulmonary function growth by age, gender, and category of smoking. Source: Gold et al. 1996. Reprinted with permission from the Massachusetts Medical Society, ©1996. Note: Mean rates of pulmonary-function growth according to age, (more...)
For both boys and girls, the amount smoked was inversely related to the level of FEV1/FVC (forced vital capacity), as well as to the forced expiratory flow (FEF) [between 25% and 75% of the FVC (FEF25–75)] (Table 2.8). The girls reached their maximum level of lung function between the ages of 16 and 18 years, a period when lung function was still increasing in the boys. For girls at 18 years of age, maximally attained FEF25–75 was 3.80 L per second for girls who never smoked, compared with 3.65 L per second for those who smoked five or more cigarettes per day. At 17 and 18 years of age, FEV1 levels began to decline among girls who smoked, but they plateaued among girls who did not smoke.
The Vlagtwedde/Vlaardingen study in The Netherlands followed 1,818 males and 1,732 females between the ages of 15 and 35 years at 3-year intervals (Wang et al. 2004). For females, FEV1 reached a plateau by age 15, while in males, FEV1 continued to rise until about age 20. However, on average, women had a longer plateau, such that their lung function began to decline at about the same age, 25 years, as in men. Both current and cumulative cigarette smoking were significant predictors of FEV1 in males, with differences in the declines measuring −44 mL per pack per day for current smoking and −85 mL per 10 pack-years1 for cumulative smoking. Athough no effect of smoking on maximum FEV1 was found in females, gender differences in the effect of smoking were not significant, and the number of young female smokers was small. Smoking was associated with a lower level of FEV1 in both males and females. The investigators observed that the magnitude of the smoking effect seen in this younger cohort was greater than that found in cohorts older than 35 years of age studied elsewhere.
In an analysis of data from 4,554 participants in the Vlagtwedde/Vlaardingen study who were 15–54 years of age at study onset (Xu et al. 1994), after 24 years of follow-up the data showed not only that sustained smoking was associated with the size of decline of FEV1 in males and females but also that younger quitters (<45 years) benefited significantly more from smoking cessation than did older quitters (≥45 years).
In another Dutch study, quitting smoking was also associated with a smaller decline in FEV1 in a comparison with those who continued to smoke (Grol et al. 1999); the study included 199 people with allergic asthma who were recruited at 5–14 years of age and followed up at 22–32 and 32–42 years of age. The investigators described a “healthy smoker effect” (p. 1835) in this small cohort, however. Compared with those who had not taken up smoking, lung function was higher in childhood (presmoking) for those who took up smoking, and it remained higher into young adulthood. In the Amsterdam Growth and Health Study (Twisk et al. 1998) of 167 adolescents recruited at a mean age of 13 years, each with six repeated spirometry measurements during a 15-year period, smoking was associated with a decrease in FVC and FEV1; the effects of smoking on maximum lung function and the impact of quitting smoking were not evaluated.
In the CARDIA longitudinal study of 5,115 African American and European American women and men 18–30 years of age, who were healthy at enrollment (Pletcher et al. 2006), the smoking of menthol cigarettes and non-menthol cigarettes were associated with similar declines in lung function (excess decline of FEV1: 84 mL; 95% CI, 32–137 mL for menthol cigarettes and 80 mL; 95% CI, 30–129 mL for nonmenthol cigarettes per 10-pack-year increase in exposure) relative to nonsmokers after adjustment for ethnicity and other factors. In addition, in a comparison with smoking of nonmenthol cigarettes, the investigators found a significant increase in the risk of relapse among those who smoked menthol cigarettes. The results were similar among African Americans and European Americans.
More study is needed to define populations of children who are particularly susceptible to the effects of smoking on pulmonary function. In a Danish study, 85 asthmatic children 5–15 years of age were seen in follow-up 10 years after enrollment (Ulrik et al. 1995); active smoking was associated with a lower level of percentage of predicted FEV1 for the 24 participants without allergic sensitization (“intrinsic asthma”) but not for the 46 children with “extrinsic asthma.” Rates of smoking were low in this small cohort, however. In the Scandinavian Asthma Genetic Study of asthmatic children, their siblings, and their parents (Bisgaard et al. 2007), the percentage of predicted FEV1 level was inversely related to active smoking in comparison with not smoking (−3.5%; p = 0.0027).
Recent studies have demonstrated the relation of current cigarette smoking to difficult-to-treat asthma in young to middle-aged adults. In one such investigation, Chaudhuri and colleagues (2003) conducted a randomized, placebo-controlled, crossover study among participants 18–55 years of age by using oral prednisolone (40 milligrams daily) or a placebo for 2 weeks in smokers with asthma, former smokers with asthma, and never smokers with asthma. There was a significant improvement after prednisolone compared with a placebo in FEV1, morning peak expiratory flow (PEF), and in the asthma control score for never smokers with asthma, but no improvement was seen in asthmatic smokers. Former smokers with asthma who were treated with prednisolone had a significant improvement in morning and night PEF but not in FEV1. Tyc (2008) provides a review of other medically at-risk youth. Because of improving neonatal care, the population of very-low-birth-weight children has grown, but these children may be particularly susceptible to the effects of smoking, in part because of their early-life experience. These children frequently sustain lung injury as a consequence of the immaturity of their lungs at birth and the need for oxygen and mechanical ventilation. In an Australian study (Doyle 2000; Doyle et al. 2003), 60 consecutive extremely low-birth-weight (<1,000 grams [g]) children were followed longitudinally, with measurements of lung function obtained on 44 of them at a mean age of 20.2 years. The proportion with a clinically important reduction in the FEV1/FVC ratio (to <75%) was significantly higher in smokers (64%) than in nonsmokers (20%). In addition, there was a larger decrease in the FEV1/FVC ratio between the ages of 8 and 20 years in the smokers.
As detailed in the 2010 Surgeon General’s report (USDHHS 2010), the past 15 years have seen a burgeoning of information on the genetics of pulmonary diseases, with additional understanding of genes that may modify the risk of early development of COPD, but researchers are just beginning to evaluate the genetic modification of smoking’s effects on the growth of lung function, maximal attained lung function, and exercise tolerance (Harju et al. 2008).
Summary
Despite the logistical challenges of following cohorts from childhood into adolescence and then through young adulthood, a number of studies now provide a clear picture of how smoking adversely affects the growth and development of the lungs as children make the transition to adulthood. The findings are consistent for various studies of large populations. For example, in smokers, growth of lung function is slower during childhood and adolescence. In addition, there is a dose-response inverse relationship between smoking in adolescence and early adulthood and level of FEV1/FVC and also between smoking and level of FEF25–75.
For smokers, the growth of lung function ceases earlier, with lower maximal attained lung function, a briefer plateau phase, and an earlier decline in lung function. Active smoking may reduce maximal exercise tolerance in young adults. Smoking may reduce the beneficial effects of glucocorticoid therapy on lung function in young adults with asthma. Although quitting smoking at all ages can be beneficial, early quitting may be more valuable than later quitting because of its potential beneficial effect on the still-growing lung.
Both experimental and observational studies provide evidence that supports the biological basis of these findings and their plausibility. Studies of changes in lung tissue provide complementary evidence supporting the biological plausibility of the development of early airway changes in young adults who initiate smoking. Biological evidence presented in the 2010 Surgeon General’s report shows that the inflammation, oxidative stress, and proteolytic responses to active cigarette smoking begin within minutes to hours after exposure. In lungs obtained at autopsy, Niewoehner and colleagues (1974) demonstrated pathologic changes in the peripheral airways of young cigarette smokers who were victims of sudden death occurring outside of the hospital. Compared with nonsmokers, the lungs of smokers showed significant increases in mural inflammatory cells, with changes consistent with respiratory bronchiolitis. In a Southern California study with 40 apparently healthy participants 20–49 years of age that included both smokers (of tobacco or marijuana) and nonsmokers, mucosal biopsies were evaluated for the presence of vascular hyperplasia, submucosal edema, inflammatory cell infiltrates, and goblet cell hyperplasia (Roth et al. 1998). Biopsies were positive for two of these criteria for 97% of smokers, and 72% were positive for three.
When the observational evidence is assessed against the accepted criteria for causality, there is strength and consistency among the studies, and the temporal relationship between smoking and its adverse effects (i.e., smoking precedes the effects) is well documented through cohort studies. In careful multivariate analyses, potential confounding factors have been considered and controlled, such as secondhand smoke exposure, reinforcing the specificity of the association. Injury has been demonstrated in the lungs of young smokers, and the mechanisms by which smoking injures the lung at any age have been well characterized and plausibility described.
Chronic Respiratory Symptoms and Diseases in Childhood
Overview
The 1994 and 2004 Surgeon General’s reports, along with several other reports, have summarized the consistent evidence that the frequency of respiratory symptoms in children and adolescents is greater in current smokers than in nonsmokers or former smokers and that the duration and amount of smoking further increase the frequency of symptoms (USDHHS 1994, 2004; Arday et al. 1995; Larsson 1995; Lam et al. 1998; Withers et al. 1998). The 1994 Surgeon General’s report concluded that “cigarette smoking during childhood and adolescence produces significant health problems among young people, including cough and phlegm production, an increased number and severity of respiratory illnesses, (and) decreased physical fitness” (USDHHS 1994, p. 41). The 2004 report further concluded that “the evidence is sufficient to infer a causal relationship between active smoking and respiratory symptoms in children and adolescents, including coughing, phlegm, wheezing, and dyspnea” (p. 27). This section includes representative evidence from the 2004 report and several additional investigations that have confirmed and extended the conclusions relevant to respiratory symptoms and disease in childhood and adolescence.
Wheeze and Asthma
Overview
As demonstrated in the 1994 and 2004 Surgeon General’s reports (USDHHS 1994, 2004) and in more recent evidence presented below, studies have consistently documented that cigarette smoking among adolescents and young adults increases the incidence, persistence, and recurrence of wheeze symptoms in various populations. Although the 2004 Surgeon General’s report concluded that “the evidence is inadequate to infer the presence or absence of a causal relationship between active smoking and physician-diagnosed asthma in childhood and adolescence,” (p. 27) accumulating evidence suggests that in children who demonstrate early-life predisposition to wheeze before taking up smoking, starting to smoke cigarettes increases the risk of developing overt wheezing and variable airflow obstruction in adolescence, with symptoms persistent enough to be diagnosed as asthma (Yeatts et al. 2003). Cigarette smoking also increases the risk of apparent de novo development of wheeze in adolescence. Because many studies have only retrospective data on symptoms in early childhood, it often cannot be decided with certainty whether adolescents with de novo wheeze symptoms were without overt manifestations of a predisposition to disease—bronchial reactivity or allergic symptoms (wheeze, night cough, hay fever)—in earlier childhood before starting to smoke. Furthermore, whether the onset of wheezing in smokers constitutes asthma, as strictly defined, is not certain. The pathophysiological mechanism(s) by which smoking increases the risk of persistent wheeze may not be through an allergy-related pathway and, as data below suggest, may result in an asthmatic phenotype that is more refractory to gluco-corticoids and other conventional therapy. Regardless, the data presented below strongly support the conclusion that without exposure to active smoking, a significantly higher proportion of adolescents and young adults with a predisposition to allergy and asthma would likely remain quiescent or with symptoms inadequately severe or recurrent to be called current or active asthma.
Asthma has been defined as
1. “a chronic inflammatory disease of the airways in which many cell types play a role—in particular, mast cells, eosinophils, and T-lymphocytes. In susceptible persons, the inflammation causes recurrent episodes of wheezing, breathlessness, chest tightness, and cough particularly at night and/or in the early morning. These symptoms are usually associated with widespread and variable airflow obstruction that is at least partly reversible either spontaneously or with treatment. The inflammation also causes an associated increase in airway responsiveness to a variety of stimuli” (USDHHS 2010, p. 439).
Although the debate continues as to whether asthma and chronic bronchitis/emphysema, or COPD, are distinct diseases (Bleecker 2004; Barnes 2006; Kraft 2006), the predisposition toward bronchial hyperrespon-siveness is a characteristic phenotype shared by the two diseases (Bleecker 2004), with genetic as well as environmental origins that may also be shared. Both diseases manifest bronchial inflammation, but the cellular nature of the inflammation differs (USDHHS 2010). However, with exposure to active smoking superimposed on the predisposition to bronchial hyperreactivity and allergic inflammation, the nature of the bronchial inflammation in smokers may overlap more with that of COPD than with that of asthma and may result in more refractory asthmatic disease.
The evidence comes from diverse populations, with studies demonstrating the association of cigarette smoking with increased risk of wheeze in White and non-White and in non-U.S. or European teenagers.
Epidemiologic Evidence (Cross-Sectional and Case-Control Studies)
The evidence from cross-sectional studies is summarized in Table 2.9. In 1995 and again in 1998, children in 30 representative and randomly selected schools from throughout the Republic of Ireland took part in cross- sectional surveys of smoking behavior in secondary school children 13 and 14 years of age as part of the International Study of Asthma and Allergies in Childhood (ISAAC) survey (Manning et al. 2002). In 1995, 3,066 students, 634 (20.7%) of whom smoked cigarettes, completed a questionnaire, with significantly higher smoking rates among girls than among boys (23.3% vs. 17.6%). The investigators found that symptoms of bronchitis (cough and phlegm) were more commonly reported in active smokers than in nonsmokers, with an OR of 3.02 (95% CI, 2.34–3.88).
Table 2.9
Cross-sectional studies on the association of smoking with childhood cough, bronchitis symptoms, shortness of breath, wheeze, and asthma.
In a U.S. sample (1982–1989) of 26,504 high school seniors (Arday et al. 1995), regular cigarette smoking since ninth grade was associated with increased odds of at least one episode in the past 30 days of a coughing spell (OR = 2.1; 95% CI, 1.90–2.33), shortness of breath when not exercising (OR = 2.67; 95% CI, 2.38–2.99), and wheezing or gasping (OR = 2.58; 95% CI, 2.29–2.90), after adjusting for gender, use of marijuana and cocaine, parental education, and the year of the survey. A strong dose-response relationship was found between the amount smoked and most respiratory outcomes.
Between 1994 and 1995, Leung and colleagues (1997) studied 4,665 Hong Kong schoolchildren 13 and 14 years of age with the ISAAC protocol. In a comparison with epidemiologic data obtained in 1992, the prevalence of asthma and wheeze were found to have increased by 71% and 255%, respectively. In multiple logistic regression analyses, active smoking was associated with current wheeze (OR = 2.72; 95% CI, 1.38–2.89) and with severe wheeze that limited speech in the past 12 months (OR = 4.62; 95% CI, 2.43–8.75).
Also in Hong Kong, Lam and coworkers (1998) evaluated 6,304 mostly 12- to 15-year-old students from 172 classes in 61 schools and found a significant dose-response relationship between the amount smoked per week and risk for chronic cough (OR = 2.71; 95% CI, 1.95–4.69) for smoking more than six cigarettes per week versus never smoked, chronic phlegm (OR = 3.91; 95% CI, 2.77–5.53), wheeze in the past 3 months (OR = 2.91; 95% CI, 1.99–4.26), and use of asthma medicine in the past 2 days (OR = 3.07; 95% CI, 1.58–5.97). Ever having asthma, allergic rhinitis, or eczema diagnosed by a doctor was not significantly associated with smoking.
As part of the North Carolina School Asthma Survey of 128,568 seventh- and eighth-grade students primarily of African American, Native American, Mexican American, or White race/ethnicity who represented 99 of the state’s 100 counties (Sotir et al. 2003), 33,534 children reported an episode of wheezing in the previous year. Of these, 17,358 reported experiencing at least one episode of wheezing triggered by a head cold (upper respiratory infection-triggered wheezing [URI-TW]). With adjustment for gender, race/ethnicity, SES, and urban/rural residence, there was a dose-response relationship between active smoking and URI-TW for those with a history of wheezing. In that same study (Sturm et al. 2004), relationships were found between smoking 2–10 cigarettes per day in the past 30 days and both active diagnosed asthma (OR = 1.24; 95% CI, 1.17–1.31) and wheezing in the past 12 months (OR = 1.27; 95% CI, 1.21–1.32) in comparisons with no smoking. Frequent wheezing not diagnosed as asthma was independently associated with current smoking (OR = 2.60; 95% CI, 2.43–2.79), after adjustment for gender, passive smoke, SES, allergies, and ethnicity (Yeatts et al. 2003).
Among 4,738 Chilean adolescents (mean age = 13 years) who responded to the ISAAC video questionnaire (Mallol et al. 2007), the prevalence of tobacco smoking in the last 12 months was 16.2%. Persistent smokers had higher rates of wheeze, wheeze with exercise, severe wheeze, and dry nocturnal cough than former smokers and nonsmokers. The investigators estimated that more than 27% of asthma symptoms in these adolescents were attributable to active smoking of tobacco.
Lewis and colleagues (1996) used data from two national British birth cohorts to compare the prevalence of wheezing illness (asthma and wheezy bronchitis) at 16 years of age between 1974 and 1986. The prevalence of asthma and/or wheezy bronchitis at 16 years of age increased from 3.8% to 6.5% during this 12-year period. Smoking by these young people was associated with increased odds of asthma and/or wheezy bronchitis, with an OR of 1.44 (95% CI, 1.14–1.82) associated with smoking at levels of 40 or more cigarettes per week (versus nonsmoking), but changes in smoking behavior did not explain the increase in asthma rates between 1974 and 1986.
In a sample of 14,578 French adolescents, active smoking of more than one cigarette per day (9.3% prevalence in this population) was associated with increased odds of wheezing, current asthma, lifetime asthma, current rhinoconjunctivitis, lifetime hay fever, and current eczema after controlling for age, gender, geographic region, familial allergy, and exposure to secondhand smoke (Annesi-Maesano et al. 2004).
A number of studies indicate that having asthma is often not a deterrent to active cigarette smoking (Tyc 2008). For example, in a study of 38,047 young adult military conscripts in Israel, whose mean age was 18.6 years at baseline (Zimlichman et al. 2004), the prevalence of smoking among those with asthma increased from 20% to 22% in the mid-1980s to an estimated 30% in the late 1990s. And in a French family-based, case-control study of 200 adult asthmatic cases, 265 nonasthmatic controls, and 586 relatives of asthmatics (147 with asthma), the investigators found that in cases with asthma, active smoking was associated with greater severity of that disorder (Siroux et al. 2000). In that study, having asthma in childhood was not associated with a reduced uptake of smoking, but persons with asthma who smoked quit more often than did controls. Adult-onset asthma was unrelated to ever having been a smoker, although as mentioned earlier in this chapter, retrospective data based on recall regarding childhood asthma may be limited. Finally among asthmatics, current smokers, compared with never smokers and former smokers, had more asthma symptoms, more frequent asthma attacks (OR = 2.39; 95% CI, 1.06–5.36), and higher asthma severity scores (Siroux et al. 2000).
Epidemiologic Evidence (Prospective Cohort Studies)
The relation of starting to smoke to the prevalence of asthma, wheezy bronchitis, or wheezing was studied in 18,559 people born March 3–9, 1958, in England, Scotland, or Wales, of whom 5,801 contributed information at 7, 11, 16, 23, and 33 years of age (Table 2.10; Strachan et al. 1996). Potential bias due to attrition was evaluated by using information obtained on 14,571 of the original 18,559 participants. Active cigarette smoking was associated with increased incidence of asthma or wheezing illness at 17–33 years of age (OR = 4.42; 95% CI, 3.31–5.92) in adjusted models. Moreover, relapse after prolonged remission of childhood wheezing was more common among current smokers than among nonsmokers. Further follow-up was reported at 42–45 years of age (Butland and Strachan 2007). The proportions of incident “asthma” and incident “wheeze without asthma” sensitivity associated with cigarette smoking, adjusted for gender and atopy (heightened sensitivity to allergic reactions), were estimated to be 13% (95% CI, 0–26) and 34% (95% CI, 27–40), respectively.
