NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Siponimod is an orally available immunomodulatory drug used to treat relapsing forms of multiple sclerosis. Siponimod is associated with transient serum enzyme elevations during therapy but has not been linked to instances of clinically apparent liver injury with jaundice, although experience with its use has been limited.
Background
Siponimod (si pon’ i mod) is an immunomodulatory agent used in the treatment of multiple sclerosis that is believed to act by modulating sphingosine-1-phosphate (S1P) receptors. Siponimod is a derivative of myriocin, a metabolite of the fungus Isaria sinclairii and is a structural analogue of sphingosine. Related in structure to fingolimod, the first S1P receptor inhibitor approved for use in multiple sclerosis, siponimod appears to have greater selectivity of S1P receptors 1 and 5, and thus might have equivalent potency but fewer off-target side effects. Once phosphorylated intracellularly, siponimod acts as a S1P receptor modulator which renders T and B cells insensitive to signals necessary for egress from lymphoid tissue. In animal models of multiple sclerosis, siponimod resulted in reduced recirculation of autoaggressive lymphocytes to the central nervous system. Subsequently, in several large, randomized controlled trials, siponimod was shown to reduce relapse rates and improve neuro-radiologic outcomes in adult patients with relapsing multiple sclerosis. Siponimod was approved for use in the United States in 2019 and is available in tablets of 0.25 and 2 mg under the brand name Mayzent. Like with other S1P receptor modulators, an initial titration period (5 days) is recommended for initiation of therapy to achieve a maintenance dose in adults of 2 mg orally once daily. As with other S1P receptor modulators, common side effects of siponimod are lymphopenia, headache, diarrhea, cough, dyspnea, hypertension, rhinorrhea and back and abdominal pain. Rare, but potentially severe adverse events include severe viral, bacterial or fungal infections, atrial arrhythmias and bradycardia, macular edema, decrease in pulmonary function, progressive multifocal leukoencephalopathy (PML), and embryonal-fetal toxicity.
Hepatotoxicity
In large controlled trials of siponimod in patients with multiple sclerosis, serum ALT elevations were common, typically arising during the first 3 months of treatment. The elevations were generally mild and asymptomatic, and they often returned to baseline values even with continuation of treatment or within 3 months of stopping. Aminotransferase elevations above 3 times upper limit of normal (ULN) were reported in 6% to 8% of siponimod recipients compared to less than 2% of placebo recipients. In these prelicensure clinical trials, there were no cases of acute hepatitis or clinically apparent liver injury but elevations in liver tests led to discontinuation in 1% if subjects. While siponimod is associated with lymphopenia and long term therapy is associated with risk for reactivation of herpes simplex and zoster infections, it has not been linked to cases of reactivation of hepatitis B, although one such instance has been reported with fingolimod. Thus, mild-to-moderate and transient serum enzyme elevations during therapy are not uncommon, but clinically apparent liver injury with jaundice due to siponimod has not been reported, although the clinical experience with its use has been limited.
Likelihood score: E* (suspected but unproven cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which siponimod might cause liver injury is not known. It is extensively metabolized in the liver; an injury might be mediated by intermediates of its metabolism. Siponimod is metabolized via the cytochrome P450 system, predominantly CYP 2C9 and 3A4 and drug-drug interactions with agents that induce or inhibit these enzymes are likely to occur.
Outcome and Management
While chronic therapy with siponimod can be associated with mild-to-moderate serum aminotransferase elevations, it has not been linked to any cases of clinically apparent liver injury. Because of the frequency of enzyme elevations detected during therapy, the product label for siponimod recommends obtaining baseline liver tests before initiation of treatment. However, no specific recommendations for monitoring liver tests during treatment have been made. There is no known cross sensitivity of the hepatic injury from siponimod with other agents used to treat multiple sclerosis. Because of the similarity in chemical structure and mechanism of action, there may be cross sensitivity to side effects with fingolimod, ozanimod or ponesimod.
