NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996.
GENERAL CONCEPTS
Classification
Helminth is a general term for a parasitic worm. The helminths include the Platyhelminthes or flatworms (flukes and tapeworms) and the Nematoda or roundworms.
Characteristics
All helminths are relatively large (> 1 mm long); some are very large (> 1 m long). All have well-developed organ systems and most are active feeders. The body is either flattened and covered with plasma membrane (flatworms) or cylindrical and covered with cuticle (roundworms). Some helminths are hermaphrodites; others have separate sexes.
Epidemiology
Helminths are worldwide in distribution; infection is most common and most serious in poor countries. The distribution of these diseases is determined by climate, hygiene, diet, and exposure to vectors.
Infection
The mode of transmission varies with the type of worm; it may involve ingestion of eggs or larvae, penetration by larvae, bite of vectors, or ingestion of stages in the meat of intermediate hosts. Worms are often long-lived.
Pathogenesis
Many infections are asymptomatic; pathologic manifestations depend on the size, activity, and metabolism of the worms. Immune and inflammatory responses also cause pathology.
Host Defenses
Nonspecific defense mechanisms limit susceptibility. Antibody- and cell-mediated responses are important, as is inflammation. Parasites survive defenses through many evasion strategies.
Introduction
Helminths - worms - are some of the world's commonest parasites (see Ch. 86). They belong to two major groups of animals, the flatworms or Platyhelminthes (flukes and tapeworms) and the roundworms or Nematoda. All are relatively large and some are very large, exceeding one meter in length.
Their bodies have well-developed organ systems, especially reproductive organs, and most helminths are active feeders. The bodies of flatworms are flattened and covered by a plasma membrane, whereas roundworms are cylindrical and covered by a tough cuticle. Flatworms are usually hermaphroditic whereas roundworms have separate sexes; both have an immense reproductive capacity.
The most serious helminth infections are acquired in poor tropical and subtropical areas, but some also occur in the developed world; other, less serious, infections are worldwide in distribution. Exposure to infection is influenced by climate, hygiene, food preferences, and contact with vectors. Many potential infections are eliminated by host defenses; others become established and may persist for prolonged periods, even years. Although infections are often asymptomatic, severe pathology can occur. Because worms are large and often migrate through the body, they can damage the host's tissues directly by their activity or metabolism. Damage also occurs indirectly as a result of host defense mechanisms. Almost all organ systems can be affected.
Host defense can act through nonspecific mechanisms of resistance and through specific immune responses. Antibody-mediated, cellular, and inflammatory mechanisms all contribute to resistance. However, many worms successfully avoid host defenses in a variety of ways, and can survive in the face of otherwise effective host responses.
Infection
Transmission of Infection
Helminths are transmitted to humans in many different ways (Fig. 87-1). The simplest is by accidental ingestion of infective eggs (Ascaris, Echinococcus, Enterobius, Trichuris) or larvae (some hookworms). Other worms have larvae that actively penetrate the skin (hookworms, schistosomes, Strongyloides). In several cases, infection requires an intermediate host vector. In some cases the intermediate vector transmits infective stages when it bites the host to take a blood meal (the arthropod vectors of filarial worms); in other cases, the larvae are contained in the tissues of the intermediate host and are taken in when a human eats that host (Clonorchis in fish, tapeworms in meat and fish, Trichinella in meat). The levels of infection in humans therefore depend on standards of hygiene (as eggs and larvae are often passed in urine or feces), on the climate (which may favor survival of infective stages), on the ways in which food is prepared, and on the degree of exposure to insect vectors.
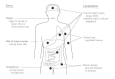
Figure 87-1
Entry and localization of pathogenic helminths.
Host Factors Influencing Susceptibility
Human behavior is a major factor influencing susceptibility to infection. If the infective stages of helminths are present in the environment, then certain ways of behaving, particularly with regard to hygiene and food, will result in greater exposure. Because helminths, with few exceptions (Strongyloides, Trichinella, some tapeworm larvae), do not increase their numbers by replication within the same host, the level of infection is directly related to the number of infective stages encountered. Obviously, not every exposure results in the development of a mature infection. Many infective organisms are killed by the host's nonspecific defense mechanisms. Of those that do become established, many are destroyed or eliminated by specific defenses. The number of worms present at any one time therefore represents a dynamic balance between the rate of infection and the efficiency of defense. This balance (which reflects the host's overall susceptibility) is altered by changes in the host's behavior and ability to express forms of defense. Children are more susceptible to many helminths than are adults, and frequently are the most heavily infected members of a community. The waning of immune competence with age may also result in increased levels of infection. Individuals differ genetically in their ability to resist infection, and it is well known that in infected populations, some individuals are predisposed to heavier infections than others. Changes in diet may affect susceptibility, as do the hormonal-immune changes accompanying pregnancy and lactation. An important cause of increased susceptibility is the immune suppression that accompanies concurrent infections with some other pathogens and the development of certain tumors. Similarly, immunosuppressive therapies (irradiation, immunosuppressant drugs) may enhance susceptibility to helminth infection. A particular hazard in immunocompromised patients is the development of disseminated strongyloidiasis, in which large numbers of larvae develop in the body by autoinfection from relatively small numbers of adult Strongyloides stercoralis. It is interesting that the human immunodeficiency virus does not result in an overall increase in susceptibility to helminth infection.
