NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996.
General Concepts
Viral Activation of Immunity
Immunity to viral infection is caused by a variety of specific and nonspecific mechanisms. The activation of different immune functions and the duration and magnitude of the immune response depend on how the virus interacts with host cells (on whether it is a cytolytic, steady-state, latent, and/or integrated infection) and on how the virus spreads (by local, primary hematogenous, secondary hematogenous, and/or nervous system spread). Therefore, viral antigens may be present in different parts of the body depending on the route of spread and phase of infection. Local infections at surfaces such as the mucosa can elicit local cell-mediated and humoral (IgA) immune responses, but not necessarily systemic immunity. The host has multiple immune defense functions that can eliminate virus and/or viral disease.
Humoral Immunity: Virus and/or virus-infected cells can stimulate B lymphocytes to produce antibody (specific for viral antigens) Antibody neutralization is most effective when virus is present in large fluid spaces (e.g., serum) or on moist surfaces (e.g., the gastrointestinal and respiratory tracts). IgG, IgM, and IgA have all been shown to exert antiviral activity. Antibody can neutralize virus by: 1) blocking virus-host cell interactions or 2) recognizing viral antigens on virus-infected cells which can lead to antibody-dependent cytotoxic cells (ADCC) or complement-mediated lysis. IgG antibodies are responsible for most antiviral activity in serum, while IgA is the most important antibody when viruses infect mucosal surfaces.
Cell-Mediated Immunity: The term cell-mediated immunity refers to (1) the recognition and/or killing of virus and virus-infected cells by leukocytes and (2) the production of different soluble factors (cytokines) by these cells when stimulated by virus or virus-infected cells. Cytotoxic T lymphocytes, natural killer (NK) cells and antiviral macrophages can recognize and kill virus-infected cells. Helper T cells can recognize virus-infected cells and produce a number of important cytokines. Cytokines produced by monocytes (monokines), T cells, and NK cells (lymphokines) play important roles in regulating immune functions and developing antiviral immune functions.
Virus-Induced Immunopathology
Immune-mediated disease may develop in certain virus infections in which viral antigens and uncontrolled immune hypersensitivity to them persist for a long period. Immune-mediated disease can be mediated by both humoral and cell-mediated immune functions. Immune-complex syndrome can be mediated by virus/virus antigen antibody complexes. T cells (cytotoxic and helper) can also mediate immunopathologic injuries via a number of mechanisms. Immunopathology can result from tissue/organ damage via cytotoxic T cells, inflammation induced via cytokines, antibody plus complement, antibody-antigen complexes and/or ADCC.
Roles of Immune Functions during Viral Infections
The early, nonspecific responses (nonspecific inhibition, natural killer cell activity, and interferon) limit virus multiplication during the acute phase of virus infections. The later specific immune (humoral and cell-mediated) responses function to help eliminate virus at the end of the acute phase, and subsequently to maintain specific resistance to reinfection.
Introduction
The general principles of immunology are presented in Chapter 1. The present chapter discusses viral activation of immunity, humoral and cell-mediated immunity, virus-induced immunopathology, and roles of immune functions during viral infections.
Viral Activation of Immunity
The term immunity as used in this chapter covers the mechanisms by which a host may specifically recognize and react to viruses. The nonspecific defenses are considered in Chapter 49. The host immune response may be beneficial, detrimental, or both. An immune response to a virus appears first during the primary infection of a susceptible, nonimmune host (Table 50-1) and increases during reinfection of an immune host. The specific immune responses that are effective against viruses are (1) cell-mediated immunity involving T lymphocytes and cytotoxic effector T lymphocytes, (2) antibody, with and without its interaction with complement and antibody-dependent cell-mediated cytotoxicity (ADCC), (3) natural killer (NK) cells and macrophages, and (4) lymphokines and monokines (Fig. 50-1). Some of these immune functions may interact, often synergistically, with nonimmune defense mechanisms (see Ch. 49).
Table 50-1
Host Effector Functions Important against Primary Viral Infections.

Figure 50-1
The immune system response to a virus. (1) Virus bearing an antigenic epitope. (2) Processing of antigen to fragments. (3) Presentation of antigen (Ag) to T cells (on the infected cell surface) and B cells (free antigenic pieces or viruses). (4 and 5) Regulator (more...)