Drug Class: Multiple Sclerosis Agents
Other Drugs in the Subclass, S1P Receptor Modulators: Fingolimod, Ozanimod, Ponesimod
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Siponimod – Mayzent®
DRUG CLASS
Multiple Sclerosis Agents
Product labeling at DailyMed, National Library of Medicine, NIH
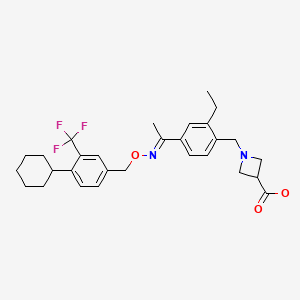
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Siponimod | 1230487-00-9 | C29-H35-F3-N2-O3 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 15 July 2021
Abbreviations: HBV, hepatitis B virus; S1P, sphingosine-1-phosphate.
- Zimmerman HJ. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 697-8.(Expert review of hepatotoxicity published in 1999 before the availability of siponimod).
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Multi-authored textbook of hepatotoxicity published in 2013 does not discuss the drugs for multiple sclerosis).
- Krensky AM, Azzi JR, Hafler DA. Immunotherapy for multiple sclerosis. Immunosuppressants and Tolerogens. In, Brunton LL, Halal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 649-52.(Textbook of pharmacology and therapeutics).
- FDA: Center for Drug Evaluation and Research. 209884Orig1s000. Clinical Review(s). pp: 166-73. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2019/209884Orig1s000MedR.pdf. (FDA website with product labels and medical review of the efficacy and safety of siponimod; mentions that serum aminotransferase elevations are common during siponimod therapy occurring in almost half of patients but are typically mild and resolve rapidly with stopping, and that serious hepatotoxicity was not encountered; ALT elevations above 3 times the ULN occurred in 6-8% of subjects). - Kappos L, Radue EW, O'Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, et al. FREEDOMS Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. [PubMed: 20089952](Among 1272 patients with relapsing multiple sclerosis treated with fingolimod [0.5 or 1.25 mg daily] or placebo for 24 months, 8.5-12.5% of fingolimod, but only 1.7% of placebo recipients developed ALT elevations above 3 times ULN, and ALT levels fell to normal with or without discontinuation, and serum bilirubin levels did not change).
- Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, et al. TRANSFORMS Study Group. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–15. [PubMed: 20089954](Among 1280 patients with relapsing multiple sclerosis treated with fingolimod [0.5 or 1.25 mg daily] or interferon beta [30 mg weekly] for 12 months, relapse rates were lower, but ALT elevations above 3 times ULN were more common with fingolimod [7% and 8%] than interferon beta [2%], although there were no clinically apparent episodes of liver injury).
- Oral fingolimod (gilenya) for multiple sclerosis. Med Lett Drugs Ther. 2010;52(1353-1354):98–9. [PubMed: 21344782](Concise review of mechanism of action, efficacy, safety and costs of fingolimod shortly after is approval for use for multiple sclerosis in the US, mentions that common side effects are headache, cough, diarrhea, back pain and aminotransferase elevations; no mention of clinically apparent liver injury).
- New drugs for relapsing multiple sclerosis. Med Lett Drugs Ther. 2012;54(1403):89–91. [PubMed: 23183318](Concise review of efficacy, safety and costs of new disease modifying drugs for multiple sclerosis lists side effects in a table including "transaminase elevations" for interferon beta, fingolimod and teriflunomide and "hepatotoxicity" for natalizumab, but not for glatiramer or mitoxantrone).
- Oh J, O'Connor PW. Safety, tolerability, and efficacy of oral therapies for relapsing-remitting multiple sclerosis. CNS Drugs. 2013;27:591–609. [PubMed: 23801528](Review of efficacy and safety or oral agents for multiple sclerosis, including fingolimod, teriflunomide, dimethyl fumarate, laquinimod and cladribine, none of which have raised major issues of hepatotoxicity).
- Kappos L, O’Connor P, Radue E-W, Polman C, Hohlfeld R, Selmaj K, Ritter S, et al. Long-term effects of fingolimod in multiple sclerosis: the randomized FREEDOMS extension trial. Neurology. 2015;84:1582–91. [PMC free article: PMC4408283] [PubMed: 25795646](Among 920 patients with multiple sclerosis enrolled in an extension study of fingolimod [0.5 vs 1.25 mg daily], beneficial effects were sustained and no new safety concerns arose, serum enzyme elevations occurring in 13% and 19% of subjects during the first and 7% and 8% during the second year of fingolimod therapy).