Table 2.10
Longitudinal studies on the association of smoking with cough, bronchitis symptoms, shortness of breath, wheeze, and asthma in cohorts followed since childhood.
Also in the United Kingdom, in a case-control study of persons 39–45 years of age who were part of an Aberdeen, Scotland, community cohort of 2,056 asymptomatic children (originally studied in 1964) (Bodner et al. 1998), current smoking was associated with an increased risk of adult-onset wheeze (relative risk [RR] = 2.01; 95% CI, 1.08–3.74) in analyses adjusting for atopy, family history of atopy, education, and gender.
Withers and colleagues (1998), who followed a cohort of 2,289 children from Southampton, England, who were initially studied at 6–8 years of age, administered a repeat questionnaire when the participants were 14–16 years of age. Regular smoking by these adolescents (at least one cigarette per week during the past 12 months) was associated with current cough (OR = 1.71; 95% CI, 1.21–2.43), onset of cough between surveys (OR = 4.35; 95% CI, 1.12–3.25), persistent wheeze in boys (OR = 4.35; 95% CI = 1.20–3.25), and a new report of wheezing (OR = 1.65; 95% CI, 1.14–2.39). Regular smoking was not, however, associated with physician-diagnosed asthma.
In Germany, the incidence of asthma during adolescence was studied in a cohort study from two cities: Dresden and Munich (Genuneit et al. 2006). As part of ISAAC, the study population of 2,936 persons was studied in 1995–1996 at 9–11 years of age and then in 2002–2003 at 16–18 years of age. The adjusted incidence rate ratio (IRR) for incident wheeze for active smokers compared with nonsmokers was 2.30 (95% CI, 1.88–2.82). The adjusted IRRs were slightly higher for incident wheeze without having a cold (IRR = 2.76; 95% CI, 1.99–3.84) and for diagnosed asthma (IRR = 2.56; 95% CI, 1.55–4.21). Dose-dependent associations were demonstrated for all three problems when stratified by both duration of active smoking (in years) and intensity of smoking. In this same study, an observed inverse relationship between reduced physical activity and new onset of wheeze was explained by differences in active smoking (Vogelberg et al. 2007).
In Norway (Tollefsen et al. 2007), 2,300 adolescents were evaluated for wheeze and asthma at 13–15 years of age and in follow-up at 17–19 years of age. For those with no respiratory symptoms at baseline, current smoking predicted development of wheeze at follow-up, which was significant for girls (girls: OR = 2.8; 95% CI, 1.6–4.9; boys: OR = 1.8; 95% CI, 0.9–3.9).
In New Zealand, a cohort of 1,037 children born in 1972–1973 in the city of Dunedin (Sears et al. 2003) was followed repeatedly from 9 to 26 years of age. Study members with persistent or relapsing wheezing had higher prevalence rates of sensitivity to house dust, mites, and cat allergen, higher airway hyperresponsiveness, and lower lung-function measurements (p <0.001 for all associations). The 613 participants with complete outcome data were found to be generally representative of the population. In univariate and multivariate models, smoking at 21 years of age predicted persistence of wheeze from the study’s onset (adjusted OR = 1.84; 95% CI, 1.13–3.00). Relapse of wheezing at 26 years of age after being wheeze free was significantly associated with smoking at 21 years of age in a univariate model (OR = 1.84; 95% CI, 1.11–3.04), but the relationship with smoking was not significant in a multivariate model. In this case, however, smoking may have led to relapse of wheeze by increasing an intermediate phenotype, bronchial hyperresponsive-ness (BHR). Therefore, adjustment for BHR in multivariate models may have led to the reduction of the estimate for the effects of smoking because BHR was in the causal pathway as a mediator rather than a confounder.
A Swedish study followed 89 of 101 children hospitalized with wheezing before the age of 2 years up to the ages of 17–20 years (Goksör et al. 2006). The study compared their risk of asthma with that of 401 age-matched, randomly selected controls; current asthma was increased in active smokers (OR = 3.2; 95% CI, 1.2–8.4) in the final multivariate model. This finding is notable because passive smoking, which was associated with active smoking, was included in the model.
Finally, in California, a prospective cohort study was conducted among 2,609 children with no lifetime history of asthma or wheezing who were recruited from fourth- and seventh-grade classrooms and followed annually in 12 Southern California communities (Gilliland et al. 2006). Smoking 300 or more cigarettes per year was associated with a RR for new-onset asthma of 3.9 (95% CI, 1.7–8.5) when no smoking was the referent. The increased risk of asthma associated with this level of smoking was greater in children with no history of allergies, but allergic sensitization was not evaluated (Table 2.10).
Summary
Since the 1994 and 2004 Surgeon General’s reports on smoking and health, additional investigations have been published that confirm and extend the conclusions of those reports in demonstrating the association between starting to smoke and increased risk of the respiratory symptoms of cough, phlegm, and wheeze, as well as reduced exercise tolerance among children and young adults (Tables 2.9 and 2.10). Moreover, additional longitudinal data support the association of smoking with recurrence or persistence of childhood wheeze that preceded the start of smoking and with new-onset wheeze in adolescence and young adulthood.
Accumulating longitudinal evidence suggests that smoking contributes to incident asthma in susceptible children, adolescents, and young adults by increasing the already greater risk of recurrent, persistent, or new-onset persistent wheeze in children with underlying airway hyperreactivity and atopy. Although children who have allergic sensitization and chronic allergic airway inflammation may be particularly susceptible to the effects of smoking, the data do not consistently support the hypothesis that smoking increases atopy or allergic sensitization. Even so, the additional airway inflammation caused by smoking in atopic adolescents and young adults may be more resistant to conventional therapy for asthma. In addition, adolescents with atopy may be less likely to become smokers.
Cardiovascular Effects of Tobacco Use
Introduction
Atherosclerotic cardiovascular disease is a chronic process with origins in youth, and smoking is strongly and causally associated with cardiovascular morbidity and mortality (USDHHS 2004). The adverse cardiovascular effects of smoking begin with the fetus, which is exposed to components of tobacco smoke from active smoking by the mother or from her exposure to secondhand smoke. Permanent effects of smoking on the cardiovascular system have been found in children, adolescents, and young adults who smoke, and these effects are antecedents of incident cardiovascular disease in later adulthood. This section reviews findings of studies directed at the consequences of tobacco exposure for youth, extending from exposures in utero through young adulthood. The range of outcomes covered is diverse, and this section will review direct assessment of atherosclerosis, noninvasive imaging of subclinical atherosclerosis, assessment of endothelial cell function, and observations of physiological effects. The section also addresses the effects of smoking as they act in combination with other risk factors for cardiovascular disease.
The processes that lead to cardiovascular morbidity and mortality may be initiated by exposures during pregnancy, which act on the fetus, and by subsequent exposures across childhood and young adulthood (Napoli et al. 2006; McGill et al. 2008). Studies illustrating the fetal and childhood origins of cardiovascular diseases are considered here, as is the role of smoking across the life course.
Conclusions of Prior Surgeon General’s Reports
Cardiovascular diseases have been considered in the Surgeon General’s reports since the landmark report of 1964 (USDHEW 1964). Many of the subsequent reports have direct relevance to the present report, and cardiovascular diseases specifically were the topic of the 1983 report (USDHHS 1983). The 1994 report addressed the consequences of tobacco use in young people; effects on premature atherosclerosis, lipid profiles, physical fitness, left ventricular mass, and heart rate were described in that report (USDHHS 1994). At that time, however, the number of studies conducted in youth was still small.
The 2004 Surgeon General’s report on the health consequences of smoking concluded that smoking does “adversely affect the homeostatic balance in the cardiovascular system, thus explaining the well-documented relationship between smoking and both subclinical and clinical manifestations of atherosclerosis” (USDHHS 2004, p. 371). “Research during the past decade has produced further evidence that tobacco smoking is causally related to all of the major clinical cardiovascular diseases” (USDHHS 2004, p. 397). The 2006 Surgeon General’s report on involuntary exposure to secondhand smoke concluded that such exposure was associated with “increased risks of coronary heart disease morbidity and mortality among both men and women” and that accumulated evidence was suggestive but not conclusive in indicating a causal relationship between this exposure and both stroke and subclinical atherosclerosis (USDHHS 2006, p. 15).
The 2010 report of the Surgeon General reviewed the biological basis of the association between tobacco use and cardiovascular disease. Its findings are particularly relevant for the present report in documenting that smoking is linked to the early phases of cardiovascular injury, even before disease is evident. Additional conclusions not covered in the current report include (1) “cigarette smoking leads to endothelial injury and dysfunction in both coronary and peripheral arteries. There is consistent evidence that oxidizing chemicals and nicotine are responsible for endothelial dysfunction”; (2) “cigarette smoking produces a chronic inflammatory state”; (3) “cigarette smoking produces insulin resistance”; and (4) “cigarette smoking produces an atherogenic lipid profile, primarily due to an increase in triglycerides and a decrease in high-density lipoprotein cholesterol” (USDHHS 2010, pp. 10–11).
Atherosclerosis underlies much of adult cardiovascular morbidity and mortality, leading to the clinical consequences of angina pectoris and myocardial infarction, sudden death, stroke, abdominal aortic aneurysm, and symptomatic atherosclerotic peripheral vascular disease. The next section reviews the evidence on smoking and atherosclerosis in children, adolescents, and young adults, giving emphasis to findings since the 1994 report. The section addresses the links between the initiation of atherosclerosis and endothelial injury in youth and risk for disease during adulthood.
Mechanisms of Tobacco-Induced Vascular Injury in Children
Mechanisms of vascular injury related to tobacco exposure as reviewed in the 2004 and 2010 Surgeon General’s reports include direct endothelial injury, induction of a prothrombotic state, promotion of inflammation, and the promotion of oxidative stress (USDHHS 2004, 2010). Some studies have addressed these mechanisms directly in fetuses, infants, children, and young adults, including the consequences of exposure to secondhand smoke and of active smoking.
Comparisons of schoolchildren exposed to tobacco smoke with an unexposed group showed increased oxidative stress and lower antioxidant levels among those who were exposed (Kosecik et al. 2005; Zalata et al. 2007). In a Korean study comparing 19 adolescent smokers with a mean duration of tobacco use of about 3 years with 19 nonsmoking adolescents, evidence of oxidative stress was obtained in assessments of multiple markers, as the researchers found lower selenium glutathione peroxidase activity, lower glutathione reductase, lower extracellular superoxide dismutase activity, and higher serum thiobarbituric acid-reactive substances (Kim et al. 2003). Thus, the available, but limited, evidence suggests that active smoking by youth is linked to oxidative stress.
There are as yet few studies on inflammatory markers and thrombosis in infants and children. In one population-based study, the authors did not show a relationship between the concentration of C-reactive protein and exposure to secondhand smoke (Cook et al. 2000). Thrombotic events in childhood are rare, and no studies have found a relationship between the risk for such events and use of tobacco or exposure to secondhand smoke. In adults, studies have linked both active tobacco use and exposure to secondhand smoke to prothrombotic effects and laboratory markers of endothelial injury (USDHHS 2004, 2006).
Methods for the Evidence Review
The evidence considered for this review was identified by a series of PubMed searches merging the terms “tobacco” or “smoking” with relevant subjects covered here, including atherosclerosis, endothelial dysfunction, vascular injury, and lipids. These searches were then further refined, adding the terms “children,” “fetus,” or “pregnancy” to the search string. Results were cross-checked with reference lists from prior relevant reports of the Surgeon General, including the 1994, 2004, 2006, and 2010 reports. Reference lists from review articles on atherosclerosis and tobacco-related morbidity in children were also used for cross-checking (e.g., McGill et al. 2008). Finally, references from articles identified in the search strategy described above and published since 2004 were reviewed to identify any articles not found with this approach.
Vascular Injury in the Fetus
Review of Evidence
Evidence of vascular injury in the fetus that was associated with tobacco use was first identified in studies of human umbilical artery specimens and other placental vascular structures (Asmussen and Kjeldsen 1975; Bylock et al. 1979; Asmussen 1982a, b; Pittilo 1990). Structural abnormalities were most commonly found in the endothelium of many different vascular structures; evidence of attempts at vascular repair was also found. Clinical support for the relevance of these experimental findings is suggested by an ultrasound study of resistance to blood flow in the umbilical artery—a measure of fetal well-being. Ultrasound studies performed at 20–24 weeks of gestation showed that fetuses exposed to tobacco smoke had evidence of increased vascular resistance (Kalinka et al. 2005). In utero exposure to tobacco smoke may also be associated with subclinical atherosclerosis. A recent study comparing neonates with and without intrauterine exposure to components of tobacco smoke from maternal smoking showed increased thickness of the aortic wall in those exposed to tobacco smoke (Gunes et al. 2007).
Animal studies confirm the vascular injury after exposure to secondhand smoke. A controlled study of fetal exposure of apolipoprotein E (Apo E) knockout mice—a genetic model of accelerated atherosclerosis—to secondhand smoke showed increased atherosclerosis in the exposed mice as adults, and the increase in atherosclerosis was linked to mitochondrial injury and oxidative stress (Yang et al. 2004). Specifically, exposed mice had increased formation of atherosclerotic lesions, damage to mitochondrial DNA, increased antioxidant activity, and increased oxidant load compared with controls. A similar controlled study in Apo E knockout mice showed that the pups of those exposed to tobacco smoke while pregnant had atherosclerotic changes after birth, but the unexposed did not (Gairola et al. 2001). Earlier animal studies of fetal exposure to secondhand smoke have shown abnormal vascular reactivity and endothelial dysfunction after birth. They also showed increased size of myocardial infarction after exposure to smoke, beginning in utero and extending up to 12 weeks after birth, when the infarction occurred (Zhu et al. 1997; Hutchison 1998).
In the past few years, there has been intense interest in markers of oxidative stress in relation to exposure to tobacco smoke. Several case-control studies have demonstrated oxidant stress in fetuses and infants exposed to tobacco smoke both in utero and postnatally (Aycicek et al. 2005; Noakes et al. 2007; Aycicek and Ipek 2008); these studies have included measurements of the oxidative stress index, total antioxidant capacity, lipid peroxidation, and F2-isoprostane. Measurement of F2-isoprostane was positively correlated with maternal cotinine levels in one study (Noakes et al. 2007).
Low Birth Weight
The association between maternal use of tobacco and low birth weight is well documented (USDHHS 2001, 2004). Low birth weight, in turn, is associated with future cardiovascular mortality, particularly in women. This association may reflect, among other risk factors, contributions of maternal smoking and of exposure to secondhand smoke during pregnancy (Davey Smith et al. 2007; Newnham and Ross 2009).
Summary
There is evidence that exposure of the fetus to tobacco smoke causes vascular injury; oxidative stress may be one of the mechanisms responsible for this effect. Because these exposures generally produce early grades of atherosclerosis that are reversible, this evidence does not imply that fetal exposure to components of tobacco smoke alone causes adult cardiovascular disease. Nonetheless, there is substantial evidence suggesting that early exposure to smoke is important in the context of lifelong exposure to cardiovascular risk factors in contemporary society. This evidence includes the following: (1) there is an association between low birth weight and future cardiovascular mortality (maternal use of tobacco lowers birth weight); (2) relationships between passive exposure to smoke and vascular injury are likely to continue postnatally with further exposure to passive smoke from parents who smoke; and (3) children of parents who smoke are more likely to smoke in the future. Thus, vascular injury of the fetus may be the first insult in a sequence of continuous exposures to risk factors.
Physiological Effects of Smoking
The relationship of left ventricular mass, an independent predictor of cardiovascular morbidity and mortality, to active use of tobacco has been assessed in several studies in young adults. In the CARDIA study, among young adults 23–35 years of age, smokers had greater left ventricular mass by 3 to 8 g, indexed to body size and depending on race/gender group (Gidding et al. 1995). In older individuals (mean age = 62 years) with left ventricular mass assessed by magnetic resonance imaging, in a comparison of active smokers with nonsmokers and after adjustment for body size, the smokers had greater mass (by 7.7 g) (Heckbert et al. 2006). In two studies of the relationship of left ventricular mass to hypertension, the recording of ambulatory blood pressure identified a relationship of higher left ventricular mass to smoking. This relationship was not found, however, when single daytime blood pressures were used to compare smokers with nonsmokers (Verdecchia et al. 1995; Majahalme et al. 1996). This difference in findings may be explained by the capturing through ambulatory monitoring of transient increases in blood pressure that are associated with smoking. A study of U.S. Army recruits involving measurement of left ventricular mass before and after an exercise intervention did not find an association between this measurement and smoking at baseline, but it showed a larger increase in left ventricular mass in those soldiers using tobacco during the intervention (Payne et al. 2006, 2007). Complementary findings were obtained in an animal study comparing smoke-exposed and unexposed rats with exposures of 2 and 6 months’ duration. Increased left ventricular mass and greater left atrial size were found in the smoke-exposed group, and duration of exposure (2 vs. 6 months) did not influence the magnitude of the effect (Castardeli et al. 2008).
A number of other physiological effects of smoking related to myocardial energetics, oxygen delivery, and exercise have been studied in children and young adults. In the CARDIA study, young adult smokers had increased resting heart rate, and those who were female had greater cardiac wall stress, both consistent with increased resting consumption of myocardial oxygen. In addition, young adult smokers had poorer endurance and lower peak heart rate with exercise compared with nonsmokers (Sidney et al. 1993). These findings could reflect an effect of smoking and/or a lower level of fitness among smokers. Finally, children exposed to secondhand smoke have abnormal concentrations of 2,3-diphosphoglycerate, an effect suggesting stressed delivery of oxygen to the tissues and increased risk for developing premature coronary heart disease (Moskowitz et al. 1990).
Atherosclerosis
Postmortem Studies
Three major studies have assessed atherosclerosis in young people at autopsy with the intent of characterizing the relationship of the presence and degree of atherosclerosis to cardiovascular risk factors, including smoking (Table 2.11). Descriptions of these studies follow.
Table 2.11
Relationship between tobacco use and atherosclerosis or subclinical atherosclerosis.
In the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study, specimens of coronary arteries and the abdominal aorta were obtained from a group of almost 3,000 15- to 34-year-olds (Whites and Blacks) who had died of external causes (accidents, homicides, suicides) (McGill et al. 2008). The prevalence and severity of atherosclerosis were measured directly and quantified by the American Heart Association (AHA) grading system. Grades I and II reflect early lesions, including fatty streaks, that are considered reversible. Grade III reflects intermediate lesions, and grades IV and V reflect advanced lesions and plaque. Each 5-year increment in age from 15 to 34 years was associated with increased coverage of surface areas by atherosclerosis of the coronary arteries and aorta and also with increasing grade of atherosclerosis; 15- to 19-year-olds had mostly grade I and II lesions, while advanced lesions associated with cardiovascular risk factors were found in some 25- to 34-year-olds. In females, these changes occurred 5–10 years later than in males; thus, the vasculature of a 25- to 34-year-old woman resembled that of a 20-year-old man (McGill et al. 2008). Risk factors for atherosclerosis were measured in the postmortem period; tobacco use was defined by an elevated serum thiocyanate level (≥90 micromoles/L).