Viral Antigens
The degree to which viral antigens are exposed to the host immune defenses is governed by the intracellular replication of viruses and by the several possible types of virus-host cell interaction (Fig. 50-2; see also Ch. 1).

Figure 50-2
Virus-host cell interactions. The degree to which viral antigens are exposed to the host immune defenses is governed by the obligate intracellular replication of viruses. This exposure varies according to the virus-host cell interactions shown here; i.e., (more...)
Acute Cytolytic Infection
Acute cytolytic infection, the most common form of virus-host cell interaction (Fig. 50-2a-c), results in destruction of the infected cell. There are three ways in which the immune system can encounter the virus or virus-specific antigens of cytolytic viruses. In some cases, the immune system encounters viral antigen only when cell lysis releases the virions (Fig. 50-2a). Many viruses (e.g., reoviruses and coxsackieviruses), however, also induce virus-specific antigens on the cell surface before cell death occurs and sometimes before viral multiplication is complete (Fig. 50-2b). In the third type of cytolytic infection (Fig. 50-2c), common among enveloped viruses (e.g., herpesviruses, poxviruses, paramyxoviruses), virus-specific antigens are present on the cell surface and the cells release the infectious virions by budding for a short period before cell death. These viruses (e.g., herpesviruses, poxviruses, and paramyxoviruses) sometimes are disseminated by contiguous spread from cell to cell without exposure to extracellular antibody. Cell-mediated immune responses are believed to be important in controlling the local spread of this type of infection.
Persistent Infections
Some viruses produce a chronic (steady-state) infection rather than an acute infection of the host cell: progeny virions are released continuously, with little adverse effect on cellular metabolism. These cells express virus-specific antigens on their surface and produce abundant virus progeny, but are not killed by the infectious process. In some steady-state infections the progeny virus is released by budding through the cell membrane, and virus can spread from cell to cell without being exposed to the extracellular environment. DNA viruses do not produce steady-state infections, but some RNA viruses (paramyxoviruses and retroviruses) do.
Latent Infections
Latent infections result when an infecting virus (e.g., a herpesvirus) is maintained within a cell for a long time (sometimes years) without giving rise to progeny virus or damaging the cell. Cells infected in this way may express virus-specific antigens on their cell surface. Months to years after infection, the virus in these cells can be reactivated, replicate, and cause disease. The mechanisms by which viruses are maintained intracellularly for long periods and then reactivated are only incompletely understood. Many latent infections occur in sequestered areas of the body (such as the nervous system), where recognition of infected cells by the immune system is believed to be difficult. In addition, any cell that harbors a virus but does not express viral antigens is not recognized by the immune system.
Integrated Virus Infection
There is another type of persistent virus-host cell interaction, integrated virus infection, in which all or part of the viral nucleic acid becomes integrated into the genome of the host cell. Progeny virions may never be assembled or released from the host cell. New virus-specific antigens, however, can be detected within the cell or on the cell surface. Infection with retroviruses is a classic example of this mechanism.
In most cases, the immune system is activated because the virus and its antigens appear in the extracellular fluid or on the cell membrane.
Virus Spread
Another important consideration in how viral infections trigger an immune response is the way in which a particular virus spreads in the host. In animal hosts, four types of viral spread are recognized: (1) local spread, in which the infection is confined largely to a mucosal surface or organ (as in infection of the respiratory epithelium by rhinoviruses or of the gastrointestinal epithelium by rotaviruses); (2) primary hematogenous spread, in which the virus is inoculated directly into the bloodstream (e.g., insect-transmitted viruses) and then disseminates to target organs; (3) secondary hematogenous spread, in which the initial virus infection and replication (often relatively asymptomatic) occur on a mucosal surface with subsequent dissemination to target organs via the bloodstream (e.g., common viral exanthems, poliomyelitis, and mumps); and (4) nervous system spread, in which viruses (such as herpesviruses and rabiesviruses) disseminate via the nervous system. Therefore, viral antigens may be present in different parts of the body depending on the route of spread and phase of infection. Different immune mechanisms may operate at the various sites of virus spread and infection .
