NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Methenamine is a urinary tract antiseptic that is used as suppressive therapy for chronic or recurrent urinary tract infections. Methenamine has not been linked to serum enzyme elevations or to instances of clinically apparent acute liver injury.
Background
Methenamine (meth” en a mene’) also known as hexamethylenetetramine or urotropin is a heterocyclic molecule used as long term prophylaxis of recurrent urinary tract infections. Methenamine is rapidly absorbed and excreted in the urine where it decomposes at an acidic pH into formaldehyde and ammonia. Formaldehyde is directly bactericidal. Almost all bacteria are sensitive to formaldehyde and resistance to it does not develop. For these reasons, methenamine is particularly helpful in suppressing recurrent or chronic urinary tract infections. The breakdown of methenamine to formaldehyde in the bladder requires an acidic pH, for which reason it is usually given as a mandelic acid or hippurate salt or with agents that acidify the urine such as ammonium or vitamin C. Methenamine was approved for use in the United States in 1976 but is not widely used. The current indications are for prevention of frequently recurrent urinary tract infections. It is not considered effective in treating acute urinary infections. Methenamine is available generically as either a mandelate or hippuric salt in tablets of 1000 mg. The typical dose in adults is 1 gram twice daily (methenamine hippurate) to four times daily (methenamine mandelate). Methenamine can cause dysuria, urinary frequency, and hematuria particularly at high doses. Other uncommon side effects are nausea, anorexia, indigestion, abdominal discomfort and rash. Severe adverse events are rare but may include hypersensitivity reactions. Methenamine is contraindicated in patients with advanced liver disease because of the generation of ammonia.
Hepatotoxicity
In prospective controlled trials, methenamine was generally well tolerated at conventional doses and no episodes of serum aminotransferase elevations or clinically apparent liver injury were reported. Since its approval and in over 100 years of general use, there have been no reports of clinically apparent liver injury attributable to methenamine. Thus, clinically apparent liver injury from methenamine must be rare if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The reason why methenamine rarely causes liver injury is probably due to its lack of hepatic metabolism and rapid unchanged urinary excretion.
Drug Class: Antiinfective Agents, Urinary Tract Infection Agents
Other Drugs in the Subclass: Fosfomycin, Nitrofurantoin, Sulfamethoxazole-Trimethoprim
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Methenamine hippurate – Generic, Hiprex®
Methenamine mandelate – Generic, Mandelamine®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
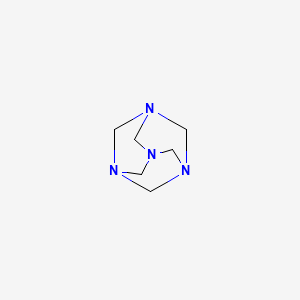
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Methenamine | 100-97-0 | C6-H12-N4 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 22 January 2021
- Zimmerman HJ. Hepatic injury from the treatment of infectious and parasitic diseases. In, Zimmerman HH. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 589-637.(Textbook of hepatotoxicity published in 1999; methenamine is not discussed).
- Moseley RH. Nitrofurantoin. Hepatotoxicity of antimicrobial and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd Edition. Amsterdam: Elsevier, 2013. p. 469-70.(Expert review of antibiotic induced liver injury does not discuss methenamine).
- MacDougall C. Methenamine. Sulfonamides, trimethoprim-sulfamethoxazole, quinolones, and agents for urinary tract infections. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1018.(Textbook of pharmacology and therapeutics mentions that methenamine is a urinary antiseptic that generates formaldehyde in the bladder when urine pH is <6 and is helpful in treating chronic, recurrent urinary tract infections not having the problem of bacterial resistance).
- Methenamine hippurate (Hiprex). Med Lett Drugs Ther. 1968;10(15):58–60. [PubMed: 4875552](Concise review of the mechanism of action, clinical efficacy, and safety of methenamine hippurate shortly after its introduction into clinical practice, mentions that uncontrolled studies have shown that both the hippurate and mandelate methenamine improve symptoms and sterilize urine in patients with recurrent bacterial cystitis and that no serious adverse events have been reported with either).
- Harding GK, Ronald AR. A controlled study of antimicrobial prophylaxis of recurrent urinary infection in women. N Engl J Med. 1974;291:597–601. [PubMed: 4603995](Among 40 women or girls with recurrent urinary tract infections treated in rotating 3 month courses of different antibiotic regimens or placebo, recurrent infections were lowest with trimethoprim-sulfamethoxazole [0.1], intermediate with sulfamethazole alone [1.6] or methenamine and ascorbic acid [1.6] and highest with placebo [3.2 per patient-year], and all regimens “were well tolerated” with no mention of ALT elevations or hepatoxicity).
- Gleckman R, Alvarez S, Joubert DW, Matthews SJ. Drug therapy reviews: methenamine mandelate and methenamine hippurate. Am J Hosp Pharm. 1979;36:1509–12. [PubMed: 391033](Review of the mechanism of action, pharmacology, safety and clinical efficacy of methenamine for recurrent urinary tract infections mentions that its antimicrobial action depends upon conversion to formaldehyde and ammonia in the bladder and depends upon acidic urine of pH less than 6.5, that hippurate and mandelate reduce urinary pH but also had weak antibacterial activity; adverse events are infrequent, mild and reversible and are largely gastrointestinal intolerance and rash, urticaria or pruritus; no mention of ALT elevations or hepatotoxicity).