In the PDAY study, tobacco use was positively associated with the prevalence of the early lesions of atherosclerosis (grades I and II) in the abdominal aorta in 15- to 19-year-olds and with all AHA grades of atherosclerosis in 30- to 34-year-olds (McGill et al. 2000b; McMahan et al. 2005, 2006). The abdominal aorta was more severely affected than were the coronary arteries by tobacco use. A case-control study of a subset of the PDAY cohort, comparing 50 smokers with 50 nonsmokers (randomly selected White men 25–34 years of age), found that smokers were twice as likely to have advanced lesions as were nonsmokers and that smokers had more advanced lesions than intermediate lesions (Zieske et al. 1999). A more complete analysis of atherosclerosis of the left anterior descending coronary artery found increased atherosclerosis in this vessel in smokers compared with nonsmokers, and it also found that smoking contributed to more rapid progression of lesions to advanced AHA grades (Zieske et al. 2005).
In the 1980s, the World Health Organization and the World Heart Federation initiated an international study in five countries in North America, Asia, and Europe that was comparable in design to the PDAY study (Kádár et al. 1999). Although this international study included 214 individuals, only 68, all from Hungary, provided information on tobacco use; a strong relationship between abdominal aortic atherosclerosis and smoking was found, with smokers more likely than nonsmokers to have advanced lesions in the descending aorta (46% vs. 14%, p <0.02).
From 1972 to 1992, the Bogalusa Heart Study collected population-based data on cardiovascular risk factors from a cohort of White and Black children living in Bogalusa, Louisiana (Berenson et al. 1998), at enrollment. Data on these risk factors, obtained at multiple follow-ups for most participants, was available beginning at 5 years of age and up to 38 years of age for some of the original participants. Smoking status was unknown for those without an assessment in late adolescence or young adulthood. Berenson and colleagues (1998) reported on an assessment at autopsy of atherosclerosis in original participants who died accidentally and for whom information on smoking was available; this sample included 49 of the 204 deceased participants, with 15 known smokers and 34 known nonsmokers. Compared with nonsmokers, involvement of the aortic surface area with fibrous plaque was greater in smokers (1.22% vs. 0.12%, p = 0.02), and fatty streaks in the surface area of the coronary arteries were more common in smokers (8.3% vs. 2.9%, p = 0.04).
The PDAY and Bogalusa studies also demonstrated that the presence of multiple cardiovascular risk factors accelerates atherosclerosis (Berenson et al. 1998; McMahan et al. 2005). With regard to smoking, the combination of tobacco use and other causal risk factors is associated with acceleration of progression from the earliest stages of atherosclerosis to more advanced lesions. Figure 2.5 shows the relationship of age and the number of cardiovascular risk factors to the severity of atherosclerosis in the right coronary artery among males in the PDAY study. The column on the right provides the percentage of the cohort with each level of risk. The slope of the rate of development of atherosclerosis is increased with the addition of each risk factor. Thus, each additional risk factor (including smoking) increases the amount of atherosclerosis at any given age; accordingly, a smoker with other risk factors will experience further acceleration of the damage from those risk factors. These changes in slope are consistent with independent actions of the major risk factors, including smoking, in promoting the development of atherosclerosis.

Figure 2.5
Relationship of age and the number of cardiovascular risk factors with severity of atherosclerosis in the right coronary artery in males in the Pathobiological Determinants of Atherosclerosis in Youth study.
Summary
There are now three studies on the associations of atherosclerosis measured at postmortem examination in children and young adults who had had cardiovascular risk factors; two were based on postmortem measurement of risk factors, while the Bogalusa Heart Study used ante-mortem assessments of risk factors obtained at varying intervals before accidental death. These cohorts included Whites and Blacks in the United States and individuals from Hungary. Because atherosclerosis results from a chronic process and cardiovascular risk factors are known to track (or to be stable predictors over time) for individuals, the atherosclerotic lesions measured in these studies can be reasonably assumed to result from chronic exposure to tobacco smoke (McGill et al. 2008). Tobacco use and addiction to nicotine typically begin in adolescence, leading to the potential for lengthy exposure to tobacco smoke across the life course, and tobacco smoking has long been causally associated with atherosclerosis in adults (USDHHS 2004). The three studies show that smoking in adolescence and young adulthood contributes to the atherosclerotic process that manifests as incident cardiovascular disease in adults and that the association of smoking with atherosclerosis, so readily identified in adulthood, is also evident shortly after youth start to smoke. Over time, cigarette smoking is associated with a rapid acceleration of the atherosclerosis grade in both the abdominal aorta and left anterior descending coronary artery.
The evidence that tobacco use contributes to atherosclerosis, even in young adults, is striking. The early appearance of atherosclerosis suggests that vascular injury is initiated in association with the onset of smoking, with rapid acceleration to more advanced atherosclerotic lesions by 25 to 34 years of age. These preclinical observations in young adults parallel findings in older individuals with manifest disease. For example, the attributable risk of mortality from abdominal aortic aneurysm for tobacco use is more than 80%, and the association of smoking in youth with abdominal atherosclerosis at autopsy is strong. The findings of the PDAY study show that smoking advances the grade/severity of atherosclerosis when controlling for other risk factors (Zieske et al. 2005).
In these studies, smoking was associated at every age with atherosclerosis, and the results were consistent across all studies, particularly for abdominal aortic atherosclerosis. The mechanisms by which smoking causes atherosclerosis have been studied extensively, and multiple significant pathways for vascular injury have been documented (USDHHS 2010). Therefore, the relationship of tobacco use to abdominal aortic atherosclerosis can be considered causal. Only the PDAY study had sufficient statistical power to assess the relationship of tobacco use to atherosclerosis of the coronary arteries; these data show an association and are highly suggestive of a causal relationship as well.
Subclinical Atherosclerosis
Epidemiologic Studies
Measurements of coronary artery calcium by computed tomography (CT) scan and of the thickness of the carotid artery intima-media by ultrasound are established techniques to detect subclinical atherosclerotic disease that predict future clinical risk (Simon et al. 2007). Tobacco use in adults is associated with changes in these measures that are indicative of adverse effects from smoking (USDHHS 2004, 2010). The CARDIA and Cardiovascular Risk in Young Finns studies collected data on cardiovascular risk factors beginning in young adulthood and childhood, respectively. These data were examined as predictors of the extent of subclinical atherosclerosis on follow-up in young adulthood. Analyses in these two studies have compared profiles of risk factors measured at young ages with risk-factor profiles measured in adulthood with regard to the strength of association with the preclinical markers. These analyses provide an indication of the importance of early exposure to smoking for subsequent risk of disease (Table 2.11).
The CARDIA study measured cardiovascular risk factors at 18–30 years of age (baseline) in a cohort made up of African Americans and Whites, both male and female, and assessed coronary calcium by CT scanning 15 years later. The multivariate adjusted OR for the presence of coronary artery calcium at follow-up was 1.5 (95% CI, 1.3–1.7) per 10 cigarettes per day smoked at 18–30 years of age; this risk estimate was greater than the estimate for coronary calcium associated with cigarette use at the time of the scan (Loria et al. 2007). A second analysis of this data set used a risk score derived from the PDAY study (Gidding et al. 2006); this score incorporated the relative contributions of all risk factors, including tobacco use, into a single value. Gidding and associates (2006) found that the score was strongly associated with the presence of coronary calcium in CARDIA participants. The association was similar in strength to that obtained in the PDAY study data set, thereby showing comparability between effects estimated in the autopsy data and in data from young adults. In addition to documenting the relationship of risk factors measured early in life to subsequent risk for atherosclerosis, this analysis highlights the contribution of multiple risk factors and how each additional risk factor, such as initiating tobacco use, adds to the subsequent risk of coronary artery calcium (Gidding et al. 2006).
In the Cardiovascular Risk in Young Finns Study, which measured risk factors in adolescence and in young adulthood (24–39 years of age) (Raitakari et al. 2003; Juonala et al. 2005), thickness of the carotid intima-media was strongly associated with smoking status in adolescence, and this relationship persisted after adjustment for smoking status at the time of the ultrasound study to determine thickness. Elasticity of the carotid arteries—an index of carotid artery compliance measured in young adulthood—was more abnormal in individuals who had cardiovascular risk factors and smoked than in those with a similar cardiovascular risk factor profile who did not smoke.
Finally, in the Bogalusa Heart Study, determinants of carotid artery intima-media thickness were assessed among participants at 27–43 years of age (Bhuiyan et al. 2006). Active smoking was significantly and positively associated with this index of atherosclerosis.
Summary
In adults, a causal relationship of tobacco use with subclinical atherosclerosis has been established (USDHHS 2004). Both the CARDIA and Cardiovascular Risk in Young Finns studies have shown further that tobacco use at a younger age is associated with subclinical atherosclerosis later in life and that the response is time and dose dependent. The effects of tobacco use and other cardiovascular risk factors measured at a young age on subclinical atherosclerosis are stronger than the effect of tobacco use and other risk factors assessed at the same time as the measurement of subclinical atherosclerosis. This temporal profile of risk suggests that the effect of tobacco smoking begins at a young age and is cumulative. The effect of smoking is enhanced in individuals with more than one risk factor. The occurrence of demonstrable effects of smoking in young adults is consistent with the chronic nature of atherosclerosis and the current understanding of the underlying processes that produce this disease (USDHHS 2010) as well as with the observation that active smoking causes rapid acceleration of atherosclerosis grade because advanced lesions are thicker than early lesions and are more likely to incorporate calcium into plaques (McGill et al. 2008). Thus, tobacco use at a young age can be considered to be a cause of future subclinical atherosclerosis (USDHHS 2004, 2010).
Endothelial Dysfunction
Review of Evidence
Ultrasound assessment of vascular reactivity in the brachial artery provided the first documented evidence of a direct effect of tobacco exposure on the cardiovascular system in youth (Celermajer et al. 1993, 1996). Vascular reactivity, as assessed by this mechanism, is considered an index of endothelial health; that is, nitric-oxide-dependent vasodilation can occur. Adverse effects of both active and passive smoking have been demonstrated on measures of endothelial function. Endothelial dysfunction has been demonstrated in young current smokers with a dose-response relationship and also among young persons exposed to secondhand smoke (Table 2.12; Celermajer et al. 1993, 1996).
Table 2.12
Endothelial dysfunction in young smokers.
The initial observations discussed above in adolescents and young adults have been confirmed in other populations (Table 2.12). For example, young Chinese workers chronically exposed to tobacco smoke in the workplace had impaired endothelial function (Woo et al. 2000). A larger British study on the impact of low birth weight on endothelial function confirmed the association of active smoking with endothelial dysfunction at 20–28 years of age (Leeson et al. 2001). A comparison of smoking and nonsmoking young Chinese adults living in Hong Kong or the United States showed impaired flow-mediated dilation in smokers compared with nonsmokers in both locations (Thomas et al. 2008). In a study of young Australian adults exposed to secondhand smoke who were categorized as nonsmokers (no passive or active smoking), passive smokers, or former passive smokers, the former passive smokers had better endothelial function than did those with persistent current passive exposure (Raitakari et al. 1999). A study in young Japanese adults (mean age = 32 years) demonstrated endothelial dysfunction in response to exposure to active or passive smoking; both endothelial dysfunction and exposure to smoke were correlated with plasma levels of 8-isoprostane, a measure of oxidative stress (Kato et al. 2006). In Australia, pregnant women who smoked were found to have impaired flow-mediated dilation, and the degree of impairment was associated with risk for low birth weight of their babies (Quinton et al. 2008). In California, a controlled-exposure study in young nonsmoking adults (Heiss et al. 2008) demonstrated endothelial dysfunction after brief exposure to secondhand smoke. Following the exposure, increased numbers of dysfunctional endothelial progenitor cells appeared in the circulation. Because endothelial progenitor cells are involved in vascular repair after injury, Celermajer and Ng (2008) proposed that the effects of secondhand smoke on endothelial cells may contribute to cardiovascular risk.
One key finding on endothelial dysfunction and early exposure to tobacco smoke comes from a cohort study of cardiovascular risk in Finland that began at 6 months of age. Parental smoking history and children’s cotinine levels were measured sequentially during 11 years of follow-up. Exposure to parental smoking, as assessed by cotinine levels, was associated with impairment in endothelial function at 11 years of age, and the response was dose dependent (Kallio et al. 2007). In another study, however, a large, population-based, cross-sectional assessment of 9- to 11-year-old boys and girls in which salivary cotinine was used as the biomarker for exposure to secondhand smoke, endothelial function, as assessed by brachial reactivity, was not associated with salivary cotinine level (Leeson et al. 1997).
Another noninvasive ultrasound vascular measure, aortic pulse wave velocity, is used to assess stiffness of the large vessels. Stiffer vessels (more rapid transmission of the pulse) are abnormal and are associated with cardiovascular mortality. In a Japanese study, endothelial dysfunction in smokers (mean age = 30.4 ± 5.7 years) was associated with increased arterial pulse wave velocity (Yufu et al. 2007). Aortic stiffness was also found to be increased in young Turkish smokers (Levent et al. 2004).
Li and colleagues (2005) examined a number of indicators of vascular function in Bogalusa Heart Study participants at a mean age of 36.3 years. Compliance of large and small arteries and systemic vascular resistance were assessed by noninvasively recorded radial artery waveforms. In a comparison of smokers with nonsmokers, compliance of small arteries was significantly lower and systemic vascular resistance significantly higher in smokers. The reduction in the compliance of small arteries was significantly associated with duration of smoking.
Summary
With regard to endothelial injury, the 2004 Surgeon General’s report concluded: “A substantial body of laboratory and experimental evidence now demonstrates that cigarette smoking in general and some specific components of cigarette smoke affect a number of basic patho-physiological processes at the critical interface between circulating blood components and the inner arterial wall. Smoking leads to endothelial injury and cell dysfunction” (USDHHS 2004, p. 371). Some of the studies supporting this conclusion were performed in young people, and studies have now been conducted around the world in children and young adults showing associations of endothelial dysfunction with active and passive exposure to tobacco smoke. The association is stronger at higher doses. Active smokers have chronic endothelial dysfunction, which means that their function remains reduced after a period of abstinence and does not change after they smoke a cigarette. Nonsmokers develop acute endothelial dysfunction equivalent to that of a chronic smoker after exposure to secondhand smoke; the time course of recovery has not been well characterized but is probably 1 to 2 days.
Several studies have linked endothelial dysfunction to oxidative stress and injury to endothelial progenitor cells. The association between use of tobacco and endothelial dysfunction is supported by evidence from animal models in fetuses and pups. In these studies, vascular effects after exposure to smoke were examined. One study indicated a possible long-term effect of early involuntary exposure to smoke in childhood on endothelial dysfunction in late childhood (Kallio et al. 2007). A cross-sectional, population-based study did not confirm this finding, however (Leeson et al. 1997).
Interactions of Smoking with Other Cardiovascular Risk Factors
Lipids
The evidence for a connection between tobacco smoking and dyslipidemia covers both active and passive smoking. There are now several studies linking exposure to secondhand smoke to lipid abnormalities in children. A cohort study of twins (White and Black) found lower high-density lipoprotein (HDL) cholesterol in children with chronic exposure to secondhand smoke at baseline, and this difference persisted over time after controlling for other cardiovascular risk factors, overweight, and family history of heart disease (Moskowitz et al. 1990, 1999). A study of high school athletes that used measures of plasma cotinine as a marker of exposure to secondhand smoke found lower HDL cholesterol in those with a level indicative of exposure (Feldman et al. 1991). Similarly, in a cross-sectional study of 104 children, lower HDL cholesterol was associated with living in a household having at least one smoker (Neufeld et al. 1997). In a study of 194 children, exposure to secondhand smoke was associated with unfavorable lipid profiles, but this effect was attenuated by adjustment for SES (Işcan et al. 1996). A meta-analysis of data from seven studies on 8- to 19-year-olds comparing smokers with nonsmokers (N = >4,600 total subjects; the kinds of lipid measures obtained varied among studies) showed adverse lipid changes in smoking versus nonsmoking children, including higher triglycerides, lower HDL cholesterol, and higher low-density lipoprotein (LDL) cholesterol in children who smoked compared with those who did not (Craig et al. 1990).
Effects on lipids in the fetus have also been observed from maternal smoking during pregnancy. Two studies have shown more adverse lipid profiles in the cord blood of fetuses with mothers who smoked than in mothers who did not, including lower HDL cholesterol and a higher ratio of total cholesterol to HDL cholesterol (Adam et al. 1993; Işcan et al. 1997). Jaddoe and colleagues (2008) followed a cohort of 350 people enrolled at 5–19 years of age for at least 10 years with baseline and follow-up lipid measurements; participants with exposure to tobacco smoke in utero tended to have a higher rate of rise of total cholesterol over follow-up and a more adverse lipid profile.
Findings of two cohort studies have suggested a relationship between active smoking by youth and worsening lipid profiles. In the Bogalusa Heart Study, initiation of tobacco use was associated with higher LDL cholesterol, very-low-density lipoprotein (VLDL) cholesterol and lower HDL cholesterol in Whites, and higher VLDL cholesterol in Blacks (Clarke et al. 1986). In the Beaver County Lipid Study, individuals with higher cholesterol, at 11–14 years of age who did not become smokers were less likely than those who became smokers to have elevated cholesterol levels as adults (Stuhldreher et al. 1991).
Insulin Resistance
The relationship of tobacco use to insulin resistance has been of increasing interest in recent years (Weitzman et al. 2005; Chiolero et al. 2008). In the CARDIA study, tobacco use was associated with future glucose intolerance in a graded fashion: continuous tobacco use predicted the highest likelihood of future glucose intolerance, while prior smoking and exposure to secondhand smoke were associated with this risk but at a lower likelihood (Houston et al. 2006). Elsewhere, a meta-analysis of the relationship of smoking to diabetes, which included 1.2 million persons, confirmed a 60% increase in the likelihood of type 2 diabetes in heavy smokers, and lower but still significantly increased risk of type 2 diabetes in lighter smokers (Willi et al. 2007). These studies involved multiple ages (16–60 years at baseline), but no data were presented specifically for adolescents and young adults.
Summary
There are numerous adverse interactions between use of tobacco and other established cardiovascular risk factors. The evidence from studies of children and young adults is consistent with studies in adults showing a relationship between exposure to tobacco smoke in youth and worsening lipid profiles (USDHHS 2010). The possibility of confounding of the effect of smoking by other health behaviors needs to be considered in interpreting this evidence, however. There is also evidence for interactions of exposure to secondhand smoke with other cardiovascular risk factors in youth. These interactions could contribute to atherogenesis in youth or increased cardiovascular morbidity later in life.
In the development of this section on the cardiovascular effects of tobacco use, evidence for an association between exposure to tobacco in youth and cardiovascular morbidity has been reviewed. Studies in the fetus, child, adolescent, and young adult have been considered as well as animal studies of fetuses and pups. When relevant, studies in older individuals have been used. Evidence supporting the causal relationship of both passive and active exposure to tobacco smoke with the development of atherosclerosis and cardiovascular morbidity, beginning as early as fetal life, has been found in a wide array of studies, including those using direct measurement of atherosclerosis in humans and animals, noninvasive measurement of injury to cardiovascular end organs, and measurement of associations with biomarkers known to be associated with atherosclerosis and other forms of cardiovascular disease.
Evidence Summary
The evidence reviewed in this chapter covers how smoking adversely affects the health of children, adolescents, and young adults. Evidence reviewed in this report and in earlier reports shows that the adverse effects of smoking can begin before the onset of active smoking. For example, smoking by the mother during pregnancy is linked to vascular injury in the fetus, and exposure of youth to secondhand smoke is associated with an unfavorable lipid profile and endothelial dysfunction.