Virus Location
The location of the virus in the host is important not only for understanding the immune response, but also for developing and administering a vaccine. For example, local infections on surfaces such as the mucosa of the respiratory or gastrointestinal tract may elicit local cell-mediated and humoral (IgA) immune responses, but not necessarily systemic immunity. The reverse is also true: systemic immunity does not always lead to local mucosal immunity. For example, the Salk polio vaccine, which consists of killed virus administered systemically, elicits serum IgG as the major antibody and induces little or no secretory response. As a result, the immunized individual resists systemic infection, but may become a temporary carrier, with virus persisting at the intestinal portal of entry because of the lack of secretory antibody. The orally administered, live Sabin polio vaccine, on the other hand, induces secretory antibody in the intestine and is effective in preventing replication and subsequent mucosal penetration by the virus.
Multiplicity of Immune Defenses
Recent studies have revealed a great complexity of host immune defenses against viral infections. This complexity arises from the many components of the host immune defenses and their interactions with one another. The existence of a variety of defenses is not surprising in view of the diversity of viruses, hosts, routes of infection, body compartments, cells, and mechanisms of virus multiplication and spread. The situation is further complicated by the varying effectiveness of the different host defenses during the different phases of the primary viral infection (implantation, spread to target organs, and subsequent recovery of each of the infected tissues), as well as during resistance to reinfection. Furthermore, the activated host defenses can actually cause disease manifestations. The presence of multiple defenses against each infection helps explain why impairment of one or a few defenses does not entirely abrogate host resistance to viral infections. Several immune and nonimmune host defenses may operate to control viral infections or, at times, add to the disease process.
Many of the immune defenses against viral invasion are fairly well understood, but the relative effectiveness of each requires additional research. In particular, as this chapter attempts to make clear, humoral and cell-mediated immunity are not independent, but interact intimately to influence the duration and magnitude of each type of immune response.
Humoral Immunity: B Lymphocytes
As described in Chapter 1, specific B lymphocytes respond to viral antigen introduced by immunization or infection. Binding of antigen to the cell surface immunoglobulin receptors, followed by interaction of the B cell with macrophages and helper T lymphocytes, causes the B cell to differentiate into clones of antibody-secreting plasma cells, each capable of secreting antigen-specific immunoglobulin of one of five major classes: IgG, IgM, IgA, IgD, and IgE (Fig. 50-1). Antibodies act against viruses primarily by binding to and neutralizing virions and by directing the lysis of infected cells by complement or killer leukocytes.
Antibody-Mediated Reactions
Neutralization of virion infectivity
At least three immunoglobulin classes have been demonstrated to exert antiviral activity: IgG, IgM, and IgA. These antibodies can neutralize the infectivity of virtually all known viruses. Antibody binds to the virus extracellularly, either neutralizing it immediately or blocking its interaction with host cells. Antibody that has bound to virus can block the infection of a cell at one of three steps: (1) attachment of virus to the cell surface, (2) penetration of virus into the cell, and (3) uncoating of virus inside the cell (Fig. 50-3). The mechanism of viral neutralization involves the binding of antibody to virus coat proteins; this usually alters the viral receptor for the target cell. More rarely, bound antibody may also interfere with penetration or uncoating.

Figure 50-3
Mechanisms of virus neutralization by antibody at the cellular level. At the cellular level, antibody can block the following steps associated with a virus infection: (1) virus attachment and adsorption to the cell surface, (2) penetration of the virus into (more...)
The exact mechanism of neutralization is unclear, but it probably involves changes in the steric conformation of the virus surface. These antibody-virus interactions can take place independently of complement.
Antibody also can neutralize virus by causing aggregation (Fig. 50-4), thus preventing adsorption of virus to cells and decreasing the number of infectious particles. Antibody and complement acting together can inactivate certain viruses (in most cases, enveloped viruses). Antibody is most effective against virus in large fluid spaces (e.g., serum) and on moist body surfaces (e.g., the respiratory and gastrointestinal tracts), where the virus is exposed to antibody for a relatively long period before escaping into cells. Consequently, viruses that spread by viremia are effectively eliminated by low levels of circulating antibody. Much higher levels of antibody are needed to prevent the spread of viruses that do not travel in the blood plasma (such as herpesviruses and rabiesviruses), because these viruses spend only a brief period traversing the small extracellular spaces between cells in solid tissue.

Figure 50-4
Extracellular neutralization of virus by antibody. Antibody can reduce the number of infectious particles by linking virions and thereby causing aggregation. Antibody alone or with complement can also inactivate viruses.