- Cronberg S, Welin CO, Henriksson L, Hellsten S, Persson KM, Stenberg P. Prevention of recurrent acute cystitis by methenamine hippurate: double blind controlled crossover long term study. Br Med J (Clin Res Ed). 1987;294:1507–8. [PMC free article: PMC1246667] [PubMed: 3111615](Among 21 women with recurrent urinary tract infections treated with methenamine or placebo for 2 years, interchanging placebo and therapy every 6 months, 41 infections occurred during placebo therapy but only 11 while on methenamine [2.1 vs 0.8 per patient year]; no discussion of adverse events).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 [11%] were attributed to drug induced liver injury, of which none were attributed to methenamine).
- Lee BS, Bhuta T, Simpson JM, Craig JC. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10:CD003265. [PMC free article: PMC7144741] [PubMed: 23076896](Systematic review of controlled trials of methenamine hippurate for prevention of urinary tract infections, identified 13 studies with 2037 patients, and identified a suppressive effect of the therapy, and that adverse event rates were low, with uncommon side effects being nausea, diarrhea, bladder pain and rash, but ALT elevations and hepatotoxicity were not mentioned).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to methenamine).
- Lo TS, Hammer KD, Zegarra M, Cho WC. Methenamine: a forgotten drug for preventing recurrent urinary tract infection in a multidrug resistance era. Expert Rev Anti Infect Ther. 2014;12:549–54. [PubMed: 24689705](Review of methenamine which has been used to treat urinary tract infections for more than 100 years and is available in the US as hippurate or mandelate salts but is currently rarely used, although it has the advantage of excellent tolerability and lack of bacterial resistance; mentions that adverse reactions of nausea, dyspepsia and diarrhea occur in less than 4% of patients, but also that increased ALT and AST levels “have been rarely described and seem reversible upon drug discontinuation”).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to methenamine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to methenamine).
- Sihra N, Goodman A, Zakri R, Sahai A, Malde S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat Rev Urol. 2018;15:750–76. [PubMed: 30361493](Extensive review of the evidence for efficacy of non-antibiotic methods for prevention of recurrent urinary tract infections including use of behavioral, dietary, herbal, and probiotic approaches, and d-mannose, NSAIDs, methenamine, estrogens, immunostimulants and vaccines states that methenamine hippurate has been shown to be beneficial and that it “was very well tolerated and the most common adverse events was nausea”).
- Barea BM, Veeratterapillay R, Harding C. Nonantibiotic treatments for urinary cystitis: an update. Curr Opin Urol. 2020;30:845–52. [PubMed: 33009152](Review of the non-antibiotic approaches to urinary tract infections mentions that therapy with methenamine reduces the risk of recurrence of infections and is safe with a low rate of side effects; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Methenamine for Recurrent Urinary Tract Infections in Solid Organ Transplantation.[Prog Transplant. 2022]Methenamine for Recurrent Urinary Tract Infections in Solid Organ Transplantation.Sweiss H, Bhayana S, Hall R, Nelson J, Kincaide E. Prog Transplant. 2022 Mar; 32(1):67-72. Epub 2021 Dec 3.
- Methenamine hippurate compared with antibiotic prophylaxis to prevent recurrent urinary tract infections in women: the ALTAR non-inferiority RCT.[Health Technol Assess. 2022]Methenamine hippurate compared with antibiotic prophylaxis to prevent recurrent urinary tract infections in women: the ALTAR non-inferiority RCT.Harding C, Chadwick T, Homer T, Lecouturier J, Mossop H, Carnell S, King W, Abouhajar A, Vale L, Watson G, et al. Health Technol Assess. 2022 May; 26(23):1-172.
- Review Methenamine: a forgotten drug for preventing recurrent urinary tract infection in a multidrug resistance era.[Expert Rev Anti Infect Ther. 2...]Review Methenamine: a forgotten drug for preventing recurrent urinary tract infection in a multidrug resistance era.Lo TS, Hammer KD, Zegarra M, Cho WC. Expert Rev Anti Infect Ther. 2014 May; 12(5):549-54. Epub 2014 Apr 1.
- Methenamine hippurate to prevent recurrent urinary tract infections in older women: protocol for a randomised, placebo-controlled trial (ImpresU).[BMJ Open. 2022]Methenamine hippurate to prevent recurrent urinary tract infections in older women: protocol for a randomised, placebo-controlled trial (ImpresU).Heltveit-Olsen SR, Sundvall PD, Gunnarsson R, Snaebjörnsson Arnljots E, Kowalczyk A, Godycki-Cwirko M, Platteel TN, Koning HAM, Groen WG, Åhrén C, et al. BMJ Open. 2022 Nov 1; 12(11):e065217. Epub 2022 Nov 1.
- Review Evaluation of methenamine for urinary tract infection prevention in older adults: a review of the evidence.[Ther Adv Drug Saf. 2019]Review Evaluation of methenamine for urinary tract infection prevention in older adults: a review of the evidence.Chwa A, Kavanagh K, Linnebur SA, Fixen DR. Ther Adv Drug Saf. 2019; 10:2042098619876749. Epub 2019 Sep 23.
- Methenamine - LiverToxMethenamine - LiverTox
- Eczema - StatPearlsEczema - StatPearls
- Public Health Consequences of E-CigarettesPublic Health Consequences of E-Cigarettes
- PTEN AND (alive[prop]) (8765)Gene
- Consequences of Gestational Weight Gain for the Mother - Weight Gain During Preg...Consequences of Gestational Weight Gain for the Mother - Weight Gain During Pregnancy
Your browsing activity is empty.
Activity recording is turned off.
See more...