Smoking causes addiction to nicotine, and the evidence reviewed in this report shows that this addiction can begin in childhood and adolescence. Adolescents become addicted to nicotine along differing trajectories of increasing intensity of smoking. Peer and parental influences have been repeatedly identified as risk factors for initiating smoking, and emerging evidence now indicates a potential role for genetic factors as well (see Chapter 4). Adolescents and young adults who stop smoking experience withdrawal, although the symptoms are variable and not uniformly comparable to those of older smokers who quit.
One reason that some adolescents and young adults start to smoke is that the tobacco industry implies through its marketing that smoking is effective for weight control (see Chapter 5, “The Tobacco Industry’s Influences on the Use of Tobacco Among Youth”). This long-used strategy continues to the present, and the belief that smoking is effective for weight control remains prevalent among adolescents and may contribute to the initiation of smoking. The evidence reviewed in this report, however, shows that smoking by adolescents and young adults has no weight-lowering effect. However, smoking cessation among adolescents and young adults is associated with weight gain, similar to adults.
Active smoking causes cancer, cardiovascular disease, COPD, and other diseases. The evidence reviewed in this chapter indicates that smoking by adolescents and young adults initiates the injurious processes that lead to cardiovascular disease and COPD. Smoking by the mother during pregnancy is associated with vascular injury to the fetus and a reduction in birth weight, a risk factor for future cardiovascular disease. Exposure to secondhand smoke across infancy and childhood has a well-documented harmful effect on lung growth, and research also indicates that exposure to secondhand smoke is associated with a less favorable lipid profile.
For COPD and cardiovascular disease, strong evidence demonstrates that active smoking across adolescence and young adulthood increases the development of atherosclerosis and limits lung growth while also accelerating the onset of decline in lung function. By early middle age, the more rapid progression of atherosclerosis and the rapid decline of lung function in some smokers lead to increasing occurrence of the corresponding clinical diseases: coronary heart disease and stroke, and COPD, respectively. These diseases are major contributors to the premature mortality of middle-aged and elderly smokers.
This chapter does not cover the various cancers caused by tobacco use; these cancers do not occur until adulthood. Epidemiologic studies, reviewed in earlier reports, indicate that duration of smoking, which reflects the age of starting to smoke, is a powerful determinant of risk for many of these cancers (USDHHS 1990, 2004). The mechanisms by which smoking causes cancer were reviewed in the 2010 report. Current understanding of these mechanisms indicates that they are first put in place with the initiation of active smoking, regardless of age.
Conclusions
- The evidence is sufficient to conclude that there is a causal relationship between smoking and addiction to nicotine, beginning in adolescence and young adulthood.
- The evidence is suggestive but not sufficient to conclude that smoking contributes to future use of marijuana and other illicit drugs.
- The evidence is suggestive but not sufficient to conclude that smoking by adolescents and young adults is not associated with significant weight loss, contrary to young people’s beliefs.
- The evidence is sufficient to conclude that there is a causal relationship between active smoking and both reduced lung function and impaired lung growth during childhood and adolescence.
- The evidence is sufficient to conclude that there is a causal relationship between active smoking and wheezing severe enough to be diagnosed as asthma in susceptible child and adolescent populations.
- The evidence is sufficient to conclude that there is a causal relationship between smoking in adolescence and young adulthood and early abdominal aortic atherosclerosis in young adults.
- The evidence is suggestive but not sufficient to conclude that there is a causal relationship between smoking in adolescence and young adulthood and coronary artery atherosclerosis in adulthood.
References
- Acierno R, Kilpatrick DG, Resnick H, Saunders B, DeArellano M, Best C. Assault, PTSD, family substance use, and depression as risk factors for cigarette use in youth: findings from the National Survey of Adolescents. Journal of Traumatic Stress. 2000;13(3):381–96. [PubMed: 10948480]
- Adam B, Cetinkaya F, Malatyalioglu E, Gürses N. Cigarette smoking and lipids and lipoproteins in cord plasma. Japanese Heart Journal. 1993;34(6):759–62. [PubMed: 8164343]
- Akbartabartoori M, Lean MEJ, Hankey CR. Relationships between cigarette smoking, body size and body shape. International Journal of Obesity. 2005;29(2):236–43. [PubMed: 15505632]
- Albright CL, Altman DG, Slater MD, Maccoby N. Cigarette advertisements in magazines: evidence for a differential focus on women’s and youth magazines. Health Education Quarterly. 1988;15(2):225–33. [PubMed: 3378906]
- Al-Riyami AA, Afifi MM. The relation of smoking to body mass index and central obesity among Omani male adults. Saudi Medical Journal. 2003;24(8):875–80. [PubMed: 12939676]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Arlington (VA): American Psychiatric Association; 2000. (text rev.)
- Amos A, Haglund M. From social taboo to “torch of freedom”: the marketing of cigarettes to women. Tobacco Control. 2000;9(1):3–8. [PMC free article: PMC1748294] [PubMed: 10691743]
- Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, Ding Q, Zhang Q, Gu X, Vijayakrishnan J, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nature Genetics. 2008;40(5):616–22. [PMC free article: PMC2713680] [PubMed: 18385676]
- Annesi-Maesano I, Oryszczyn MP, Raherison C, Kopferschmitt C, Pauli G, Taytard A, Tunon de Lara M, Vervloet D, Charpin D. Increased prevalence of asthma and allied diseases among active adolescent tobacco smokers after controlling for passive smoking exposure: a cause for concern? Clinical and Experimental Allergy. 2004;34(7):1017–23. [PubMed: 15248844]
- Anzengruber D, Klump KL, Thorton L, Brandt H, Craw-ford S, Fichter MM, Halmi KA, Johnson C, Kaplan AS, LaVia M. Smoking in eating disorders. Eating Behaviors. 2006;7(4):291–9. [PubMed: 17056404]
- Arday DR, Giovino GA, Schulman J, Nelson DE, Mowery P, Samet JM. Cigarette smoking and self-reported health problems among U.S. high school seniors, 1982–1989. American Journal of Health Promotion. 1995;10(2):111–6. [PubMed: 10160044]
- Asmussen I. Ultrastructure of human umbilical arteries from newborn children of smoking and non-smoking mothers. Acta Pathologica, Microbiologica, et Immunologica Scandinavica, Section A, Pathology. 1982;90(5):375–83. [PubMed: 7148456]
- Asmussen I. Ultrastructure of the umbilical artery from a newborn delivered at term by a mother who smoked 80 cigarettes per day. Acta Pathologica, Microbiologica, et Immunologica Scandinavica, Section A, Pathology. 1982;90(6):397–404. [PubMed: 7164814]
- Asmussen I, Kjeldsen K. Intimal ultrastructure of human umbilical arteries: observations on arteries from newborn children of smoking and nonsmoking mothers. Circulation Research. 1975;36(5):579–89. [PubMed: 1122569]
- Audrain-McGovern J, Al Koudsi N, Rodriguez D, Wileyto PE, Shields PG, Tyndale RF. The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics. 2007;119(1):e264–e274. [PubMed: 17130279]
- Audrain-McGovern J, Rodriguez D, Tercyak KP, Epstein LH, Goldman P, Wileyto EP. Applying a behavioral economic framework to understanding adolescent smoking. Psychology of Addictive Behaviors. 2004;18(1):64–73. [PubMed: 15008687]
- Austin SB, Gortmaker SL. Dieting and smoking initiation in early adolescent girls and boys: a prospective study. American Journal of Public Health. 2001;91(3):446–50. [PMC free article: PMC1446587] [PubMed: 11236412]
- Aycicek A, Erel O, Kocyigit A. Decreased total antioxidant capacity and increased oxidative stress in passive smoker infants and their mothers. Pediatrics International. 2005;47(6):635–9. [PubMed: 16354215]
- Aycicek A, Ipek A. Maternal active or passive smoking causes oxidative stress in cord blood. European Journal of Pediatrics. 2008;167(1):81–5. [PubMed: 17297611]
- Bagot KS, Heishman SJ, Moolchan ET. Tobacco craving predicts lapse to smoking among adolescent smokers in cessation treatment. Nicotine & Tobacco Research. 2007;9(6):647–52. [PubMed: 17558821]
- Baker TB, Conti DV, Moffit TE, Caspi A. Phenotypes and Endophenotypes: Foundations for Genetic Studies of Nicotine Use and Dependence. Bethesda (MD): U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2009. The nicotine-dependence phenotype: translating theoretical perspectives and extant data into recommendations for genetic mapping; pp. 73–131. Tobacco Control Monograph No. 20. NIH Publication No. 09-6366.
- Bamia C, Trichopoulou A, Lenas D, Trichopoulos D. Tobacco smoking in relation to body fat mass and distribution in a general population sample. International Journal of Obesity. 2004;28(8):1091–6. [PubMed: 15197410]
- Barker DJP. The developmental origins of adult disease. Journal of the American College of Nutrition. 2004;23(6 Suppl):588S–595S. [PubMed: 15640511]
- Barker DJP, Osmond C, Forsén TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. New England Journal of Medicine. 2005;353(17):1802–9. [PubMed: 16251536]
- Barnes PJ. Against the Dutch hypothesis: asthma and chronic obstructive pulmonary disease are distinct diseases. American Journal of Respiratory and Critical Care Medicine. 2006;174(3):240–3. [PubMed: 16864717]
- Barrett-Connor E, Khaw KT. Cigarette smoking and increased central adiposity. Annals of Internal Medicine. 1989;111(10):783–7. [PubMed: 2817625]
- Bean MK, Mitchell KS, Speizer IS, Wilson DB, Smith BN, Fries EA. Rural adolescent attitudes toward smoking and weight loss: relationship to smoking status. Nicotine & Tobacco Research. 2008;10(2):279–86. [PubMed: 18236292]
- Benowitz NL. Mechanisms of disease: nicotine addiction. New England Journal of Medicine. 2010;362(24):2295–03. [PMC free article: PMC2928221] [PubMed: 20554984]
- Berenson GS, Srinivasan SR, Bao W, Newman WP 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults: the Bogalusa Heart Study. New England Journal of Medicine. 1998;338(23):1650–6. [PubMed: 9614255]
- Bernat DH, Erickson DJ, Widome R, Perry CL, Forster JL. Adolescent smoking trajectories: results from a population-based cohort study. Journal of Adolescent Health. 2008;43(4):334–40. [PMC free article: PMC2743902] [PubMed: 18809130]
- Berscheid E, Walster E, Bohrnstedt G. The happy American body: a survey report. Psychology Today. 1973;7(6):119–31.
- Bhuiyan AR, Srinivasan SR, Chen W, Paul TK, Berenson GS. Correlates of vascular structure and function measures in asymptomatic young adults: the Bogalusa Heart Study. Atherosclerosis. 2006;189(1):1–7. [PubMed: 16569409]
- Bierut LJ. Genetic vulnerability and susceptibility to substance dependence. Neuron. 2011;69(4):618–27. [PMC free article: PMC3095110] [PubMed: 21338875]
- Bierut LJ, Madden PAF, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, Swan GE, Rutter J, Bertelsen S, Fox L, et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Human Molecular Genetics. 2007;16(1):24–35. [PMC free article: PMC2278047] [PubMed: 17158188]
- Bisgaard H, Pedersen S, Anhøj J, Agertoft L, Hedlin G, Gulsvik A, Bjermer L, Carlsen KH, Nordvall L, Lundbäck B, et al. Determinants of lung function and airway hyperresponsiveness in asthmatic children. Respiratory Medicine. 2007;101(7):1477–82. [PubMed: 17336509]
- Bleecker ER. Similarities and differences in asthma and COPD: the Dutch hypothesis. Chest. 2004;126(2 Suppl):93S–95S. [PubMed: 15302768]
- Blitstein JL, Robinson LA, Murray DM, Klesges RC, Zbikowski SM. Rapid progression to regular cigarette smoking among nonsmoking adolescents: interactions with gender and ethnicity. Preventive Medicine. 2003;36(4):455–63. [PubMed: 12649054]
- Bodner CH, Ross S, Little J, Douglas JG, Legge JS, Friend JAR, Godden DJ. Risk factors for adult onset wheeze: a case control study. American Journal of Respiratory and Critical Care Medicine. 1998;157(1):35–42. [PubMed: 9445276]
- Boles SM, Johnson PB. Gender, weight concerns, and adolescent smoking. Journal of Addictive Diseases. 2001;20(2):5–14. [PubMed: 11318397]
- Brandon TH, Baker TB. The Smoking Consequences Questionnaire: the subjective expected utility of smoking in college students. Psychological Assessment. 1991;3(3):484–91.
- Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behavior Genetics. 1995;25(2):95–101. [PubMed: 7733862]
- Breslau N, Fenn N, Peterson EL. Early smoking initiation and nicotine dependence in a cohort of young adults. Drug and Alcohol Dependence. 1993;33(2):129–37. [PubMed: 8261877]
- Breslau N, Kilbey MM, Andreski P. DSM-III-R nicotine dependence in young adults: prevalence, correlates and associated psychiatric disorders. Addiction. 1994;89(6):743–54. [PubMed: 8069175]
- Breton CV, Byun H-M, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. American Journal of Respiratory and Critical Care Medicine. 2009;180(5):462–7. [PMC free article: PMC2742762] [PubMed: 19498054]
- Brook JS, Duan T, Zhang C, Cohen PR, Brook DW. The association between attention deficit hyperactivity disorder in adolescence and smoking in adulthood. American Journal on Addictions. 2008;17(1):54–9. [PMC free article: PMC3668564] [PubMed: 18214723]
- Brown JD, Witherspoon EM. The mass media and American adolescents’ health. Journal of Adolescent Health. 2002;31(6):153–70. [PubMed: 12470911]
- Brown RA, Lewinsohn PM, Seeley JR, Wagner EF. Cigarette smoking, major depression, and other psychiatric disorders among adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(12):1602–10. [PubMed: 8973066]
- Budd GM, Preston DB. College students’ attitudes and beliefs about the consequences of smoking: development and normative scores of a new scale. Journal of the American Academy of Nurse Practitioners. 2001;13(9):421–7. [PubMed: 11930854]
- Bulik CM, Sullivan PF, Epstein LH, McKee M, Kaye WH, Dahl RE, Weltzin TE. Drug use in women with anorexia and bulimia nervosa. International Journal of Eating Disorders. 1992;11(3):213–25.
- Butland BK, Strachan DP. Asthma onset and relapse in adult life: the British 1958 birth cohort study. Annals of Allergy, Asthma & Immunology. 2007;98(4):337–43. [PubMed: 17458429]
- Bylock A, Bondjers G, Jansson I, Hansson HA. Surface ultrastructure of human arteries with special reference to the effects of smoking. Acta Pathologica, Microbiologica, et Immunologica Scandinavica, Section A, Pathology. 1979;87A(3):201–9. [PubMed: 463566]
- Cachelin FM, Weiss JW, Garbanati JA. Dieting and its relationship to smoking, acculturation, and family environment in Asian and Hispanic adolescents. Eating Disorders. 2003;11(1):51–61. [PubMed: 16864287]
- Calderon LL, Yu CK, Jambazian P. Dieting practices in high school students. Journal of the American Dietetic Association. 2004;104(9):1369–74. [PubMed: 15354152]
- Califano JA Jr. The wrong way to stay slim. New England Journal of Medicine. 1995;333(18):1214–6. [PubMed: 7565980]
- Camp DE, Klesges RC, Relyea G. The relationship between body weight concerns and adolescent smoking. Health Psychology. 1993;12(1):24–32. [PubMed: 8462495]
- Carroll SL, Lee RE, Kaur H, Harris KJ, Strother ML, Huang TT-K. Smoking, weight loss intention and obesity-promoting behaviors in college students. Journal of the American College of Nutrition. 2006;25(4):348–53. [PubMed: 16943457]
- Castardeli E, Duarte DR, Minicucci MF, Azevedo PS, Matsubara BB, Matsubara LS, Campana AO, Paiva SAR, Zornoff LA. Exposure time and ventricular remodeling induced by tobacco smoke exposure in rats. Medical Science Monitor. 2008;14(3):BR62–BR66. [PubMed: 18301351]
- Cavallo DA, Duhig AM, McKee S, Krishnan-Sarin S. Gender and weight concerns in adolescent smokers. Addictive Behaviors. 2006;31(11):2140–6. [PubMed: 16567058]
- Cawley J, Markowitz S, Tauras J. Lighting up and slimming down: the effects of body weight and cigarette prices on adolescent smoking initiation. Journal of Health Economics. 2004;23(2):293–311. [PubMed: 15019756]
- Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilation in healthy young adults. New England Journal of Medicine. 1996;334(3):150–4. [PubMed: 8531969]
- Celermajer DS, Ng MK. Where there’s smoke… Journal of the American College of Cardiology. 2008;51(18):1772–4. [PubMed: 18452783]
- Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88(5 Pt 1):2149–55. [PubMed: 8222109]
- Cepeda-Benito A, Ferrer AR. Smoking Consequences Questionnaire—Spanish. Psychology of Addictive Behaviors. 2000;14(3):219–30. [PubMed: 10998948]
- Charlton A. Smoking and weight control in teenagers. Public Health. 1984;98(5):277–81. [PubMed: 6505135]
- Chassin L, Presson CC, Pitts SC, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood in a midwestern community sample: multiple trajectories and their psychosocial correlates. Health Psychology. 2000;19(3):223–31. [PubMed: 10868766]
- Chaudhuri R, Livingston E, McMahon AD, Thomson L, Borland W, Thomson NC. Cigarette smoking impairs the therapeutic response to oral corticosteroids in chronic asthma. American Journal of Respiratory and Critical Care Medicine. 2003;168(11):1308–11. [PubMed: 12893649]
- Chesley EB, Roberts TA, Auinger P, Kreipe RE, Klein JD. Longitudinal impact of weight-related intentions with the initiation and maintenance of smoking among adolescents. Journal of Adolescent Health. 2004;34(2):130.