Besides binding directly to virus, antibodies may enhance phagocytosis. Three types of antibody interactions with phagocytic cells are seen: direct binding of antibody to the surface of the phagocytic cells (cytophilic antibody), uptake of antigen antibody complexes through the Fc receptor, and uptake of antigen-antibody-complement complexes through the C3b receptor (see Ch. 1). This phagocytosis of virions may result in inactivation of virus (see Ch. 49), and in the activation of the phagocytic cell which can lead to cytokine production.
Antibody effects on virus-infected cells
Antibody also can act on virus-infected cells by recognizing virus-specific antigens on the surface of infected cells (Fig. 50-3). Complement can then cause lysis of these cells. This complement-mediated lysis occurs both by the classic and the alternative complement pathways. Antibody-coated infected cells also can be destroyed by various effector cells via ADCC. Alternatively, however, some antibodies can mask viral antigens on the surface of infected cells, thereby removing or covering antigens on the surfaces of these infected cells.
Physical barriers to antibody
Before antibody can combine with and neutralize the virus, it must reach the site of virus replication. Barriers to the distribution of antibody include the cell membrane, which excludes antibody, and anatomic tissue barriers, which limit the distribution of macromolecules into certain organs such as the central nervous system.
IgG Antibodies
IgG is the most thoroughly studied antibody class and is responsible for most antiviral activity in serum. IgG antibodies reach infected (inflamed) sites by transduction (leakage) from capillaries. IgG is particularly protective in generalized viral infections that have a viremic phase (e.g., measles, polio, and hepatitis), perhaps because virions in serum are exposed to antibody. IgG antibodies are transferred passively from mother to offspring through the placenta and usually provide temporary protection against generalized viral infections during the first 6 to 9 months of life. Antibody is most protective when present before infection or during the spread of virus to target organs.
Production and the roles of antibody classes
After immunization or infection with viruses, various classes of antibody appear sequentially. For example, during primary infection or immunization, most antigens first elicit IgM (early antibody) responses; IgA and IgG responses follow within a few days. Reinfection, in contrast, stimulates production mainly of IgG, although some IgM and IgA are generated. When the primary antigenic stimulation is in the respiratory or gastrointestinal tract, IgA antibody is predominant, accompanied by some IgM. These antibodies are secreted locally at mucosal surfaces and are important in protecting the host against localized surface viral infections such as the common cold, influenza, and enteric viral infections. When viral replication is confined to a mucosal surface, resistance to infection is determined primarily by secretory IgA; serum IgG antibody provides less protection. Viral infections that begin on a mucosal surface and then spread hematogenously (e.g., measles, rubella, and polio) can be prevented at the mucosal stage by local secretory antibody and at the viremic stage by IgG antibodies. If serum IgG only is induced in a host, hematogenous spread can be prevented, but viral replication still may occur on the mucosal surface.
IgE antibodies and immediate hypersensitivity
Recent information suggests that viruses that bind to IgE antibodies may trigger immediate hypersensitivity responses through the release of vasoactive mediators (see Ch. 1). These observations may explain many of the apparent allergic manifestations, such as wheezing and urticaria, that accompany some viral infections.
Complement
Complement enhances the phagocytosis of many viruses. This enhanced phagocytosis is due to coating (opsonization) of virions by complement or by complement bound to antibody. Complement also can neutralize virus by enhancing either antibody-mediated steric changes on the virus or aggregation of the virus via antibody. In addition, complement can directly inactivate antibody-coated, enveloped virions.
Hypogammaglobulinemia
A small minority of patients with impaired B-lymphocyte function (hypogammaglobulinemia limited to impairment of humoral immunity) have a significantly increased frequency of severe poliovirus and enterovirus infections of the nervous system (in addition to more frequent and severe infections with pyogenic bacteria). The risk of central nervous system invasion is related to the duration of viremia, as has been shown in immunosuppressed animals. The course of most viral infections is typically benign in most of these hypogammaglobulinemic patients, indicating that their weak antibody response and other defense mechanisms may be effective. The development of normal specific resistance to reinfection in hypogammaglobulinemic patients may result, in part, from their ability eventually to produce low levels of serum antibody to virus, as well as from the action of their intact cell-mediated immune system.