- Selmaj K, Li DK, Hartung HP, Hemmer B, Kappos L, Freedman MS, Stüve O, et al. Siponimod for patients with relapsing-remitting multiple sclerosis (BOLD): an adaptive, dose-ranging, randomised, phase 2 study. Lancet Neurol. 2013;12:756–67. [PubMed: 23764350](Among 297 patients with relapsing multiple sclerosis treated with siponimod [0.25, 0.50,1.25, 2 or 10 mg] or placebo once daily for 6 months, there was a dose related increase in clinical responses and higher rates of adverse events including ALT elevations [0% to 6-8%], but levels were rarely above 3 times ULN and there were no hepatic serious adverse events).
- English C, Aloi JJ. New FDA-approved disease-modifying therapies for multiple sclerosis. Clin Ther. 2015;37:691–715. [PubMed: 25846320](Systematic review of efficacy and safety of the newer disease modifying therapies of multiple sclerosis lists ALT elevations as adverse events associated with fingolimod, teriflunomide and dimethyl fumarate, but not peginterferon beta or alemtuzumab).
- Subei AM, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis. CNS Drugs. 2015;29:565–75. [PMC free article: PMC4554772] [PubMed: 26239599](Review of the function of the S1P receptors [subtypes 1 to 5] and the clinical implications of their differential modulation by different inhibitors).
- Kappos L, Li DK, Stüve O, Hartung HP, Freedman MS, Hemmer B, Rieckmann P, et al. Safety and Efficacy of siponimod (BAF312) in patients with relapsing-remitting multiple sclerosis: dose-blinded, randomized extension of the phase 2 BOLD study. JAMA Neurol. 2016;73:1089–98. [PubMed: 27380540](Among 252 patients with relapsing multiple sclerosis continued in an extension of a phase 2 study of siponimod [Selmaj 2013], there was a dose related increase in frequency of ALT elevations [2% to 17%] but there were no hepatic serious adverse events).
- Hammond ER. Perspectives on safety and efficacy-the BOLD phase 2 extension study of siponimod in relapsing-remitting multiple sclerosis. JAMA Neurol. 2016;73:1052–4. [PubMed: 27380179](Editorial in response to the analyses of efficacy and safety of siponimod in an extension study [Kappos 2016]).
- Shakeri-Nejad K, Aslanis V, Veldandi UK, Gardin A, Zaehringer A, Dodman A, Su Z, et al. Pharmacokinetics, safety, and tolerability of siponimod (BAF312) in subjects with different levels of hepatic impairment: a single-dose, open-label, parallel-group study. Int J Clin Pharmacol Ther. 2017;55:41–53. [PubMed: 27443658](Single dose studies of siponimod done in 40 subjects found little difference in pharmacokinetics among patients with or without hepatic impairment).
- Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, Rocca MA. Multiple sclerosis. Nat Rev Dis Primers. 2018;4:43. [PubMed: 30410033](Review of the pathogenesis, clinical features, natural history, management and therapy of multiple sclerosis).
- Kappos L, Bar-Or A, Cree BAC, Fox RJ, Giovannoni G, Gold R, Vermersch P, et al. EXPAND Clinical Investigators. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263–73. [PubMed: 29576505](Among 1645 patients with secondary progressive multiple sclerosis enrolled in a controlled trial for up to 3 years, disability progression was less frequent with siponimod than placebo [26% vs 32%] but liver related “investigations” were more common [12% vs 4%], although none resulted in clinically apparent liver injury with jaundice ).
- Jin Y, Borell H, Gardin A, Ufer M, Huth F, Camenisch G. In vitro studies and in silico predictions of fluconazole and CYP2C9 genetic polymorphism impact on siponimod metabolism and pharmacokinetics. Eur J Clin Pharmacol. 2018;74:455–64. [PMC free article: PMC5849655] [PubMed: 29273968](In vitro metabolic studies using human liver microsomes demonstrated possible functional consequences of CYP2C9*2/*2 and CYP2C9*3/*3 polymorphisms on the metabolism of siponimod which predict significant reductions in clearance and possible drug accumulation to toxic levels).