Parasite Factors Influencing Susceptibility
The ability of hosts to control infection is offset by the ability of parasites to avoid the host's defenses and increase their survival. In addition to their ability to evade specific immune defenses (see below), many worms are unaffected by the host's attempts to limit their activities or to destroy them simply because they are large and mobile. Many important species measure several centimeters in length or diameter (Ascaris, hookworms, hydatid cysts, Trichuris) and others may exceed one meter in length (tapeworms). Size alone renders many defense mechanisms inoperative, as does the tough cuticle of adult roundworms. The ability of worms to move actively through tissues enables them to escape inflammatory foci.
Many of the pathogenic consequences of worm infections are related to the size, movement and longevity of the parasites, as the host is exposed to long-term damage and immune stimulation, as well as to the sheer physical consequences of being inhabited by large foreign bodies.
Pathogenesis
Direct Damage from Worm Activity
The most obvious forms of direct damage are those resulting from the blockage of internal organs or from the effects of pressure exerted by growing parasites (Fig. 87-2). Large Ascaris or tapeworms can physically block the intestine, and this may occur after some forms of chemotherapy; migrating Ascaris may also block the bile duct. Granulomas that form around schistosome eggs may block the flow of blood through the liver, and this may lead to pathological changes in that organ and elsewhere. Blockage of lymph flow, leading to elephantiasis, is associated with the presence of adult Wuchereria in lymphatics. Pressure atrophy is characteristic of larval tapeworm infections (hydatid cyst, the larva of Echinococcus granulosus) where the parasite grows as a large fluid-filled cyst in the liver, brain, lungs, or body cavity. The multilocular hydatid cysts caused by Echinococcus multilocularis have a different growth form, metastasizing within organs and causing necrosis. The larvae of Taenia solium, the pork tapeworm, frequently develop in the central nervous system (CNS) and eyes. Some of the neurological symptoms of the resulting condition, called cysticercosis, are caused by the pressure exerted by the cysts.

Figure 87-2
Pathogenesis: direct damage caused by large helminths.
Intestinal worms cause a variety of pathologic changes in the mucosa, some reflecting physical and chemical damage to the tissues, others resulting from immunopathologic responses. Hookworms (Ancylostoma and Necator) actively suck blood from mucosal capillaries. The anticoagulants secreted by the worms cause the wounds to bleed for prolonged periods, resulting in considerable blood loss. Heavy infections in malnourished hosts are associated with anemia and protein loss. Protein-losing enteropathies may also result from the inflammatory changes induced by other intestinal worms. Diversion of host nutrients by competition from worms is probably unimportant, but interference with normal digestion and absorption may well aggravate undernutrition. The tapeworm Diphyllobothrium latum can cause vitamin B12 deficiency through direct absorption of this factor.
Many helminths undertake extensive migrations through body tissues, which both damage tissues directly and initiate hypersensitivity reactions. The skin, lungs, liver, and intestines are the organs most affected. Petechial hemorrhages, pneumonitis, eosinophilia, urticaria and pruritus, organomegaly, and granulomatous lesions are among the signs and symptoms produced during these migratory phases.
Feeding by worms upon host tissues is an important cause of pathology, particularly when it induces hyperplastic and metaplastic changes in epithelia. For example, liver fluke infections lead to hyperplasia of the bile duct epithelium. Chronic inflammatory changes around parasites (for example, the granulomas around schistosome eggs in the bladder wall) have been linked with neoplasia, but the nature of the link is not known. The continuous release by living worms of excretory-secretory materials, many of which are known to have direct effects upon host cells and tissues, may also contribute to pathology.