- Chilcoat HD, Breslau N. Pathways from ADHD to early drug use. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(11):1347–54. [PubMed: 10560220]
- Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. American Journal of Clinical Nutrition. 2008;87(4):801–9. [PubMed: 18400700]
- Chiriboga DE, Ma Y, Li W, Olendzki BC, Pagoto SL, Merriam PA, Matthews CE, Herbert JR, Ockene IS. Gender differences in predictors of body weight and body weight change in healthy adults. Obesity. 2008;16(1):137–45. [PMC free article: PMC4355617] [PubMed: 18223626]
- Choi WS, Gilpin EA, Farkas AJ, Pierce JP. Determining the probability of future smoking among adolescents. Addiction. 2001;96(2):313–23. [PubMed: 11182877]
- Clark DB, Wood DS, Martin CS, Cornelius JR, Lynch KG, Shiffman S. Multidimensional assessment of nicotine dependence in adolescents. Drug and Alcohol Dependence. 2005;77(3):235–42. [PubMed: 15734223]
- Clarke WR, Srinivasan SR, Shear CL, Hunter SM, Croft J, Webber LS, Berenson GS. Cigarette smoking initiation and longitudinal changes in serum lipids and lipoproteins in early adulthood: the Bogalusa Heart Study. American Journal of Epidemiology. 1986;124(2):207–19. [PubMed: 3728437]
- Colby SM, Tiffany ST, Shiffman S, Niaura RS. Measuring nicotine dependence among youth: a review of available approaches and instruments. Drug and Alcohol Dependence. 2000;59(Suppl 1):S23–S39. [PubMed: 10773436]
- Colder CR, Mehta P, Balanda K, Campbell RT, Mayhew KP, Stanton WR, Pentz MA, Flay BR. Identifying trajectories of adolescent smoking: an application of latent growth mixture modeling. Health Psychology. 2001;20(2):127–35. [PubMed: 11315730]
- Conners CK, Levin ED, Sparrow E, Hinton SC, Erhardt D, Meck WH, Rose JE, March J. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD). Psychopharmacology Bulletin. 1996;32(1):67–73. [PubMed: 8927677]
- Cook DG, Mendall MA, Whincup PH, Carey IM, Ballam L, Morris JE, Miller GJ, Strachan DP. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis. 2000;149(1):139–50. [PubMed: 10704625]
- Cooper TV, Klesges RC, Robinson LA, Zbikowski SM. A prospective evaluation of the relationships between smoking dosage and body mass index in an adolescent, biracial cohort. Addictive Behaviors. 2003;28(3):501–12. [PubMed: 12628622]
- Copeland AL, Carney CE. Smoking expectancies as mediators between dietary restraint and disinhibition and smoking in college women. Experimental and Clinical Psychopharmacology. 2003;11(3):247–51. [PubMed: 12940504]
- Copeland AL, Diefendorff JM, Kendzor DE, Rash CJ, Businelle MS, Patterson SM, Williamson DA. Measurement of smoking outcome expectancies in children: the Smoking Consequences Questionnaire-Child. Psychology of Addictive Behaviors. 2007;21(4):469–77. [PubMed: 18072829]
- Covey LS, Tam D. Depressive mood, the single-parent home, and adolescent cigarette smoking. American Journal of Public Health. 1990;80(11):1330–3. [PMC free article: PMC1404887] [PubMed: 2240299]
- Craig WY, Palomaki GE, Johnson AM, Haddow JE. Cigarette smoking-associated changes in blood lipid and lipoprotein levels in the 8- to 19-year-old age group: a meta-analysis. Pediatrics. 1990;85(2):155–8. [PubMed: 2136949]
- Crawley HF, While D. The diet and body weight of British teenage smokers at 16–17 years. European Journal of Clinical Nutrition. 1995;49(12):904–14. [PubMed: 8925792]
- Crisp A, Sedgwick P, Halek C, Joughin N, Humphrey H. Why may teenage girls persist in smoking? Journal of Adolescence. 1999;22(5):657–72. [PubMed: 10527537]
- Crisp AH, Halek C, Sedgewick P, Stravraki C, Williams E, Kiossis I. Smoking and pursuit of thinness in schoolgirls in London and Ottawa. Postgraduate Medical Journal. 1998;74(874):473–9. [PMC free article: PMC2360886] [PubMed: 9926121]
- Crocker P, Kowalski N, Kowalski K, Chad K, Humbert L, Forrester S. Smoking behaviour and dietary restraint in young adolescent women: the role of physical self-perceptions. Canadian Journal of Public Health. 2001;92(6):428–32. [PMC free article: PMC6979877] [PubMed: 11799546]
- Croll J, Neumark-Sztainer D, Story M, Ireland M. Prevalence and risk and protective factors related to disordered eating behaviors among adolescents: relationship to gender and ethnicity. Journal of Adolescent Health. 2002;31(2):166–75. [PubMed: 12127387]
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nature Neuroscience. 2005;8(11):1465–70. [PubMed: 16251989]
- Davey Smith G, Hyppönen E, Power C, Lawlor DA. Offspring birth weight and parental mortality: prospective observational study and meta-analysis. American Journal of Epidemiology. 2007;166(2):160–9. [PubMed: 17485730]
- Davis RM. Current trends in cigarette advertising and marketing. New England Journal of Medicine. 1987;316(12):725–32. [PubMed: 3547127]
- de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Australian & New Zealand Journal of Obstetrics & Gynaecology. 2006;46(1):4–14. [PubMed: 16441686]
- Dierker LC, Avenevoli S, Merikangas KR, Flaherty BP, Stolar M. Association between psychiatric disorders and the progression of tobacco use behaviors. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(10):1159–67. [PubMed: 11589528]
- DiFranza JR, Rigotti NA, McNeill AD, Ockene JK, Savageau JA, St Cyr D, Coleman M. Initial symptoms of nicotine dependence in adolescents. Tobacco Control. 2000;9(3):313–9. [PMC free article: PMC1748379] [PubMed: 10982576]
- Ding L, Pallonen UE, Migneault JP, Velicer WF. Development of a measure to assess adolescents’ temptation to smoke [abstract] Annals of Behavioral Medicine. 1994;16(Suppl):S175.
- Doll R, Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. Journal of Epidemiology and Community Health. 1978;32(4):303–13. [PMC free article: PMC1060963] [PubMed: 744822]
- Donovan JE, Jessor R, Costa FM. Syndrome of problem behavior in adolescence: a replication. Journal of Consulting and Clinical Psychology. 1988;56(5):762–5. [PubMed: 3192793]
- Dowdell EB, Santucci ME. Health risk behavior assessment: nutrition, weight, and tobacco use in one urban seventh-grade class. Public Health Nursing. 2004;21(2):128–36. [PubMed: 14987212]
- Doyle LW. Growth and respiratory health in adolescence of the extremely low-birth weight survivor. Clinics in Perinatology. 2000;27(2):421–32. [PubMed: 10863658]
- Doyle LW, Olinsky A, Faber B, Callanan C. Adverse effects of smoking on respiratory function in young adults born weighing less than 1000 grams. Pediatrics. 2003;112(3):565–9. [PubMed: 12949285]
- Drewnowski A, Yee DK, Kurth CL, Krahn DD. Eating pathology and DSM-III-R bulimia nervosa: a continuum of behavior. American Journal of Psychiatry. 1994;151(8):1217–9. [PubMed: 8037258]
- DuRant RH, Smith JA, Kreiter SR, Krowchuk DP. The relationship between early age of onset of initial substance use and engaging in multiple health risk behaviors among young adolescents. Archives of Pediatrics & Adolescent Medicine. 1999;153(3):286–91. [PubMed: 10086407]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Research Part C Embryo Today. 2008;84(1):30–44. [PubMed: 18383130]
- Elisaf M, Papanikolaou N, Letzaris G, Siamopoulos KC. Smoking habit in female students of northwestern Greece: relation to other cardiovascular risk factors. Journal of the Royal Society for the Promotion of Health. 1996;116(2):87–90. [PubMed: 8627593]
- Ernster VL. Mixed messages for women: a social history of cigarette smoking and advertising. New York State Journal of Medicine. 1985;85(7):335–40. [PubMed: 3900827]
- Ernster V, Kaufman N, Nichter M, Samet J, Yoon SY. Women and tobacco: moving from policy to action. Bulletin of the World Health Organization. 2000;78(7):891–901. [PMC free article: PMC2560804] [PubMed: 10994262]
- Facchini M, Rozensztejn R, González C. Smoking and weight control behaviors. Eating and Weight Disorders. 2005;10(1):1–7. [PubMed: 15943165]
- Fagerström KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerström Tolerance Questionnaire. Journal of Behavioral Medicine. 1989;12(2):159–82. [PubMed: 2668531]
- Fairburn CG, Cooper Z. The eating disorder examination. In: Fairburn CG, Wilson GT, editors. Binge Eating: Nature, Assessment, and Treatment. New York: Guilford Press; 1993.
- Farrell M, Marshall EJ. Epidemiology of tobacco, alcohol and drug use. Psychiatry. 2006;5(12):427–30.
- Feldman J, Shenker IR, Etzel RA, Spierto FW, Lilienfield DE, Nussbaum M, Jacobson MS. Passive smoking alters lipid profiles in adolescents. Pediatrics. 1991;88(2):259–64. [PubMed: 1861923]
- Fergusson DM, Lynskey MT, Horwood LJ. Comorbidity between depressive disorders and nicotine dependence in a cohort of 16-year-olds. Archives of General Psychiatry. 1996;53(11):1043–7. [PubMed: 8911227]
- Fidler JA, West R, Van Jaarsveld CHM, Jarvis MJ, Wardle J. Does smoking in adolescence affect body mass index, waist or height: findings from a longitudinal study. Addiction. 2007;102(9):1493–501. [PubMed: 17645429]
- Field AE, Austin SB, Frazier AL, Gillman MW, Camargo CA Jr, Colditz GA. Smoking, getting drunk, and engaging in bulimic behaviors: in which order are the behaviors adopted? Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(7):846–53. [PubMed: 12108810]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service; 2008. Clinical Practice Guideline.
- Fogarty AW, Glancy C, Jones S, Lewis SA, McKeever TM, Britton JR. A prospective study of weight change and systemic inflammation over 9 y. American Journal of Clinical Nutrition. 2008;87(1):30–5. [PubMed: 18175734]
- Forman VL, Morello P. Weight concerns, postexperimental smoking, and perceived difficulty in quitting in Argentinean adolescents. Eating Behaviors. 2003;4(1):41–52. [PubMed: 15000987]
- Frank RE, Serdula MK, Adame D. Weight loss and bulimic eating behavior: changing patterns within a population of young adult women. Southern Medical Journal. 1991;84(4):457–60. [PubMed: 2014429]
- Freedman DS, Serdula MK, Percy CA, Ballew C, White L. Obesity, levels of lipids and glucose, and smoking among Navajo adolescents. Journal of Nutrition. 1997;127(10):2120S–2127S. [PubMed: 9339179]
- French SA, Jeffery RW. Weight concerns and smoking: a literature review. Annals of Behavioral Medicine. 1995;17(3):234–44. [PubMed: 24203535]
- French SA, Jeffery RW, Klesges LM, Forster JL. Weight concerns and change in smoking behavior over two years in a working population. American Journal of Public Health. 1995;85(5):720–2. [PMC free article: PMC1615425] [PubMed: 7733437]
- French SA, Jeffery RW, Pirie PL, McBride CM. Do weight concerns hinder smoking cessation efforts? Addictive Behaviors. 1992;17(3):219–26. [PubMed: 1636469]
- French SA, Perry CL, Leon GR, Fulkerson JA. Weight concerns, dieting behavior, and smoking initiation among adolescents: a prospective study. American Journal of Public Health. 1994;84(11):1818–20. [PMC free article: PMC1615224] [PubMed: 7977924]
- Fulkerson JA, French SA. Cigarette smoking for weight loss or control among adolescents: gender and racial/ethnic differences. Journal of Adolescent Health. 2003;32(4):306–13. [PubMed: 12667735]
- Fulton JE, Shekelle RB. Cigarette smoking, weight gain, and coronary mortality: results from the Chicago Western Electric Study. Circulation. 1997;96(5):1438–44. [PubMed: 9315529]
- Gairola CG, Drawdy ML, Block AE, Daugherty A. Sidestream cigarette smoke accelerates atherogenesis in apolipoprotein E−/− mice. Atherosclerosis. 2001;156(1):49–55. [PubMed: 11368996]
- Garner DM. Eating Disorder Inventory-2: Professional Manual. Odessa (FL): Psychological Assessment Resources; 1991.
- Garner DM, Olmsted MP. Manual for Eating Disorders Inventory (EDI). Odessa (FL): Psychological Assessment Resources; 1984.
- Garner DM, Olmsted MP, Polivy J. Development and validation of a multidimensional eating disorder inventory for anorexia nervosa and bulimia. International Journal of Eating Disorders. 1983;2(2):15–34.
- Genuneit J, Weinmayr G, Radon K, Dressel H, Windstetter D, Rzehak P, Vogelberg C, Leupold W, Nowak D, von Mutius E, et al. Smoking and the incidence of asthma during adolescence: results of a large cohort study in Germany. Thorax. 2006;61(7):572–8. [PMC free article: PMC2104663] [PubMed: 16537668]
- George VA, Johnson P. Weight loss behaviors and smoking in college students of diverse ethnicity. American Journal of Health Behavior. 2001;25(2):115–24. [PubMed: 11297041]
- Gicquel C, El-Osta A, Le Bouc Y. Epigenetic regulation and fetal programming. Best Practice & Research Clinical Endocrinology & Metabolism. 2008;22(1):1–16. [PubMed: 18279777]
- Gidding SS, McMahan CA, McGill HC, Colangelo LA, Schreiner PF, Williams OD, Liu K. Prediction of coronary artery calcium in young adults using the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) risk score: the CARDIA study. Archives of Internal Medicine. 2006;166(21):2341–7. [PubMed: 17130387]
- Gidding SS, Xie X, Liu K, Manolio T, Flack JM, Gardin JM. Cardiac function in smokers and nonsmokers: the CARDIA Study. Journal of the American College of Cardiology. 1995;26(1):211–6. [PubMed: 7797754]
- Gilliland FD, Islam T, Berhane K, Gauderman WJ, McConnell R, Avol E, Peters JM. Regular smoking and asthma incidence in adolescents. American Journal of Respiratory and Critical Care Medicine. 2006;174(10):1094–100. [PMC free article: PMC2648110] [PubMed: 16973983]
- Glasgow RE, Strycker LA, Eakin EG, Boles SM, Whitlock EP. Concern about weight gain associated with quitting smoking: prevalence and association with outcome in a sample of young female smokers. Journal of Consulting and Clinical Psychology. 1999;67(6):1009–11. [PubMed: 10596524]
- Goksör E, Åmark M, Alm B, Gustafsson PM, Wennergren G. Asthma symptoms in early childhood—what happens then? Acta Paediatrica. 2006;95(4):471–8. [PubMed: 16720497]
- Gold DR, Wang X, Wypij D, Speizer FE, Ware JH, Dockery DW. Effects of cigarette smoking on lung function in adolescent boys and girls. New England Journal of Medicine. 1996;335(13):931–7. [PubMed: 8782500]
- Goodman E, Capitman J. Depressive symptoms and cigarette smoking among teens. Pediatrics. 2000;106(4):748–55. [PubMed: 11015518]
- Granner ML, Abood DA, Black DR. Racial differences in eating disorder attitudes, cigarette, and alcohol use. American Journal of Health Behavior. 2001;25(2):83–99. [PubMed: 11297045]
- Grant BF. Age at smoking onset and its association with alcohol consumption and DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse. 1998;10(1):59–73. [PubMed: 9720007]
- Grol MH, Gerritsen J, Vonk JM, Schouten JP, Koëter GH, Rijcken B, Postma DS. Risk factors for growth and decline of lung function in asthmatic individuals up to age 42 years: a 30-year follow-up study. American Journal of Respiratory and Critical Care Medicine. 1999;160(6):1830–7. [PubMed: 10588593]
- Grucza RA, Bierut LJ. Cigarette smoking and the risk for alcohol use disorders among adolescent drinkers. Alcoholism, Clinical and Experimental Research. 2006;30(12):2046–54. [PMC free article: PMC2431150] [PubMed: 17117970]
- Gunes T, Koklu E, Yikilmaz A, Ozturk MA, Akcakus M, Kurtoglu S, Coskun A, Koklu S. Influence of maternal smoking on neonatal aortic intima-media thickness, serum IGF-I and IGFBP-3 levels. European Journal of Pediatrics. 2007;166(10):1039–44. [PubMed: 17203279]
- Haberstick BC, Timberlake D, Ehringer MA, Lessem JM, Hopfer CJ, Smolen A, Hewitt JK. Genes, time to first cigarette and nicotine dependence in a general population sample of young adults. Addiction. 2007;102(4):655–65. [PubMed: 17309537]
- Hanna EZ, Grant BF. Parallels to early onset alcohol use in the relationship of early onset smoking with drug use and DSM-IV drug and depressive disorders: findings from the National Longitudinal Epidemiologic Survey. Alcoholism, Clinical and Experimental Research. 1999;23(3):513–22. [PubMed: 10195827]
- Hanson K, Allen S, Jensen S, Hatsukami D. Treatment of adolescent smokers with the nicotine patch. Nicotine & Tobacco Research. 2003;5(4):515–26. [PubMed: 12959789]
- Harju T, Mazur W, Merikallio H, Soini Y, Kinnula VL. Glutathione-S-transferases in lung and sputum specimens, effects of smoking and COPD severity. Respiratory Research. 2008;9:80. [PMC free article: PMC2654438] [PubMed: 19077292]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–27. [PubMed: 1932883]
- Heckbert SR, Post W, Pearson GDN, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. Journal of the American College of Cardiology. 2006;48(11):2285–92. [PMC free article: PMC1794681] [PubMed: 17161261]
- Heiss C, Amabile N, Lee AC, Real WM, Schick SF, Lao D, Wong ML, Jahn S, Angeli FS, Minasi P, et al. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. Journal of the American College of Cardiology. 2008;51(18):1760–71. [PubMed: 18452782]
- Herman CP. Restrained eating. Psychiatric Clinics of North America. 1978;1(3):593–607.
- Herman CP, Mack D. Restrained and unrestrained eating. Journal of Personality. 1975;43(4):647–60. [PubMed: 1206453]
- Herman CP, Polivy J. Restrained eating. In: Stunkard AJ, editor. Obesity. Philadelphia: Saunders; 1980.
- Ho MK, Tyndale RF. Overview of the pharmacogenomics of cigarette smoking. Pharmacogenomics Journal. 2007;7(2):81–98. [PubMed: 17224913]
- Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, Salmeron BJ, Srivastava V, Thaker GK, Goldman D, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(30):13509–14. [PMC free article: PMC2922167] [PubMed: 20643934]
- Hong T, Cody MJ. Presence of pro-tobacco messages on the Web. Journal of Health Communication. 2002;7(4):273–307. [PubMed: 12356288]
- Honjo K, Siegel M. Perceived importance of being thin and smoking initiation among young girls. Tobacco Control. 2003;12(3):289–95. [PMC free article: PMC1747738] [PubMed: 12958390]
- Hops H, Andrews JA, Duncan SC, Duncan TE, Tildesley E. Adolescent drug use development: a social interactional and contextual perspective. In: Sameroff AJ, Lewis M, Miller SM, editors. Handbook of Developmental Psychopathology. 2nd ed. New York: Kluwer Academic; 2000. pp. 589–605.