- Siponimod (Mayzent)--a new drug for multiple sclerosis. Med Lett Drugs Ther. 2019;61(1571):70–2. [PubMed: 31169805](Concise review of the mechanism of action, clinical efficacy, safety and costs of siponimod in comparison to other agents used to treat multiple sclerosis; mentions that serum aminotransferase elevations can occur with siponimod therapy and that patients should be tested for liver function tests and have CYP 2C9 genotype testing before starting treatment).
- Al-Salama ZT. Siponimod: First global approval. Drugs. 2019;79:1009–15. [PubMed: 31144287](Review of the mechanism of action, history of development, clinical efficacy and safety of siponimod; mentions the common side effects include headache, hypertension, aminotransferase elevations, peripheral edema, nausea, dizziness, diarrhea, bradycardia and musculoskeletal pain but that the aminotransferase levels were rarely severely elevated).
- Lu MC, Shih YL, Hsieh TY, Lin JC. Flare of hepatitis B virus after fingolimod treatment for relapsing and remitting multiple sclerosis. J Formos Med Assoc. 2020;119:886–7. [PubMed: 31679907](Letter describing 41 year old Taiwanese woman with relapsing multiple sclerosis and inactive HBsAg carrier state who developed reactivation of hepatitis B after 35 months of treatment with fingolimod [ALT 385 U/L, HBV DNA 8 log10 IU/mL, bilirubin not given], who responded to tenofovir with resolution of ALT elevations and decrease of HBV DNA levels to undetectable despite continuation of fingolimod).
- Drugs for multiple sclerosis. Med Lett Drugs Ther. 2021;63(1620):42–8. [PubMed: 33976089](Concise review of the relative clinical efficacy, safety and costs of drugs for relapsing multiple sclerosis including parenteral agents [such as interferon-beta, glatiramer acetate, natalizumab, alemtuzumab, ocrelizumab, ofatumumab, rituximab and mitoxantrone] and the oral agents [such as the S1P receptor modulators, cladribine, fumarates, and teriflunomide], many of which are associated with serum ALT elevations and several have been reported to cause clinically apparent liver injury or reactivation of hepatitis B).
- McGinley MP, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet 2021 Jun 24: S0140-6736(21)00244-0. Epub ahead of print. [PubMed: 34175020](Review of the function of S1P receptors and the mechanism of action of S1P receptor modulators in affecting lymphocyte tracking out of lymph nodes into the circulation and tissues; fingolimod is a nonspecific modulator affecting S1P receptors 1, 3, 4 and 5, whereas siponimod and ozanimod act predominantly on S1P receptors 1 and 5 and ponesimod against S1P receptor 1 alone, the activity against S1P receptor-1 accounting for most of the beneficial effects in multiple sclerosis and the restricted specificity perhaps accounting for the lower rate of cardiac, lung and eye adverse events driven mostly by the inhibition of the other S1P receptor subtypes).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Ozanimod.[LiverTox: Clinical and Researc...]Review Ozanimod.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Ponesimod.[LiverTox: Clinical and Researc...]Review Ponesimod.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Multiple Sclerosis Agents.[LiverTox: Clinical and Researc...]Review Multiple Sclerosis Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Fingolimod.[LiverTox: Clinical and Researc...]Review Fingolimod.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Glatiramer Acetate.[LiverTox: Clinical and Researc...]Review Glatiramer Acetate.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Siponimod - LiverToxSiponimod - LiverTox
- STK_RS01260 [Sulfurisphaera tokodaii str. 7]STK_RS01260 [Sulfurisphaera tokodaii str. 7]Gene ID:1458116Gene
- cirA [Escherichia coli str. K-12 substr. MG1655]cirA [Escherichia coli str. K-12 substr. MG1655]Gene ID:949042Gene
- UNC13C unc-13 homolog C [Homo sapiens]UNC13C unc-13 homolog C [Homo sapiens]Gene ID:440279Gene
- OR5H14 olfactory receptor family 5 subfamily H member 14 [Homo sapiens]OR5H14 olfactory receptor family 5 subfamily H member 14 [Homo sapiens]Gene ID:403273Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...