Indirect Damage from Host Response
As with all infectious organisms, it is impossible to separate the pathogenic effects caused strictly by mechanical or chemical tissue damage from those caused by the immune response to the parasite. All helminths are “foreign bodies” not only in the sense of being large and invasive but also in the immunologic sense: they are antigenic and therefore stimulate immunity. An excellent illustration of this interrelation between direct and indirect damage is seen in the pathology associated with schistosome infections, especially with Schistosoma mansoni (Fig. 87-3). Hypersensitivity-based, granulomatous responses to eggs trapped in the liver cause a physical obstruction to blood flow, which leads to liver pathology. Hypersensitivity-based inflammatory changes probably also contribute to the lymphatic blockage associated with filarial infections (Brugia, Wuchereria).

Figure 87-3
Pathogenesis: indirect damage caused by immunopathologic responses (for example, in Schistosomiasis).
Immune-mediated inflammatory changes occur in the skin, lungs, liver, intestine, CNS, and eyes as worms migrate through these structures. Systemic changes such as eosinophilia, edema, and joint pain reflect local allergic responses to parasites. The pathologic consequences of immune-mediated inflammation are seen clearly in intestinal infections (especially Strongyloides and Trichinella infections). Structural changes, such as villous atrophy, develop. The permeability of the mucosa changes, fluid accumulates in the gut lumen, and intestinal transit time is reduced. Prolonged changes of this type may lead to a protein-losing enteropathy. The inflammatory changes that accompany the passage of schistosome eggs through the intestinal wall also cause severe intestinal pathology. Heavy infections with the whipworm Trichuris in the large bowel can lead to inflammatory changes, resulting in blood loss and rectal prolapse.
The severity of these indirect changes is a result of the chronic nature of the infection. The fact that many worms are extremely long-lived means that many inflammatory changes become irreversible, producing functional changes in tissues. Three examples are the hyperplasia of bile ducts in long-term liver fluke infections, the extensive fibrosis associated with chronic schistosomiasis, and the skin atrophy associated with onchocerciasis. Severe pathology may also result when worms stray into abnormal body sites.
Defenses Against Infection
Nonspecific Resistance
Infective stages attempting to enter via the mouth or through the skin are opposed by the same non-specific defenses that protect humans from invasion of other pathogens. Following oral ingestion, parasites must survive passage through the acid stomach to reach the small bowel. The natural parasites of humans are adapted to do this, but opportunistic parasites may be killed. Similarly, natural parasites are adapted to the environmental conditions of the bowel (and in many cases require them as cues for development), but accidental parasites may find them inappropriate. Penetration into the intestinal wall may trigger inflammatory responses that immobilize and kill the worm. This may itself lead to serious pathology (as in Anisakis infection). Worms entering through the skin must survive the skin secretions, penetrate the epidermal layers, and avoid inflammatory trapping in the dermis. Invasion of humans by the larvae of dog and cat hookworms (Ancylostoma spp.) results in dermatitis and “creeping eruption” as the worms become the focus of inflammatory reactions that form trails in the skin.
Once in the tissues, worms need the correct sequence of environmental signals to mature. Absent or incomplete signals constitute a form of nonspecific resistance that may partially or completely prevent further development. The parasite may not die, however; indeed, prolonged survival at a larval stage may result in pathology from the continuing inflammatory response (e.g. Toxocara infection).
Specific Acquired Immunity
There is no doubt that specific immunity is responsible for the most effective forms of host defense, although the dividing line between nonspecific and specific mechanisms is difficult to draw with precision (Fig. 87-4). All helminths stimulate strong immune responses, which can easily be detected by measuring specific antibody or cellular immunity. Although these responses are useful for diagnosing infection, they frequently appear not to be protective. The high prevalence of helminth infection in endemic areas (sometimes approaching 100 per cent), and the fact that individuals may remain infected for many years and can easily be reinfected after they are cured by chemotherapy, suggest that protective immunity against helminths is weak or absent in humans. However, some degree of immunity does appear to operate, because the intensity of infection often declines with age, and many individuals in endemic areas remain parasitologically negative and/or clinically normal. Evidence from laboratory studies provides some clues as to the mechanisms involved. Antibodies that bind to surface antigens may focus complement- or cell-mediated effectors that can damage the worm. Macrophages and eosinophils are the prime cytotoxic effector cells, and IgM, IgG and IgE are the important immunoglobulins. Antibodies may also block enzymes released by the worm, thus interfering with its ability to penetrate tissues or to feed. Inflammatory changes may concentrate effector cells around worms, and the release of cellular mediators may then disable and kill the worm. Encapsulation of trapped worms by inflammatory cells may also result in the death of the worm, although this is not always the case. Intestinal worms can be dislodged by the structural and physiologic changes that occur in the intestine during acute inflammation. It has long been suspected that IgE-mediated hypersensitivity reactions, involving mast cells and basophils, contribute to this process, but the evidence is still circumstantial. Despite the abundance of IgA in the intestinal lumen, there is no conclusive evidence that it is involved in protective immunity in humans, although some field and laboratory data suggest it is.