- Houston TK, Person SD, Pletcher MJ, Liu K, Iribarren C, Kiefe CI. Active and passive smoking and development of glucose intolerance among young adults in a prospective cohort: CARDIA study. BMJ (British Medical Journal). 2006;332(7549):1064–9. [PMC free article: PMC1458534] [PubMed: 16603565]
- Howe H. An historical review of women, smoking and advertising. Health Education. 1984;15(3):3–9. [PubMed: 6444018]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine & Tobacco Research. 2007;9(3):315–27. [PubMed: 17365764]
- Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, et al. A susceptibility locus for lung cancer maps to nicotine acetylcholine receptor subunit genes on 15q25. Nature. 2008;452(7187):633–7. [PubMed: 18385738]
- Hurt RD, Croghan GA, Beede SD, Wolter TD, Croghan IT, Patten CA. Nicotine patch therapy in 101 adolescent smokers: efficacy, withdrawal symptom relief, and carbon monoxide and plasma cotinine levels. Archives of Pediatrics & Adolescent Medicine. 2000;154(1):31–7. [PubMed: 10632247]
- Hutchison S. Smoking as a risk factor for endothelial dysfunction. Canadian Journal of Cardiology. 1998;14(Suppl D):20D–22D. [PubMed: 9713426]
- Hutter HP, Moshammer H, Neuberger M. Smoking cessation at the workplace: 1 year success of short seminars. International Archives of Occupational and Environmental Health. 2006;79(1):1432–46. [PubMed: 16133522]
- Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. Journal of Hypertension. 2000;18(7):815–31. [PubMed: 10930178]
- Ilomäki R, Riala K, Hakko H, Lappalainen J, Ollinen T, Räsänen P, Timonen M. Study 70 Workgroup. Temporal association of onset of daily smoking with adolescent substance use and psychiatric morbidity. European Psychiatry. 2008;23(2):85–91. [PubMed: 18082380]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Tobacco Smoke and Involuntary Smoking. Vol. 8. Lyon (France): International Agency for Research on Cancer; 2004. [PMC free article: PMC4781536] [PubMed: 15285078]
- Işcan A, Uyanik BS, Vurgun N, Ece A, Yiđitođlu MR. Effects of passive exposure to tobacco, socioeconomic status and family history of essential hypertension on lipid profiles in children. Japanese Heart Journal. 1996;37(6):917–23. [PubMed: 9057686]
- Iþcan A, Yiđitođlu MR, Ece A, Ari Z, Akyildiz M. The effect of cigarette smoking during pregnancy on cord blood lipid, lipoprotein and apolipoprotein levels. Japanese Heart Journal. 1997;38(4):497–501. [PubMed: 9350146]
- Istvan J, Matarazzo JD. Tobacco, alcohol, and caffeine use: a review of their interrelationships. Psychological Bulletin. 1984;95(2):301–26. [PubMed: 6544436]
- Jaddoe VWV, de Ridder MAJ, van den Elzen APM, Hofman A, Uiterwaal CSPM, Witteman JCM. Maternal smoking in pregnancy is associated with cholesterol development in the offspring: a 27-years follow-up study. Atherosclerosis. 2008;196(1):42–8. [PubMed: 17336310]
- Janzon E, Hedblad B, Berglund G, Engström G. Changes in blood pressure and body weight following smoking cessation in women. Journal of Internal Medicine. 2004;255(2):266–72. [PubMed: 14746564]
- Jarry JL, Coambs RB, Polivy J, Herman CP. Weight gain after smoking cessation in women: the impact of dieting status. International Journal of Eating Disorders. 1998;24(1):53–64. [PubMed: 9589311]
- Jeffery RW, Boles SM, Strycker LA, Glasgow RE. Smoking-specific weight gain concerns and smoking cessation in a working population. Health Psychology. 1997;16(5):487–9. [PubMed: 9302546]
- Jeffery RW, Hennrikus DJ, Lando HA, Murray DM, Liu JW. Reconciling conflicting findings regarding postcessation weight concerns and success in smoking cessation. Health Psychology. 2000;19(3):242–6. [PubMed: 10868768]
- Jenks RA, Higgs S. Associations between dieting and smoking-related behaviors in young women. Drug and Alcohol Dependence. 2007;88(2–3):291–9. [PubMed: 17178198]
- Jitnarin N, Kosulwat V, Boonpraderm A, Haddock CK, Booth KM, Berkel LA, Poston WS. The relationship between smoking, BMI and dietary intake [abstract] Journal of the American Dietetic Association. 2006;106(8 Suppl 1):A39.
- Juonala M, Järvisalo MJ, Mäki-Torkko N, Kähönen M, Viikari JSA, Raitakari OT. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2005;12(10):1486–93. [PubMed: 16129802]
- Kádár A, Mózes G, Illyés G, Schönefeld T, Kulka J, Sipos B, Glasz T, Tõkés AM, Szik A. World Health Organization (WHO) and the World Heart Federation (WHF) pathobiological determinants of atherosclerosis in youth study (WHO/WHF/PBDAY Study) 1986–1996: histomorphometry and histochemistry of atherosclerotic lesions in coronary arteries and the aorta in a young population. Nutrition, Metabolism, and Cardiovascular Diseases. 1999;9(5):220–7. [PubMed: 10656168]
- Kalinka J, Hanke W, Sobala W. Impact of prenatal tobacco smoke exposure, as measured by midgestation serum cotinine levels, on fetal biometry and umbilical flow velocity waveforms. American Journal of Perinatology. 2005;22(1):41–7. [PubMed: 15668843]
- Kallio K, Jokinen E, Raitakari OT, Hämäläinen M, Siltala M, Volanen I, Kaitosaari T, Viikari J, Rönnemaa T, Simell O. Tobacco smoke exposure is associated with attenuated endothelial function in 11-year-old healthy children. Circulation. 2007;115(25):3205–12. [PubMed: 17548727]
- Kandel D, Schaffran C, Griesler P, Samuolis J, Davies M, Galanti R. On the measurement of nicotine dependence in adolescence: comparisons of the mFTQ and a DSM-IV-based scale. Journal of Pediatric Psychology. 2005;30(4):319–32. [PMC free article: PMC1282455] [PubMed: 15863429]
- Kandel D, Yamaguchi K. From beer to crack: developmental patterns of drug involvement. American Journal of Public Health. 1993;83(6):851–5. [PMC free article: PMC1694748] [PubMed: 8498623]
- Kandel DB, Davies M. Adult sequelae of adolescent depressive symptoms. Archives of General Psychiatry. 1986;43(3):255–62. [PubMed: 3954545]
- Kandel DB, Johnson JG, Bird H, Canino G, Goodman SH, Lahey BB, Regier DA, Schwab-Stone M. Psychiatric disorders associated with substance use among children and adolescents: findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) Study. Journal of Abnormal Child Psychology. 1997;25(2):121–32. [PubMed: 9109029]
- Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. Journal of Studies on Alcohol. 1992;53(5):447–57. [PubMed: 1405637]
- Karp I, O’Loughlin J, Paradis G, Hanley J, DiFranza J. Smoking trajectories of adolescent novice smokers in a longitudinal study of tobacco use. Annals of Epidemiology. 2005;15(6):445–52. [PubMed: 15967392]
- Kato T, Inoue T, Morooka T, Yoshimoto N, Node K. Short-term passive smoking causes endothelial dysfunction via oxidative stress in nonsmokers. Canadian Journal of Physiology and Pharmacology. 2006;84(5):523–9. [PubMed: 16902597]
- Kendzor DE, Copeland AL, Stewart TM, Businelle MS, Williamson DA. Weight-related concerns associated with smoking in young children. Addictive Behaviors. 2007;32(3):598–607. [PubMed: 16860488]
- Kenney BA, Holahan CJ. Depressive symptoms and cigarette smoking in a college sample. Journal of American College Health. 2008;56(4):409–14. [PubMed: 18316285]
- Killen JD, Ammerman S, Rojas N, Varady J, Haydel F, Robinson TN. Do adolescent smokers experience withdrawal effects when deprived of nicotine? Experimental and Clinical Psychopharmacology. 2001;9(2):176–82. [PubMed: 11518093]
- Killen JD, Robinson TN, Haydel KF, Hayward C, Wilson DM, Hammer LD, Litt IF, Taylor CB. Prospective study of risk factors for the initiation of cigarette smoking. Journal of Consulting and Clinical Psychology. 1997;65(6):1011–6. [PubMed: 9420362]
- Kim SH, Kim JS, Shin HS, Keen CL. Influence of smoking on markers of oxidative stress and serum mineral concentrations in teenage girls in Korea. Nutrition. 2003;19(3):240–3. [PubMed: 12620526]
- Klesges RC, Elliott VE, Robinson LA. Chronic dieting and the belief that smoking controls body weight in a biracial, population-based adolescent sample. Tobacco Control. 1997;6(2):89–94. [PMC free article: PMC1759548] [PubMed: 9291216]
- Klesges RC, Klesges LM. Cigarette smoking as a dieting strategy in a university population. International Journal of Eating Disorders. 1988;7(3):413–9.
- Klesges RC, Meyers AW, Klesges LM, LaVasque ME. Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychological Bulletin. 1989;106(2):204–30. [PubMed: 2678202]
- Klesges RC, Mizes JS, Klesges LM. Self-help dieting strategies in college males and females. International Journal of Eating Disorders. 1987;6(3):409–17.
- Klesges RC, Robinson LA, Zbikowski SM. Is smoking associated with lower body mass in adolescents: a large-scale biracial investigation. Addictive Behaviors. 1998;23(1):109–13. [PubMed: 9468748]
- Klesges RC, Ward KD, Ray JW, Cutter G, Jacobs DR Jr, Wagenknecht LE. The prospective relationships between smoking and weight in a young, biracial cohort: the Coronary Artery Risk Development in Young Adults Study. Journal of Consulting and Clinical Psychology. 1998;66(6):987–93. [PubMed: 9874912]
- Klesges RC, Winders SE, Meyers AW, Eck LH, Ward KD, Hultquist CM, Ray JW, Shadish WR. How much weight gain occurs following smoking cessation: a comparison of weight gain using both continuous and point prevalence abstinence. Journal of Consulting and Clinical Psychology. 1997;65(2):286–91. [PubMed: 9086692]
- Klesges RC, Zbikowski SM, Lando HA, Haddock CK, Talcott GW, Robinson LA. The relationship between smoking and body weight in a population of young military personnel. Health Psychology. 1998;17(5):454–8. [PubMed: 9776004]
- Koopmans J, vanDoornen LJP, Boomsma DI. Association between alcohol use and smoking in adolescent and young adult twins: a bivariate genetic analysis. Alcoholism, Clinical and Experimental Research. 1997;21(3):537–46. [PubMed: 9161615]
- Kosecik M, Erel O, Sevinc E, Selek S. Increased oxidative stress in children exposed to passive smoking. International Journal of Cardiology. 2005;100(1):61–4. [PubMed: 15820286]
- Kraft M. Rebuttal by Dr. Kraft. American Journal of Respiratory and Critical Care Medicine. 2006;174(3):243–4. [PubMed: 16864716]
- Krahn D, Kurth C, Demitrack M, Drewnowski A. The relationship of dieting severity and bulimic behaviors to alcohol and other drug use in young women. Journal of Substance Abuse. 1992;4(4):341–53. [PubMed: 1294277]
- Krug I, Treasure J, Anderluh M, Bellodi L, Cellini E, di Bernardo M, Granero R, Karwautz A, Nacmias B, Penelo E, et al. Present and lifetime comorbidity of tobacco, alcohol and drug use in eating disorders: a European multicenter study. Drug and Alcohol Dependence. 2008;97(1–2):169–79. [PubMed: 18571341]
- Krupka LR, Vener AM, Richmond G. Tobacco advertising in gender-oriented popular magazines. Journal of Drug Education. 1990;20(1):15–29. [PubMed: 2348303]
- Laaksonen M, Rahkonen O, Prättälä R. Smoking status and relative weight by educational level in Finland, 1978–1995. Preventive Medicine. 1998;27(3):431–7. [PubMed: 9612833]
- Lam TH, Chung SF, Betson CL, Wong CM, Hedley AJ. Respiratory symptoms due to active and passive smoking in junior secondary school students in Hong Kong. International Journal of Epidemiology. 1998;27(1):41–8. [PubMed: 9563692]
- Larsson L. Incidence of asthma in Swedish teenagers: relation to sex and smoking habits. Thorax. 1995;50(3):260–4. [PMC free article: PMC1021189] [PubMed: 7660339]
- Lasser K, Boyd JW, Woolhander S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA: the Journal of the American Medical Association. 2000;284(20):2606–10. [PubMed: 11086367]
- Laucht M, Becker K, Frank J, Schmidt MH, Esser G, Treutlein J, Skowronek MH, Schumann G. Genetic variation in dopamine pathways differentially associated with smoking progression in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(6):673–81. [PubMed: 18434921]
- Leatherdale ST, Wong SL, Manske SR, Colditz GA. Susceptibility to smoking and its association with physical activity, BMI, and weight concerns among youth. Nicotine & Tobacco Research. 2008;10(3):499–505. [PubMed: 18324569]
- Leeson CPM, Kattenhorn M, Morley R, Lucas A, Deanfield JE. Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life. Circulation. 2001;103(9):1264–8. [PubMed: 11238271]
- Leeson CPM, Whincup PH, Cook DG, Donald AE, Papacosta O, Lucas A, Deanfield JE. Flow-mediated dilation in 9- to 11-year-old children: the influence of intrauterine and childhood factors. Circulation. 1997;96(7):2233–8. [PubMed: 9337195]
- Lessov CN, Swan GE, Ring HZ, Khroyan TV, Lerman C. Genetics and drug use as a complex phenotype. Substance Use & Misuse. 2004;39(10–12):1515–69. [PubMed: 15587945]
- Lessov-Schlaggar CN, Hops H, Brigham J, Hudmon KS, Andrews JA, Tildesley E, McBride D, Jack LM, Javitz HS, Swan GE. Adolescent smoking trajectories and nicotine dependence. Nicotine & Tobacco Research. 2008;10(2):341–51. [PubMed: 18236299]
- Leung R, Wong G, Lau J, Ho A, Chan JKW, Choy D, Douglass C, Lai CKW. Prevalence of asthma and allergy in Hong Kong schoolchildren: an ISAAC study. European Respiratory Journal. 1997;10(2):354–60. [PubMed: 9042632]
- Levent E, Ozyürek AR, Ülger Z. Evaluation of aortic stiffness in tobacco-smoking adolescents. Journal of Adolescent Health. 2004;34(4):339–43. [PubMed: 15041004]
- Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, Rose JE, March J. Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology. 1996;123(1):55–63. [PubMed: 8741955]
- Levine A, Huang Y, Drisaldi B, Griffin EA Jr, Pollak DD, Xu S, Yin D, Schaffran C, Kandel DB, Kandel ER. Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Science Translational Medicine. 2011;3(107):107–109. [PMC free article: PMC4042673] [PubMed: 22049069]
- Lewinsohn P, Rohde P, Brown RA. Level of current and past adolescent cigarette smoking as predictors of future substance use disorders in young adulthood. Addiction. 1999;94(6):913–21. [PubMed: 10665079]
- Lewis S, Butland B, Strachan D, Bynner J, Richards D, Butler N, Britton J. Study of the aetiology of wheezing illness at age 16 in two national British birth cohorts. Thorax. 1996;51(7):670–6. [PMC free article: PMC472487] [PubMed: 8882071]
- Li C, Fielding R, Marcoolyn G, Wong CM, Hedley A. Smoking behavior among female cabin crew from ten Asian countries. Tobacco Control. 1994;3(1):21–9.
- Li H, Srinivasan SR, Chen W, Xu J-H, Li S, Berenson GS. Vascular abnormalities in asymptomatic, healthy young adult smokers without other major cardiovascular risk factors: the Bogalusa Heart Study. American Journal of Hypertension. 2005;18(3):319–24. [PubMed: 15797647]
- Li MD, Ma JZ, Payne TJ, Lou X-Y, Zhang D, Dupont RT, Elston RC. Genome-wide linkage scan for nicotine dependence in European Americans and its converging results with African Americans in the Mid-South Tobacco Family sample. Molecular Psychiatry. 2008;13(4):407–16. [PubMed: 17579606]
- Lissner L, Bengtsson C, Lapidus L, Björkelund C. Smoking initiation and cessation in relation to body fat distribution based on data from a study of Swedish women. American Journal of Public Health. 1992;82(2):273–5. [PMC free article: PMC1694294] [PubMed: 1739163]
- Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, Berrettini W, Knouff CW, Yuan X, Waeber G, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nature Genetics. 2010;42(5):436–40. [PMC free article: PMC3612983] [PubMed: 20418889]
- Liu P, Vikis HG, Wang D, Lu Y, Wang Y, Schwartz AG, Pinney SM, Yang P, de Andrade M, Petersen GM, et al. Familial aggregation of common sequence variants on 15q24–25.1 in lung cancer. Journal of the National Cancer Institute. 2008;100(18):1326–30. [PMC free article: PMC2538550] [PubMed: 18780872]
- Loken B. Heavy smokers’, light smokers’, and nonsmokers’ beliefs about cigarette smoking. Journal of Applied Psychology. 1982;67(5):616–22. [PubMed: 7142102]
- Loria CM, Liu K, Lewis CE, Hulley SB, Sidney S, Schreiner PJ, Williams OD, Bild DE, Detrano R. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA Study. Journal of the American College of Cardiology. 2007;49(20):2013–20. [PubMed: 17512357]
- MacPherson L, Strong DR, Myers MG. Using an item response model to examine the nicotine dependence construct characterized by the HONC and the mFTQ among adolescent smokers. Addictive Behaviors. 2008;33(7):880–94. [PMC free article: PMC5858876] [PubMed: 18384973]
- Majahalme S, Turjanmaa V, Weder A, Lu H, Tuomisto M, Virjo A, Uusitalo A. Blood pressure levels and variability, smoking, and left ventricular structure in nor-motension and in borderline and mild hypertension. American Journal of Hypertension. 1996;9(11):1110–8. [PubMed: 8931837]
- Malaiyandi V, Sellers EM, Tyndale RF. Implications of CYP2A6 genetic variation for smoking behaviors and nicotine dependence. Clinical Pharmacology and Therapeutics. 2005;77(3):145–58. [PubMed: 15735609]
- Malinauskas BM, Raedeke TD, Aeby VG, Smith JL, Dallas MB. Dieting practices, weight perceptions, and body composition: a comparison of normal weight, overweight, and obese college females. Nutrition Journal. 2006;5:11. [PMC free article: PMC1456978] [PubMed: 16579846]
- Mallol J, Castro-Rodriguez JA, Cortez E. Effects of active tobacco smoking on the prevalence of asthma-like symptoms in adolescents. International Journal of Chronic Obstructive Pulmonary Disease. 2007;2(1):65–9. [PMC free article: PMC2692110] [PubMed: 18044067]
- Manning P, Goodman P, Kinsella T, Lawlor M, Kirby B, Clancy L. Bronchitis symptoms in young teenagers who actively or passively smoke cigarettes. Irish Medical Journal. 2002;95(7):202–4. [PubMed: 12227526]
- Marti B, Tuomilehto J, Korhonen HJ, Kartovaara L, Vartiainen E, Pietinen P, Puska P. Smoking and leanness: evidence for change in Finland. BMJ (British Medical Journal). 1989;298(6683):1287–90. [PMC free article: PMC1836514] [PubMed: 2500198]
- Mayhew KP, Flay BR, Mott JA. Stages in the development of adolescent smoking. Drug and Alcohol Dependence. 2000;59(Suppl 1):S61–S81. [PubMed: 10773438]
- McGill HC Jr, McMahan CA, Gidding SS. Preventing heart disease in the 21st century: implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Study. Circulation. 2008;117(9):1216–27. [PubMed: 18316498]
- McGill HC Jr, McMahan CA, Herderick EE, Tracy RE, Malcom GT, Zieske AW, Strong JP. PDAY Research Group. Effects of coronary heart disease risk factors on atherosclerosis of selected regions of the aorta and right coronary artery. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(3):836–45. [PubMed: 10712411]
- McKee SA, Nhean S, Hinson RE, Mase T. Smoking for weight control: effect of priming for body image in female restrained eaters. Addictive Behaviors. 2006;31(12):2319–23. [PubMed: 16567057]
- McMahan CA, Gidding SS, Fayad ZA, Zieske AW, Malcom GT, Tracy RE, Strong JP, McGill HC Jr. Pathobiological Determinants of Atherosclerosis in Youth Research Group. Risk scores predict atherosclerotic lesions in young people. Archives of Internal Medicine. 2005;165(8):883–90. [PubMed: 15851639]
- McMahan CA, Gidding SS, Malcom GT, Tracy RE, Strong JP, McGill HC. Pathobiological Determinants of Atherosclerosis in Youth Research Group. Pathobiological determinants of atherosclerosis in youth risk scores are associated with early and advanced atherosclerosis. Pediatrics. 2006;118(4):1447–55. [PubMed: 17015535]
- McNeill AD, West RJ, Jarvis M, Jackson P, Bryant A. Cigarette withdrawal symptoms in adolescent smokers. Psychopharmacology (Berlin). 1986;90(4):533–6. [PubMed: 3101108]
- McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicology and Teratology. 2007;29(1):66–73. [PMC free article: PMC1847361] [PubMed: 17174067]
- Meyers AW, Klesges RC, Winders SE, Ward KD, Peterson BA, Eck LH. Are weight concerns predictive of smoking cessation: a prospective analysis. Journal of Consulting and Clinical Psychology. 1997;65(3):448–52. [PubMed: 9170768]
- Molarius A, Seidell JC, Kuulasmaa K, Dobson AJ, Sans S. Smoking and relative body weight: an international perspective from the WHO MONICA Project. Journal of Epidemiology and Community Health. 1997;51(3):252–60. [PMC free article: PMC1060469] [PubMed: 9229053]
- Moolchan ET, Robinson ML, Ernst M, Cadet JL, Pickworth WB, Heishman SJ, Schroeder JR. Safety and efficacy of the nicotine patch and gum for the treatment of adolescent tobacco addiction. Pediatrics. 2005;115(4):e407–e414. [PubMed: 15805342]
- Moskowitz WB, Mosteller M, Schieken RM, Bossano R, Hewitt JK, Bodurtha JN, Segrest JP. Lipoprotein and oxygen transport alterations in passive smoking pre-adolescent children. The MCV Twin Study. Circulation. 1990;81(2):586–92. [PubMed: 2297864]
- Moskowitz WB, Schwartz PF, Schieken RM. Childhood passive smoking, race, and coronary artery disease risk: the MCV Twin Study. Archives of Pediatrics & Adolescent Medicine. 1999;153(5):446–53. [PubMed: 10323623]
- Napoli C, Lerman LO, de Nigris F, Gossl M, Balestrieri ML, Lerman A. Rethinking primary prevention of atherosclerosis-related diseases. Circulation. 2006;114(23):2517–27. [PubMed: 17146003]
- National Cancer Institute. Changes in Cigarette-Related Disease Risks and Their Implications for Prevention and Control. Bethesda (MD): U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 1997. Smoking and Tobacco Control Monograph No. 8. NIH Publication No. 97-4213.