Cell-Mediated Immunity
Cell-mediated immunity (CMI) was once thought to be mediated solely by T lymphocytes; however, it is now clear that it is mediated by a variety of cell types, cell factors, or both. Virus-infected or virally transformed cells activate strong cell-mediated immune responses (Fig. 50-1). For some viral infections, cell-mediated immune reactions may be more important than antibody in early termination of viral infection and prevention of dissemination within the host. Recent evidence shows that cell mediated immunity functions at the body surfaces, as well as internally. Cell-mediated immune responses to viral infections involve T lymphocytes, ADCC, macrophages, natural killer (NK) cells, lymphokines, and monokines (Figs. 50-5 and 50-6).

Figure 50-5
Lysis of virus-infected cells by cytotoxic effector cells. Cytotoxic effector cells that can destroy virus-infected cells include cytotoxic T cells, natural killer cells, and activated macrophages. Cytotoxic T lymphocytes can recognize and destroy virus-infected (more...)
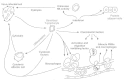
Figure 50-6
Cell-mediated events in viral infections. Soluble mediators include immune interferon, chemotactic factors, macrophage migration inhibitory factor, and lymphotoxin; other lymphokines and monokines are not depicted. Cytotoxic effector lymphocytes, macrophages, (more...)
T Lymphocytes
Much evidence indicates that T lymphocytes are important in recovery from viral infections. Of the many functional subsets of T cells, those that express specific cytotoxic activity against virus-infected or transformed cells have aroused the most interest.
Cytotoxic T lymphocytes
The generation of virus-specific cytotoxic T lymphocytes (CTLs) is believed to be important in preventing viral multiplication (Fig. 50-5). Presumably, the T lymphocytes prevent virus multiplication by destroying infected cells before mature, infectious virus particles can be assembled. This hypothesis assumes that viral antigens appear on the plasma membrane before the release of virus progeny, a view that is substantiated by studies of many, but not all, infections.
Exposure to a virus-infected cell can cause the antigen-specific T lymphocytes to differentiate into cytotoxic effector T cells, which can lyse virus infected or virally transformed cells. These cytotoxic T cells are specific not only for the viral antigen but also for self major histocompatibility antigens and will lyse virus-infected cells only if these cells also express the correct major histocompatibility complex (MHC) gene products.
Activation of cytotoxic and other T lymphocytes may be one of the earliest manifestations of an immune response. T-cell effector functions occur as early as 3 to 4 days after initiation of a viral infection. However, T-cell responses often decrease rapidly, within 5 to 10 days of elimination of the virus (although virus-specific memory T cells persist for long periods). In contrast, antibodies usually become measurable later in the viral infection (after 7 days) and persist at high levels for much longer (often for years).
Helper T cells may be as important as cytotoxic T cells in the immune response to a virus infection. Helper T cells are required for the generation of cytotoxic T cells and for optimal antibody production. In addition, helper T cells, and cytotoxic T cells produce a number of important soluble factors (lymphokines) that can recruit and influence other cellular components of the immune and inflammatory responses.
Animal studies indicate that impairing the T-cell defenses enhances infections by herpes simplex virus, poxviruses, and Sindbis virus and enhances the development of tumors induced by polyomavirus. Since the host retains some resistance to infections, T lymphocytes probably are not the sole defense against these viruses. Impairment of T lymphocytes also hinders T cell-dependent antibody production. In humans, T-cell impairment is associated mainly with more frequent and severe poxvirus and herpesvirus infections. Nevertheless, these infections still do not develop in most individuals with T-cell deficiencies, even though the prevalence of herpesviruses (and many other viruses) is great.
Antibody-Dependent Cell-Mediated Cytotoxicity
Effector leukocytes for ADCC have surface receptors that recognize and bind to the Fc portion of IgG molecules. When IgG binds to virus-specified antigens on the surface of an infected cell, the Fc portion becomes a target for effector cells capable of mediating ADCC . Binding of these effector cells to the Fc portion of IgG bound to the infected-cell surface antigens results in lysis of the infected cell. ADCC is a very efficient way of lysing virus-infected cells because it requires significantly less antibody than does antibody-complement lysis.
Lymphocytes, macrophages, and neutrophils are all capable of mediating ADCC against virus infected cells. The lymphocytes with this ability appear to be heterogeneous. Natural killer cells, as well as null lymphocytes with Fc receptors for IgG, appear to be able to mediate ADCC activity.