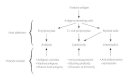
Figure 87-4
Host defense and parasite escape. A schematic diagram of the development and expression of acquired immunity to helminths and of the ways in which parasites escape the immune response.
Avoidance of Host Defenses
Despite their immunogenicity, many helminths survive for extended periods in the bodies of their hosts. Some of the reasons have already been mentioned (size, motility), but we now know that worms employ many sophisticated devices to render host defenses ineffective (Fig. 87-4). Some worms (schistosomes) disguise their outer surface by acquiring host molecules which reduce their antigenicity; intrinsic membrane changes also make these worms resistant to immune attack. Filarial nematodes acquire serum albumin on their cuticle, which may act as a disguise. Many worms release substances that depress lymphocyte function, inactivate macrophages, or digest antibodies. Larval cestodes appear to prolong their survival by producing anticomplement factors which protect their outer layers from lytic attack. Antigenic variation in the strict sense is not known to occur, but many species show a stage-specific change of antigens as they develop, and this phenomenon may delay the development of effective immune mechanisms. All helminths release relatively large amounts of antigenic materials, and this voluminous production may divert immune responses or even locally exhaust immune potential. Irrelevant antibodies produced by the host may block the activity of potentially protective antibodies, as has been shown to be the case in schistosome infections.
It is striking that many helminth infections are associated with a degree of immune suppression, which may affect specific or general responsiveness. Many explanations have been proposed for this immune suppression, including antigen overload, antigenic competition, induction of suppressor cells, and production of lymphocyte-specific suppressor factors. Reduced immune responsiveness may not only prolong the survival of the original infecting worm species but increase the host's susceptibility to other pathogens. Epidemiologic evidence also raises the possibility that infections acquired early in life—before or shortly after birth—may induce a form of immune tolerance, allowing heavy worm burdens to accumulate in the body.
The subtlety with which parasitic worms manipulate the host's immune system not only increases their importance as pathogens but also creates formidable problems for their control and eradication.
References
- Brown HA, Neva FA: Basic Clinical Parasitology. Appleton-Century-Crofts, East Norwalk, CT, 1983
- Crewe W (ed): Blacklock and Southwell's Guide to Human Parasitology. 11th Ed. Chapman and Hall, London, 1990
- Despommier DD, Karapelou JW: Parasite Life Cycles. Springer-Verlag, New York, 1988
- Despommier DD, Gwadz RW, Hotez PJ: Parasitic Diseases. 3rd Ed. Springer-Verlag, New York, 1995
- Manson-Bahr PEC, Bell DR: Manson's Tropical Diseases. 19th Ed. Balliere Tindall, London, 1987
- Muller R, Baker JR: Medical Parasitology. JB Lippincott Company, Philadelphia, 1990
- Peters W, Gilles HM: A Colour Atlas of Tropical Medicine and Parasitology. 3rd Ed. Wolfe Medical Publications, London, 1989
- Review Protozoa: Pathogenesis and Defenses.[Medical Microbiology. 1996]Review Protozoa: Pathogenesis and Defenses.Seed JR. Medical Microbiology. 1996
- Review Granulocytes in helminth infection -- who is calling the shots?[Curr Med Chem. 2012]Review Granulocytes in helminth infection -- who is calling the shots?Makepeace BL, Martin C, Turner JD, Specht S. Curr Med Chem. 2012; 19(10):1567-86.
- Review Helminths: Structure, Classification, Growth, and Development.[Medical Microbiology. 1996]Review Helminths: Structure, Classification, Growth, and Development.Castro GA. Medical Microbiology. 1996
- Review Viral Pathogenesis.[Medical Microbiology. 1996]Review Viral Pathogenesis.Baron S, Fons M, Albrecht T. Medical Microbiology. 1996
- Review Helminths and Endocrinology.[Endotext. 2000]Review Helminths and Endocrinology.Raizada N. Endotext. 2000
- Helminths: Pathogenesis and Defenses - Medical MicrobiologyHelminths: Pathogenesis and Defenses - Medical Microbiology
- Citing MedicineCiting Medicine
- Papers and Poster Sessions Presented at Meetings - Citing MedicinePapers and Poster Sessions Presented at Meetings - Citing Medicine
- Two-Letter Abbreviations for Canadian Provinces and Territories and U.S. States ...Two-Letter Abbreviations for Canadian Provinces and Territories and U.S. States and Territories - Citing Medicine
- Journals in Audiovisual Formats - Citing MedicineJournals in Audiovisual Formats - Citing Medicine
Your browsing activity is empty.
Activity recording is turned off.
See more...