- National Cancer Institute. Phenotypes and Endophenotypes: Foundations for Genetic Studies of Nicotine Use and Dependence. Bethesda (MD): U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2009. Tobacco Control Monograph No. 20. NIH Publication No. 09-6366.
- National Cancer Institute. Smokeless tobacco. 2012. [accessed: February 5, 2012]. < http://www
.cancer.gov /cancertopics/tobacco/smokeless-tobacco>. - Nelson CB, Wittchen H-U. Smoking and nicotine dependence: results from a sample of 14- to 24-year-olds in Germany. European Addiction Research. 1998;4(1–2):42–9. [PubMed: 9740816]
- Neufeld EJ, Mietus-Snyder M, Beiser AS, Baker AL, New-burger JW. Passive cigarette smoking and reduced HDL cholesterol levels in children with high-risk lipid profiles. Circulation. 1997;96(5):1403–7. [PubMed: 9315524]
- Neumark-Sztainer D, Croll J, Story M, Hannan PJ, French SA, Perry C. Ethnic/racial differences in weight-related concerns and behaviors among adolescent girls and boys: findings from Project EAT. Journal of Psychosomatic Research. 2002;53(5):963–74. [PubMed: 12445586]
- Newnham JP, Ross MG, editors. Early Life Origins of Human Health and Disease. New York: Karger; 2009.
- Nichter M, Nichter M, Vuckovic N, Tesler L, Adrian S, Ritenbaugh C. Smoking as a weight-control strategy among adolescent girls and young women: a reconsideration. Medical Anthropology Quarterly. 2004;18(3):305–24. [PubMed: 15484966]
- Nicklas BJ, Tomoyasu N, Muir J, Goldberg AP. Effects of cigarette smoking and its cessation on body weight and plasma leptin levels. Metabolism. 1999;48(6):804–8. [PubMed: 10381158]
- Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. New England Journal of Medicine. 1974;291(15):755–8. [PubMed: 4414996]
- Noakes PS, Thomas R, Lane C, Mori TA, Barden AE, Devadason SG, Prescott SL. Association of maternal smoking with increased infant oxidative stress at 3 months of age. Thorax. 2007;62(8):714–7. [PMC free article: PMC2117280] [PubMed: 17356057]
- Nuyt AM. Mechanisms underlying developmental programming of elevated blood pressure and vascular dysfunction: evidence from human studies and experimental animal models. Clinical Science (London, England). 2008;114(1):1–17. [PubMed: 18047465]
- O’Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Annals of the New York Academy of Sciences. 2004;1021:167–74. [PubMed: 15251887]
- O’Hara P, Connett JE, Lee WW, Nides M, Murray R, Wise R. Early and late weight gain following smoking cessation in the Lung Health Study. American Journal of Epidemiology. 1998;148(9):821–30. [PubMed: 9801011]
- O’Loughlin J, DiFranza J, Tyndale RF, Meshefedjian G, McMillan-Davey E, Clarke PBS, Hanley J, Paradis G. Nicotine-dependence symptoms are associated with smoking frequency in adolescents. American Journal of Preventive Medicine. 2003;25(3):219–25. [PubMed: 14507528]
- O’Loughlin J, Karp I, Henderson M, Gray-Donald K. Does cigarette use influence adiposity or height in adolescence? Annals of Epidemiology. 2008;18(5):395–402. [PubMed: 18346909]
- Pallonen UE, Prochaska JO, Velicer WF, Prokhorov AV, Smith NF. Stages of acquisition and cessation for adolescent smoking: an empirical integration. Addictive Behaviors. 1998;23(3):303–24. [PubMed: 9668929]
- Park NH, Kim JS, Lee YM. Factors associated with the stage of change of smoking cessation behavior in adolescents. Taehan Kanho Hakhoe Chi. 2003;33(8):1101–10. [PubMed: 15314356]
- Patton GC, Carlin JB, Coffey C, Wolfe R, Hibbert M, Bowes G. Depression, anxiety, and smoking initiation: a prospective study over 3 years. American Journal of Public Health. 1998;88(10):1518–22. [PMC free article: PMC1508459] [PubMed: 9772855]
- Patton GC, Carlin JB, Shao Q, Hibbert ME, Rosier M, Selzer R, Bowes G. Adolescent dieting: healthy weight control or borderline eating disorder? Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1997;38(3):299–306. [PubMed: 9232476]
- Pauly JR, Slotkin TA. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Paediatrica. 2008;97(10):1331–7. [PubMed: 18554275]
- Payne JR, Eleftheriou KI, James LE, Hawe E, Mann J, Stronge A, Kotwinski P, World M, Humphries SE, Pennell DJ, et al. Left ventricular growth response to exercise and cigarette smoking: data from LARGE Heart. Heart. 2006;92(12):1784–8. [PMC free article: PMC1861277] [PubMed: 16803937]
- Payne JR, James LE, Eleftheriou KI, Hawe E, Mann J, Stronge A, Banham K, World M, Humphries SE, Pennell DJ, et al. The association of left ventricular mass with blood pressure, cigarette smoking and alcohol consumption; data from the LARGE Heart Study. International Journal of Cardiology. 2007;120(1):52–8. [PubMed: 17079035]
- Pederson LL, Lefcoe NM. Cross-sectional analysis of variables related to cigarette smoking in late adolescence. Journal of Drug Education. 1985;15(3):225–40. [PubMed: 3878398]
- Peters JM, Ferris BG Jr. Smoking and morbidity in a college-age group. American Review of Respiratory Disease. 1967;95(5):783–9. [PubMed: 6023512]
- Peters JM, Ferris BG Jr. Smoking, pulmonary function, and respiratory symptoms in a college-age group. American Review of Respiratory Disease. 1967;95(5):774–82. [PubMed: 6023511]
- Peto R. Influence of dose and duration of smoking on lung cancer rates. IARC Scientific Publications. 1986;(74):23–33. [PubMed: 3623669]
- Pierce JP, Choi WS, Gilpin EA, Farkas AJ, Merritt RK. Validation of susceptibility as a predictor of which adolescents take up smoking in the United States. Health Psychology. 1996;15(5):355–61. [PubMed: 8891714]
- Pierce JP, Distefan JM, Kaplan RM, Gilpin EA. The role of curiosity in smoking initiation. Addictive Behaviors. 2005;30(4):685–96. [PubMed: 15833574]
- Pierce JP, Gilpin EA. A historical analysis of tobacco marketing and the uptake of smoking by youth in the United States: 1890–1977. Health Psychology. 1995;14(6):500–8. [PubMed: 8565924]
- Pierce JP, Lee L, Gilpin EA. Smoking initiation by adolescent girls, 1944 through 1988: an association with targeted advertising. JAMA: the Journal of the American Medical Association. 1994;271(8):608–11. [PubMed: 8301793]
- Pillai SG, Ge D, Zhu G, Kong X, Shianna KV, Need AC, Feng S, Hersh CP, Bakke P, Gulsvik A, et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genetics. 2009;5(3):e1000421. [PMC free article: PMC2650282] [PubMed: 19300482] [CrossRef]
- Pisinger C, Jorgensen T. Waist circumference and weight following smoking cessation in a general population: the Inter99 Study. Preventive Medicine. 2007;44(4):290–5. [PubMed: 17222450]
- Pittilo RM. Cigarette smoking and endothelial injury: a review. Advances in Experimental Medicine and Biology. 1990;273:61–78. [PubMed: 2288292]
- Pletcher MJ, Hulley BJ, Houston T, Kiefe CI, Benowitz N, Sidney S. Menthol cigarettes, smoking cessation, atherosclerosis, and pulmonary function: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Archives of Internal Medicine. 2006;166(17):1915–22. [PubMed: 17000950]
- Plummer BA, Velicer WF, Redding CA, Prochaska JO, Rossi JS, Pallonen UE, Meier KS. Stage of change, decisional balance, and temptations for smoking: measurement and validation in a large, school-based population of adolescents. Addictive Behaviors. 2001;26(4):551–71. [PubMed: 11456077]
- Polivy J, Herman CP, Howard KI. Restraint scale: assessment of dieting. In: Hersen M, Bellack AS, editors. Dictionary of Behavioral Assessment Techniques. New York: Pergamon Press; 1988. pp. 377–80. Pergamon General Psychology Series.
- Pomerleau CS, Ehrlich E, Tate JC, Marks JL, Flessland KA, Pomerleau OF. The female weight-control smoker: a profile. Journal of Substance Abuse. 1993;5(4):391–400. [PubMed: 8186673]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. Journal of Substance Abuse. 1995;7(3):373–8. [PubMed: 8749796]
- Poorthuis RB, Goriounova NA, Couey JJ, Mansvelder HD. Nicotinic actions on neuronal networks for cognition: general principles and long-term consequences. Biochemical Pharmacology. 2009;78(7):668–76. [PubMed: 19426718]
- Potter BK, Pederson LL, Chan SS, Aubut JA, Koval JJ. Does a relationship exist between body weight, concerns about weight, and smoking among adolescents: an integration of the literature with an emphasis on gender. Nicotine & Tobacco Research. 2004;6(3):397–425. [PubMed: 15203775]
- Prokhorov AV, Hudmon KS, Cinciripini PM, Marani S. “Withdrawal symptoms” in adolescents: a comparison of former smokers and never-smokers. Nicotine & Tobacco Research. 2005;7(6):909–13. [PubMed: 16298726]
- Prokhorov AV, Hudmon KS, de Moor CA, Kelder SH, Conroy JL, Ordway N. Nicotine dependence, withdrawal symptoms, and adolescents’ readiness to quit smoking. Nicotine & Tobacco Research. 2001;3(2):151–5. [PubMed: 11403729]
- Prokhorov AV, Padgett DI, Wetter DW, Le TT, Kitsman HE. Spit tobacco intervention in dental practice: recommendations for clinicians. Texas Dental Journal. 1998;115(6):59–63. [PubMed: 9667213]
- Quinton AE, Cook C-M, Peek MJ. The relationship between cigarette smoking, endothelial function and intrauterine growth restriction in human pregnancy. BJOG. 2008;115(6):780–4. [PubMed: 18355365]
- Raitakari OT, Adams MR, McCredie RJ, Griffiths KA, Celermajer DS. Arterial endothelial dysfunction related to passive smoking is potentially reversible in healthy young adults. Annals of Internal Medicine. 1999;130(7):578–81. [PubMed: 10189327]
- Raitakari OT, Juonala M, Kähönen M, Taittonen L, Laitinen T, Mäki-Torkko N, Järvisalo MJ, Uhari M, Jokinen E, Rönnemaa T, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA: the Journal of the American Medical Association. 2003;290(17):2277–83. [PubMed: 14600186]
- Ribeiro SN, Jennen-Steinmetz C, Schmidt MH, Becker K. Nicotine and alcohol use in adolescent psychiatric inpatients: associations with diagnoses, psychosocial factors, gender and age. Nordic Journal of Psychiatry. 2008;62(4):315–21. [PubMed: 18615350]
- Riggs NR, Chou CP, Li C, Pentz MA. Adolescent to emerging adulthood smoking trajectories: when do smoking trajectories diverge, and do they predict early adulthood nicotine dependence? Nicotine & Tobacco Research. 2007;9(11):1147–54. [PubMed: 17978988]
- Riggs PD, Mikulich SK, Whitmore EA, Crowley TJ. Relationship of ADHD, depression, and non-tobacco substance use disorders to nicotine dependence in substance-dependent delinquents. Drug and Alcohol Dependence. 1999;54(3):195–205. [PubMed: 10372793]
- Robbins DR, Enright PL, Sherrill DL. Lung function development in young adults: is there a plateau phase? European Respiratory Journal. 1995;8(5):768–72. [PubMed: 7656949]
- Robinson LA, Klesges RC, Zbikowski SM, Glaser R. Predictors of risk for different stages of adolescent smoking in a biracial sample. Journal of Consulting and Clinical Psychology. 1997;65(4):653–62. [PubMed: 9256567]
- Robinson LA, Murray DM, Alfano CM, Zbikowski SM, Blitstein JL, Klesges RC. Ethnic differences in predictors of adolescent smoking onset and escalation: a longitudinal study from 7th to 12th grade. Nicotine & Tobacco Research. 2006;8(2):297–307. [PubMed: 16766422]
- Robinson ML, Berlin I, Moolchan ET. Tobacco smoking trajectory and associated ethnic differences among adolescent smokers seeking cessation treatment. Journal of Adolescent Health. 2004;35(3):217–24. [PubMed: 15313503]
- Rodriguez D, Tercyak KP, Audrain-McGovern J. Effects of inattention and hyperactivity/impulsivity symptoms on development of nicotine dependence from mid adolescence to young adulthood. Journal of Pediatric Psychology. 2008;33(6):563–75. [PubMed: 17956929]
- Rojas NL, Killen JD, Haydel KF, Robinson TN. Nicotine dependence among adolescent smokers. Archives of Pediatrics & Adolescent Medicine. 1998;152(2):151–6. [PubMed: 9491041]
- Roth MD, Arora A, Barsky SH, Kleerup EC, Simmons M, Tashkin DP. Airway inflammation in young marijuana and tobacco smokers. American Journal of Respiratory and Critical Care Medicine. 1998;157(3 Pt 1):928–37. [PubMed: 9517614]
- Rubinstein ML, Thompson PJ, Benowitz NL, Shiffman S, Moscicki AB. Cotinine levels in relation to smoking behavior and addiction in young adolescent smokers. Nicotine & Tobacco Research. 2007;9(1):129–35. [PubMed: 17365743]
- Ryan YM, Gibney MJ, Flynn MA. The pursuit of thinness: a study of Dublin schoolgirls aged 15 y. International Journal of Obesity and Related Metabolic Disorders. 1998;22(5):485–7. [PubMed: 9622347]
- Saarni SE, Silventoinen K, Rissanen A, Sarlio-Lähteenkorva S, Kaprio J. Intentional weight loss and smoking in young adults. International Journal of Obesity. 2004;28(6):796–802. [PubMed: 15024402]
- Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, Cichon S, Giegling I, Han S, Han Y, Keskitalo-Vuokko K, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genetics. 2010;6(8):e1001053. [PMC free article: PMC2916847] [PubMed: 20700436] [CrossRef]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PAF, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Human Molecular Genetics. 2007;16(1):36–49. [PMC free article: PMC2270437] [PubMed: 17135278]
- Saules KK, Pomerleau CS, Snedecor SM, Mehringer AM, Shadle MB, Kurth C, Krahn DD. Relationship of onset of cigarette smoking during college to alcohol use, dieting concerns, and depressed mood: results from the Young Women’s Health Survey. Addictive Behaviors. 2004;29(5):893–9. [PubMed: 15219333]
- Schepis TS, Rao U. Epidemiology and etiology of adolescent smoking. Current Opinion in Pediatrics. 2005;17(5):607–12. [PubMed: 16160535]
- Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ, Lessem JM, McQueen MB, Rhee SH, Ehringer MA. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biological Psychiatry. 2008;63(11):1039–46. [PMC free article: PMC2526976] [PubMed: 18163978]
- Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. New England Journal of Medicine. 2003;349(15):1414–22. [PubMed: 14534334]
- Sherrill DL, Lebowitz MD, Knudson RJ, Burrows B. Smoking and symptom effects on the curves of lung function growth and decline. American Review of Respiratory Disease. 1991;144(1):17–22. [PubMed: 2064125]
- Sherrill DL, Lebowitz MD, Knudson RJ, Burrows B. Continuous longitudinal regression equations for pulmonary function measures. European Respiratory Journal. 1992;5(4):452–62. [PubMed: 1563504]
- Shiffman S, Paty JA, Gwaltney CJ, Dang Q. Immediate antecedents of cigarette smoking: an analysis of unrestricted smoking patterns. Journal of Abnormal Psychology. 2004;113(1):166–71. [PubMed: 14992670]
- Shimokata H, Muller DC, Andres R. Studies in the distribution of body fat III: effects of cigarette smoking. JAMA: the Journal of the American Medical Association. 1989;261(8):1169–73. [PubMed: 2915440]
- Shisslak CM, Renger R, Sharpe T, Crago M, McKnight KM, Gray N, Bryson S, Estes LS, Parnaby OG, Killen J, et al. Development and evaluation of the McKnight Risk Factor Survey for assessing potential risk and protective factors for disordered eating in preadolescent and adolescent girls. International Journal of Eating Disorders. 1999;25(2):195–214. [PubMed: 10065397]
- Shor RE, Williams DC, Canon LK, Latta RM, Shor MB. Beliefs of smokers and never smokers about the motives that underlie tobacco smoking. Addictive Behaviors. 1981;6(4):317–24.