Macrophages
Macrophages are important in both specific and nonspecific responses to viral infections (e.g., herpesvirus infections). Factors that modify macrophage activity can influence the outcome of an infection. Moreover, since macrophages are central to the induction of T and B lymphocyte responses, any effect on macrophages will influence B and T cells.
Macrophages confer protection against viruses through either an intrinsic or an extrinsic process. In the former, virions are disposed of within macrophages acting either as phagocytes or as nonpermissive host cells. In the latter case, macrophages retard or ablate virus multiplication in neighboring cells by destroying virus-infected cells or by producing soluble factors (interferons) that act on these cells. Phagocytosis of some viruses by macrophages decreases virus levels in body fluids (as during viremia) and thereby impedes virus spread. These effects are produced only if the virus is destroyed or contained by macrophages. If a virus replicates in macrophages, the infected macrophages may aid in transmission of the virus to other body cells. The permissiveness of macrophages for virus replication may depend on the age and genetic constitution of the host and on the specific condition of the macrophages.
Macrophage activation mediated either by products of infection (viral and cellular) or by soluble factors produced by T cells (e.g., gamma interferon) often enhance phagocytosis and the elimination of free virus particles. Another important effector mechanism of activated macrophages is their ability to recognize and destroy virus-infected and virus-transformed cells (Fig. 50-5). In addition, activated macrophages participate in virus inhibition by producing cytokines (interferon, etc.) and mediating ADCC.
Natural Killer Cells
Natural killer (NK) cells exhibit cytotoxic activity against a number of tumor cell lines, particularly against virus-infected or virus-transformed cells (Fig. 50-5). Natural killer or natural killer-like cells, which have been found in almost every mammalian species examined and even in some invertebrates, are identified as large granular lymphocytes that possess Fc receptors. They can mediate ADCC activity; their nonspecific cytotoxic activity is increased by interferon and interleukin-2 (IL-2); and they can produce a number of different cytokines including interferon when stimulated with virus or virus-infected cells.
Although natural killer cells display cytotoxic activity against virus-infected or transformed cells, they show little or no cytotoxic activity against normal cells. Unlike that of cytotoxic T lymphocytes, natural killer cell killing is not human leukocyte antigen (HLA) restricted, and natural killer cells do not exhibit conventional immunologic specificity. There is evidence that natural killer cells play an important defensive role in virus infections in humans and animals. Their importance is believed to be due to their ability to produce cytokines and to kill virus-infected cells.
Lymphokines and Monokines
Soluble factors from T lymphocytes (lymphokines) and macrophages (monokines) regulate the degree and duration of the immune responses generated by T lymphocytes, B lymphocytes, and macrophages (see Ch. 1). Interleukin-2 and gamma interferon are two such important factors produced by activated T cells. Interleukin-l is a soluble factor produced by macrophages. All three of these factors are essential for the full differentiation and proliferation of cytotoxic T cells. The two interleukins are also important for antibody production by B lymphocytes.
Macrophages and T lymphocytes also produce several other important factors that act in both the immune and the inflammatory responses. Gamma interferon can activate macrophages to become cytotoxic toward virus-infected cells and can increase the level of phagocytosis and degradation. Lymphotoxins produced by T cells also may participate in the destruction of virus-infected cells. Virus can stimulate alpha interferon production from macrophages; this enhances natural killer cell function and inhibits virus multiplication in neighboring cells.
Virus-Induced Immunopathology
A host clearly has numerous mechanisms to recognize and eliminate the viruses that it encounters. However, some viruses persist despite these mechanisms, and then the immune responses may become detrimental to the host and cause immune-mediated disease. When an antigen (virus) persists, pathologic changes and diseases result from different types of immunologic interactions, including immediate hypersensitivity, antibody-mediated immune complex syndrome, and tissue damage caused by cell-mediated effector cells and antibody plus complement. Of these mechanisms, the immune complex syndrome during viral infections has been studied most intensively. Two major complications of deposition of immune complexes are vascular damage and nephritis. Some viral diseases in which immune complexes have been demonstrated are hepatitis B, infectious mononucleosis, dengue hemorrhagic fever, and subacute sclerosing panencephalitis.
Cytotoxic T cells also mediate immunopathologic injury in murine models of human infections (i.e., infections with lymphocytic choriomeningitis virus and poxviruses). Both cytotoxic T cells and T cells responsible for delayed-type hypersensitivity have also been implicated in the pathology associated with influenza pneumonia and coxsackievirus myocarditis of mice. A delicate balance between the removal of infected cells that are the source of viral progeny and injury to vital cells probably exists for T cells as well as for the other host immune components.
Viruses may sometimes circumvent host defenses. An important factor that may impair the function of sensitized T lymphocytes is apparent from the observation that T cells activated by reaction with antigen or mitogen lose their normal resistance to many viruses. Therefore, these activated T lymphocytes develop the capacity to support the replication of viruses, leading to impairment of T lymphocyte function.
Roles of Immune functions During Viral Infections
On the basis of the mechanisms described here and in Chapter 49, a hypothetical model can be constructed that shows how the immune components defend against viruses (Fig. 50-1; Table 50-1).
Nonspecific Defenses
A primary infection in a nonimmune, susceptible host is countered first by the nonspecific defense mechanisms (see Ch. 49 ). The early nonspecific responses occur within hours and consist of interferon production, inflammation, fever, phagocytosis, and natural killer cell activity. These defenses may prevent or abort infection; if they do not, the virus is disseminated by local spread, viremia, or nerve spread. It then may seed to a number of target organs and thereby produce a generalized infection.
Specific Defenses Antibody
The events that lead to a specific immune response begin almost immediately after exposure and result in the production of antiviral antibody and cell-mediated immunity in 3 to 10 days. The disseminated antibody response in serum is predominantly IgG (preceded by IgM); the local antibody response in secretions is predominantly secretory IgA (with some IgM). The persistence of IgA antibodies in secretions is much shorter (months) than the persistence of IgG antibody in serum (years). The role of IgE in secretions is unknown, but it may mediate immediate hypersensitivity and amplify the immune response during infection. Antibodies may neutralize virus directly or destroy virus-infected cells via ADCC or complement. Clearly, serum antibody confers protection against generalized infections (e.g., measles, polio, and type A hepatitis), in which virus must spread through the antibody-containing bloodstream; inoculation of small quantities of antibody into susceptible individuals prevents viral disease but may not prevent subclinical infection at mucosal surfaces.
In localized infections of mucosal surfaces, protection does not correlate with the presence of serum antibody, but it does correlate with the presence of local IgA antibody, as has been shown in human studies of viruses restricted to the respiratory tract (e.g., respiratory syncytial virus and influenza virus) or to the gastrointestinal tract (e.g., enteroviruses). Under some conditions in which serum antibody is present but local IgA is absent, hypersensitivity instead of protective immunity may occur (e.g., respiratory syncytial virus infection). Also, serum antibody may not protect against recurrence of latent infections, such as herpes zoster (shingles) and herpes simplex, both because the virus may be shielded by its intracellular location and because cell-mediated immunity may be the more important defense. Antibody may also cause undesirable effects in certain chronic infections. Examples in which small amounts of serum antibody complex with virus and deposit in the kidneys, thereby inducing immune complex disease, are listed in Table 50-1.
Therefore, serum IgM and IgG antibody seem to be effective in preventing infections of a generalized nature; however, in localized surface infections the presence of secretory IgA antibody appears to correlate much better with protection than the presence of circulating IgG antibody. In persistent infections, serum antibody may be responsible for certain long-term sequelae.
Cell-Mediated Immunity
Cell-mediated immunity is essential in recovery from and control of viral infections, especially infections involving oncogenic viruses or viruses that spread directly from cell to contiguous cell. In these situations antibody cannot reach the virus but virally induced antigens on the surface of the infected cell can be recognized by different effector cells (e.g., cytotoxic T cells) (Fig. 50-6).
If the virus reaches target organs, it is more difficult to control. The host defenses that may play important roles in target organs are initially inflammation, fever, and interferon and subsequently cell-mediated immunity.
In some situations, cell-mediated immunity may develop before antibody production begins. For example, cytotoxic effector T cells have been found in bronchial washings 3 to 4 days after initiation of intranasal infection in mice; at this time, antibody cannot yet be detected.