- Sidney S, Sternfeld B, Gidding SS, Jacobs DR Jr, Bild DE, Oberman A, Haskell WL, Crow RS, Gardin JM. Cigarette smoking and submaximal exercise test duration in a biracial population of young adults: the CARDIA Study. Medicine and Science in Sports and Exercise. 1993;25(8):911–6. [PubMed: 8371651]
- Simon A, Chironi G, Levenson J. Comparative performance of subclinical atherosclerosis tests in predicting coronary heart disease in asymptomatic individuals. European Heart Journal. 2007;28(24):2967–71. [PubMed: 17967818]
- Siroux V, Pin I, Oryszczyn MP, Le Moual N, Kauffmann F. Relationships of active smoking to asthma and asthma severity in the EGEA study. European Respiratory Journal. 2000;15(3):470–7. [PubMed: 10759439]
- Smith AE, Cavallo DA, Dahl T, Wu R, George TP, Krishnan-Sarin S. Effects of acute tobacco abstinence in adolescent smokers compared with nonsmokers. Journal of Adolescent Health. 2008;43(1):46–54. [PMC free article: PMC2527725] [PubMed: 18565437]
- Smith AE, Cavallo DA, McFetridge A, Liss T, Krishnan-Sarin S. Preliminary examination of tobacco withdrawal in adolescent smokers during smoking cessation treatment. Nicotine & Tobacco Research. 2008;10(7):1253–9. [PMC free article: PMC3777831] [PubMed: 18629736]
- Smith TA, House RF Jr, Croghan IT, Gauvin TR, Colligan RC, Offord KP, Gomez-Dahl LC, Hurt RD. Nicotine patch therapy in adolescent smokers. Pediatrics. 1996;98(4 Pt 1):659–67. [PubMed: 8885942]
- Sneve M, Jorde R. Cross-sectional study on the relationship between body mass index and smoking, and longitudinal changes in body mass index in relation to change in smoking status: The Tromso Study. Scandinavian Journal of Public Health. 2008;36(4):397–407. [PubMed: 18539694]
- Soldz S, Cui X. Pathways through adolescent smoking: a 7-year longitudinal grouping analysis. Health Psychology. 2002;21(5):495–504. [PubMed: 12211517]
- Sonntag H, Wittchen H-U, Höfler M, Kessler RC, Stein MB. Are social fears and DSM-IV social anxiety disorder associated with smoking and nicotine dependence in adolescents and young adults? European Journal of Psychiatry. 2000;15(1):67–74. [PubMed: 10713804]
- Sotir M, Yeatts K, Shy C. Presence of asthma risk factors and environmental exposures related to upper respiratory infection-triggered wheezing in middle school-age children. Environmental Health Perspectives. 2003;111(4):657–62. [PMC free article: PMC1241460] [PubMed: 12676631]
- Stavropoulos-Kalinoglou A, Metsios GS, Panoulas VF, Douglas KMJ, Nevill AM, Jamurtas AZ, Kita M, Koutedakis Y, Kitas GD. Cigarette smoking associates with body weight and muscle mass of patients with rheumatoid arthritis: a cross-sectional, observational study. Arthritis Research & Therapy. 2008;10:R59. [PMC free article: PMC2483449] [PubMed: 18492239] [CrossRef]
- Stice E, Martinez EE. Cigarette smoking prospectively predicts retarded physical growth among female adolescents. Journal of Adolescent Health. 2005;37(5):363–70. [PubMed: 16227120]
- Stice E, Shaw H. Prospective relations of body image, eating, and affective disturbances to smoking onset in adolescent girls: how Virginia slims. Journal of Consulting and Clinical Psychology. 2003;71(1):129–35. [PubMed: 12602433]
- Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ (British Medical Journal). 1996;312(7040):1195–9. [PMC free article: PMC2350975] [PubMed: 8634562]
- Strong DR, Kahler CW, Colby SM, Griesler PC, Kandel D. Linking measures of adolescent nicotine dependence to a common latent continuum. Drug and Alcohol Dependence. 2009;99(1–3):296–308. [PMC free article: PMC2655729] [PubMed: 18938047]
- Stuhldreher WL, Orchard TJ, Donahue RP, Kuller LH, Gloninger MF, Drash AL. Cholesterol screening in childhood: sixteen-year Beaver County Lipid Study experience. Journal of Pediatrics. 1991;119(4):551–6. [PubMed: 1919885]
- Sturm JJ, Yeatts K, Loomis D. Effects of tobacco smoke exposure on asthma prevalence and medical care use in North Carolina middle school children. American Journal of Public Health. 2004;94(2):308–13. [PMC free article: PMC1448248] [PubMed: 14759947]
- Substance Abuse and Mental Health Services Administration. Results from the 2002 National Survey on Drug Use and Health: National Findings. Rockville (MD): US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2002. NHSDA Series H-22. DHHS Publication No SMA 03-3836.
- Sutherland I, Willner P. Patterns of alcohol, cigarette and illicit drug use in English adolescents. Addiction. 1998;93(8):1199–208. [PubMed: 9813901]
- Suwarna L. Virginia Slims. Philip Morris Collection. 1985. Bates No. 2026305099. < http://legacy
.library .ucsf.edu/tid/pov56b00>. - Swan GE, Hudmon KS, Jack LM, Hemberger K, Carmelli D, Khroyan TV, Ring HZ, Hops H, Andrews JA, Tildesley E, et al. Environmental and genetic determinants of tobacco use: methodology for a multidisciplinary, longitudinal family-based investigation. Cancer Epidemiology, Biomarkers & Prevention. 2003;12(10):994–1005. [PMC free article: PMC2587265] [PubMed: 14578134]
- Swan GE, Lessov-Schlagger CN, Bierut LJ, Shields AE, Bergen AW, Vanyukov M. Phenotypes and Endophenotypes: Foundations for Genetic Studies of Nicotine Use and Dependence. Bethesda (MD): U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2009. Status of genetic studies of nicotine dependence; pp. 19–69. Tobacco Control Monograph No. 20. NIH Publication No. 09-6366.
- Tager IB, Muñoz A, Rosner B, Weiss ST, Carey V, Speizer FE. Effect of cigarette smoking on the pulmonary function of children and adolescents. American Review of Respiratory Disease. 1985;131(5):752–9. [PubMed: 4003920]
- Tager IB, Segal MR, Speizer FE, Weiss ST. The natural history of forced expiratory volumes: effect of cigarette smoking and respiratory symptoms. American Review of Respiratory Disease. 1988;138(4):837–49. [PubMed: 3202458]
- Talcott GW, Fiedler ER, Pascale RW, Klesges RC, Peterson AL, Johnson RS. Is weight gain after smoking cessation inevitable? Journal of Consulting and Clinical Psychology. 1995;63(2):313–6. [PubMed: 7751493]
- Thomas GN, Chook P, Yip TWC, Kwong SK, Chan TYK, Qiao M, Huang XS, Guo DS, Feng JZ, Chan SW, et al. Smoking without exception adversely affects vascular structure and function in apparently healthy Chinese: implications in global atherosclerosis prevention. International Journal of Cardiology. 2008;128(2):172–7. [PubMed: 18199502]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, et al. A variant associated with nicotine dependence, lung cancer and peripheral artery disease. Nature. 2008;452(7187):638–42. [PMC free article: PMC4539558] [PubMed: 18385739]
- Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, Sulem P, Rafnar T, Esko T, Walter S, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nature Genetics. 2010;42(5):448–53. [PMC free article: PMC3080600] [PubMed: 20418888]
- Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nature Genetics. 2010;42(5):441–7. [PMC free article: PMC2914600] [PubMed: 20418890]
- Tollefsen E, Langhammer A, Romundstad P, Bjermer L, Johnsen R, Holmen TL. Female gender is associated with higher incidence and more stable respiratory symptoms during adolescence. Respiratory Medicine. 2007;101(5):896–902. [PubMed: 17084607]
- Townsend J, Wilkes H, Haines A, Jarvis M. Adolescent smokers seen in general practice: health, lifestyle, physical measurements, and response to antismoking advice. BMJ (British Medical Journal). 1991;303(6808):947–50. [PMC free article: PMC1671366] [PubMed: 1755876]
- True WR, Xian H, Scherrer JF, Madden PAF, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for nicotine and alcohol dependence in men. Archives of General Psychiatry. 1999;56(7):655–61. [PubMed: 10401514]
- Twisk JW, Staal BJ, Brinkman MN, Kemper HC, van Mechelen W. Tracking of lung function parameters and the longitudinal relationship with lifestyle. European Respiratory Journal. 1998;12(3):627–34. [PubMed: 9762791]
- Tyc VL. Introduction to the special issue: tobacco control strategies for medically at-risk youth. Journal of Pediatric Psychology. 2008;33(2):113–8. [PubMed: 18192299]
- Ulrik CS, Backer V, Dirksen A, Pedersen M, Koch C. Extrinsic and intrinsic asthma from childhood to adult age: a 10-yr follow-up. Respiratory Medicine. 1995;89(8):547–54. [PubMed: 7480988]
- Upadhyaya HP, Deas D, Brady KT, Kruesi M. Cigarette smoking and psychiatric comorbidity in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(11):1294–305. [PubMed: 12410071]
- US Department of Health and Human Services. The Health Consequences of Smoking: Cardiovascular Disease A Report of the Surgeon General. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Office on Smoking and Health; 1983. DHHS Publication No. (PHS) 84-50204.
- US Department of Health and Human Services. The Health Consequences of Smoking: Nicotine Addiction A Report of the Surgeon General. Atlanta (GA): US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1988. DHHS Publication No. (CDC) 88-8406.
- US Department of Health and Human Services. The Health Benefits of Smoking Cessation A Report of the Surgeon General. Atlanta (GA): US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1990. DHHS Publication No. (CDC) 90-8416. [PubMed: 32255575]
- US Department of Health and Human Services. Preventing Tobacco Use Among Young People A Report of the Surgeon General. Atlanta (GA): US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health; 1994.
- US Department of Health and Human Services. Women and Smoking A Report of the Surgeon General. Rockville (MD): U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2001.
- US Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
- US Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [PubMed: 20669524]
- US Department of Health and Human Services. How Tobacco Smoke Causes Disease—The Biology and Behavioral Basis for Tobacco-Attributable Disease: A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. [PubMed: 21452462]
- US Department of Health, Education, and Welfare. Smoking and Health Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington: U.S. Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control; 1964. PHS Publication No. 1103.
- van Strien T, Frijters JER, Bergers GPA, Defares PB. The Dutch Eating Behavior Questionnaire for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders. 1986;5(2):295–315.
- Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Zampi I, Battistelli M, Gattogigio R, Sacchi N, Porcellati C. Cigarette smoking, ambulatory blood pressure and cardiac hypertrophy in essential hypertension. Journal of Hypertension. 1995;13(10):1209–15. [PubMed: 8586813]
- Vidrine JI, Anderson CB, Pollak KI, Wetter DW. Gender differences in adolescent smoking: mediator and moderator effects of self-generated expected smoking outcomes. American Journal of Health Promotion. 2006;20(6):383–7. [PubMed: 16871816]
- Vogelberg C, Hirsch T, Radon K, Dressel H, Windstetter D, Weinmayr G, Weiland SK, von Mutius E, Nowak D, Leupold W. Leisure time activity and new onset of wheezing during adolescence. European Respiratory Journal. 2007;30(4):672–6. [PubMed: 17596269]
- Voorhees CC, Schreiber GB, Schumann BC, Biro F, Craw-ford PB. Early predictors of daily smoking in young women: the National Heart, Lung, and Blood Institute Growth and Health Study. Preventive Medicine. 2002;34(6):616–24. [PubMed: 12052022]
- Wahl SK, Turner LR, Mermelstein RJ, Flay BR. Adolescents’ smoking expectancies: psychometric properties and prediction of behavior change. Nicotine & Tobacco Research. 2005;7(4):613–23. [PubMed: 16085531]
- Wang MQ, Fitzhugh EC, Eddy JM, Westerfield RC. School dropouts’ attitudes and beliefs about smoking. Psychological Reports. 1998;82(3 Pt 1):984–6. [PubMed: 9676508]
- Wang MQ, Fitzhugh EC, Eddy JM, Westerfield RC. School dropouts’ attitudes and beliefs about smoking. Psychological Reports. 1998;82(3 Pt 1):984–6. [PubMed: 9676508]
- Wang X, Mensinga TT, Schouten JP, Rijcken B, Weiss ST. Determinants of maximally attained level of pulmonary function. American Journal of Respiratory and Critical Care Medicine. 2004;169(8):941–9. [PubMed: 15072985]
- Wechsler H, Davenport A, Dowdall G, Moeykens B, Castillo S. Health and behavioral consequences of binge drinking in college: a national survey of students at 140 campuses. JAMA: the Journal of the American Medical Association. 1994;272(21):1672–7. [PubMed: 7966895]
- Weekley CK III, Klesges RC, Relyea G. Smoking as a weight-control strategy and its relationship to smoking status. Addictive Behaviors. 1992;17(3):259–71. [PubMed: 1636473]
- Weiser M, Reichenberg A, Grotto I, Yasvitzky R, Rabinowitz J, Lubin G, Nahon D, Knobler HY, Davidson M. Higher rates of cigarette smoking in male adolescents before the onset of schizophrenia: a historical-prospective cohort study. American Journal of Psychiatry. 2004;161(7):1219–23. [PubMed: 15229054]
- Weiss RB, Baker TB, Cannon DS, von Niederhausern A, Dunn DM, Matsunami N, Singh NA, Baird L, Coon H, McMahon WM, et al. A candidate gene approach identifies the CHRNA5-A3-B4 Region as a risk factor for age-dependent nicotine addiction. PLoS Genetics. 2008;4(7):e1000125. [PMC free article: PMC2442220] [PubMed: 18618000] [CrossRef]
- Weitzman ER, Chen YY. The co-occurrence of smoking and drinking among young adults in college: national survey results from the United States. Drug and Alcohol Dependence. 2005;80(3):377–86. [PubMed: 16009507]
- Weitzman M, Cook S, Auinger P, Florin TA, Daniels S, Nguyen M, Winickoff JP. Tobacco smoke exposure is associated with the metabolic syndrome in adolescents. Circulation. 2005;112(6):862–9. [PubMed: 16061737]
- Welch SL, Fairburn CG. Smoking and bulimia nervosa. International Journal of Eating Disorders. 1998;23(4):433–7. [PubMed: 9561434]
- West R, Hargreaves M. Factors associated with smoking in student nurses. Psychology & Health. 1995;10(3):195–204.
- White HR, Pandina RJ, Chen PH. Developmental trajectories of cigarette use from early adolescence into young adulthood. Drug and Alcohol Dependence. 2002;65(2):167–78. [PubMed: 11772478]
- Wilens TE, Biederman J, Adamson JJ, Henin A, Sgambati S, Gignac M, Sawtelle R, Santry A, Monuteaux MC. Further evidence of an association between adolescent bipolar disorder with smoking and substance use disorders: a controlled study. Drug and Alcohol Dependence. 2008;95(3):188–98. [PMC free article: PMC2365461] [PubMed: 18343050]
- Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA: the Journal of the American Medical Association. 2007;298(22):2654–64. [PubMed: 18073361]
- Wiltshire S, Amos A, Haw S, McNeill A. Image, context and transition: smoking in mid-to-late adolescence. Journal of Adolescence. 2005;28(5):603–17. [PubMed: 16203198]
- Withers NJ, Low L, Holgate ST, Clough JB. The natural history of respiratory symptoms in a cohort of adolescents. American Journal of Respiratory and Critical Care Medicine. 1998;158(2):352–7. [PubMed: 9700106]
- Woo KS, Chook P, Leong HC, Huang XS, Celermajer DS. The impact of heavy passive smoking on arterial endothelial function in modernized Chinese. Journal of the American College of Cardiology. 2000;36(4):1228–32. [PubMed: 11028475]
- Worsley A, Worsley AJ, McConnon S, Silva P. The weight control practices of 15 year old New Zealanders. Journal of Paediatrics and Child Health. 1990;26(1):41–5. [PubMed: 2331417]
- Wu L-T, Anthony JC. Tobacco smoking and depressed mood in late childhood and early adolescence. American Journal of Public Health. 1999;89(12):1837–40. [PMC free article: PMC1509011] [PubMed: 10589312]
- Xu X, Weiss ST, Rijcken B, Schouten JP. Smoking, changes in smoking habits, and rate of decline in FEV1: new insight into gender differences. European Respiratory Journal. 1994;7(6):1056–61. [PubMed: 7925873]
- Yang Z, Knight CA, Mamerow MM, Vickers K, Penn A, Postlethwait EM, Ballinger SW. Prenatal environmental tobacco smoke exposure promotes adult atherogenesis and mitochondrial damage in apolipoprotein E−/− mice fed a chow diet. Circulation. 2004;110(24):3715–20. [PubMed: 15569831]
- Yeatts K, Davis KJ, Sotir M, Herget C, Shy C. Who gets diagnosed with asthma: frequent wheeze among adolescents with and without a diagnosis of asthma. Pediatrics. 2003;111(5 Pt 1):1046–54. [PubMed: 12728087]
- Young LE. Imprinting of genes and the Barker hypothesis. Twin Research. 2001;4(5):307–17. [PubMed: 11869481]
- Yufu K, Takahashi N, Hara M, Saikawa T, Yoshimatsu H. Measurements of the brachial-ankle pulse wave velocity and flow-mediated dilatation in young, healthy smokers. Hypertension Research. 2007;30(7):607–12. [PubMed: 17785928]
- Zacny JP. Behavioral aspects of alcohol-tobacco interactions. Recent Developments in Alcoholism. 1990;8:205–19. [PubMed: 2185518]
- Zalata A, Yahia S, El-Bakary A, Elsheikha HM. Increased DNA damage in children caused by passive smoking as assessed by comet assay and oxidative stress. Mutation Research. 2007;629(2):140–7. [PubMed: 17368083]
- Zhu B-Q, Sun Y-P, Sudhir K, Sievers RE, Browne AE, Gao L, Hutchison SJ, Chou TM, Deedwania PC, Chatterjee K, et al. Effects of second-hand smoke and gender on infarct size of young rats exposed in utero and in the neonatal to adolescent period. Journal of the American College of Cardiology. 1997;30(7):1878–85. [PubMed: 9385922]
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, Breslau N, Brown RA, George TP, Williams J, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine & Tobacco Research. 2008;10(12):1691–715. [PubMed: 19023823]
- Zieske AW, McMahan CA, McGill HC Jr, Homma S, Takei H, Malcolm GT, Tracy RE, Strong JP. Smoking is associated with advanced coronary atherosclerosis in youth. Atherosclerosis. 2005;180(1):87–92. [PubMed: 15823279]
- Zieske AW, Takei H, Fallon KB, Strong JP. Smoking and atherosclerosis in youth. Atherosclerosis. 1999;144(2):403–8. [PubMed: 10407501]
- Zimlichman E, Mandel D, Mimouni FB, Shochat T, Grotto I, Kreiss Y. Smoking habits in adolescents with mild to moderate asthma. Pediatric Pulmonology. 2004;38(3):193–7. [PubMed: 15274096]
- Zucker AN, Harrell ZA, Miner-Rubino K, Stewart AJ, Pomerleau CS, Boyd CJ. Smoking in college women: the role of thinness pressures, media exposure, and critical consciousness. Psychology of Women Quarterly. 2001;25(3):233–41.
Footnotes
- 1
Pack-years = the number of years of smoking multiplied by the number of packs of cigarettes smoked per day.
- The Health Consequences of Tobacco Use Among Young People - Preventing Tobacco U...The Health Consequences of Tobacco Use Among Young People - Preventing Tobacco Use Among Youth and Young Adults
- Elder MistreatmentElder Mistreatment
- Voltage-Gated Calcium Channels in Epilepsy - Jasper's Basic Mechanisms of the Ep...Voltage-Gated Calcium Channels in Epilepsy - Jasper's Basic Mechanisms of the Epilepsies
- Herbal MedicineHerbal Medicine
- Patient Assessment - Managing Chronic Pain in Adults With or in Recovery From Su...Patient Assessment - Managing Chronic Pain in Adults With or in Recovery From Substance Use Disorders
Your browsing activity is empty.
Activity recording is turned off.
See more...