Cell-mediated immune responses can cause tissue damage; the lung lesions produced in influenza may be examples. The lethal effects of lymphocytic choriomeningitis virus in mice are mediated by cytotoxic effector T cells. The rash in many exanthems (such as measles) is thought to represent a cell-mediated attack on virus localized within cells of the dermis and its vasculature.
References
- Baron S, Grossberg SE, Klimpel GR, Brunell PA: Mechanisms of action and pharmacology: the immune and interferon systems. In Galasso G (ed): Antiviral Agents and Viral Diseases of Man. Raven Press, New York, 1984 .
- Biron CA. Cytokines in the generation of immune responses to, and resolution of, virus infection. Currrent Opinion in Immunology. 1994;6:530. [PubMed: 7946039]
- Brenner BG, Grylles C, Wainberg MA. Role of antibody-dependent cellular cytotoxicity and lymphokine-activated killer cells in AIDS and related diseases. J Leukocyte Biology. 1991;50:628. [PubMed: 1940615]
- Doherty PC. Cell-mediated immunity in virus infections of the central nervous system. Ann NY Acad Sci. 1988;540:228. [PMC free article: PMC7168035] [PubMed: 3061336]
- Herberman RB, Ortaldo JR. Natural killer cells: their role in defenses against disease. Science. 1981;214:24. [PubMed: 7025208]
- Hirsch RL, Winkelstein JA, Griffin DE. The role of complement in viral infections. III. Activation of the classical and alternative complement pathways by Sindbis virus. J Immunol. 1980;124:2507. [PubMed: 7365263]
- McChesney MB, Oldstone MBA. Viruses perturb lymphocyte functions: selected principles characterizing virus-induced immunosuppression. Annu Rev Immunol. 1987;5:279. [PubMed: 3036182]
- Mogensen LA. Role of macrophages in natural resistance to virus infections. Microbiol Rev. 1979;43:1. [PMC free article: PMC281459] [PubMed: 379574]
- Ogra PL, Leibovitz EE, Zhao RG. Oral immunization and secretory immunity to viruses. Curr Top Microbiol Immunol. 1989;146:73. [PubMed: 2659276]
- Rouse BT, Norley S, Martin S. Antiviral cytotoxic T lymphocyte induction and vaccination. Rev Infect Dis. 1988;10:16. [PubMed: 2451265]
- Sissons JGP, Oldstone MBA. Antibody-mediated destruction of virus-infected cells. Adv Immunol. 1980;29:209. [PMC free article: PMC7173112] [PubMed: 6251708]
- Review Protozoa: Pathogenesis and Defenses.[Medical Microbiology. 1996]Review Protozoa: Pathogenesis and Defenses.Seed JR. Medical Microbiology. 1996
- Review [Immunopathology of chronic liver diseases].[Verh Dtsch Ges Pathol. 1995]Review [Immunopathology of chronic liver diseases].Meyer zum Büschenfelde KH. Verh Dtsch Ges Pathol. 1995; 79:186-97.
- Innate immune defenses exhibit circadian rhythmicity and differential temporal sensitivity to a bacterial endotoxin in Nile tilapia (Oreochromis niloticus).[Fish Shellfish Immunol. 2016]Innate immune defenses exhibit circadian rhythmicity and differential temporal sensitivity to a bacterial endotoxin in Nile tilapia (Oreochromis niloticus).Lazado CC, Skov PV, Pedersen PB. Fish Shellfish Immunol. 2016 Aug; 55:613-22. Epub 2016 Jun 23.
- Deficiency of the B cell-activating factor receptor results in limited CD169+ macrophage function during viral infection.[J Virol. 2015]Deficiency of the B cell-activating factor receptor results in limited CD169+ macrophage function during viral infection.Xu HC, Huang J, Khairnar V, Duhan V, Pandyra AA, Grusdat M, Shinde P, McIlwain DR, Maney SK, Gommerman J, et al. J Virol. 2015 May; 89(9):4748-59. Epub 2015 Feb 11.
- Assessing Cell-Mediated Immune Responses to HSV in Murine Systems.[Methods Mol Med. 1998]Assessing Cell-Mediated Immune Responses to HSV in Murine Systems.Banks TA, Hariharan MJ, Rouse BT. Methods Mol Med. 1998; 10:327-43.
- Immune Defenses - Medical MicrobiologyImmune Defenses - Medical Microbiology
Your browsing activity is empty.
Activity recording is turned off.
See more...
