Context and Policy Issues
Chronic pain can be defined as ongoing pain lasting longer than expected, usually more than three months.1 It was estimated that 18.9% of Canadians aged 18 years and over were affected by chronic pain in 2011.2 Chronic pain is more prevalent among the elderly than younger populations.2 Half of chronic pain patients have suffered for more than 10 years.2 A third of the patients consider their pain very severe.2 Chronic pain most commonly occurs at the lower back and is due to arthritis.2 Canadian guidelines recommend medications for chronic non-cancer pain,3 but the management strategies vary depending on the sites, durations, and causes of chronic pain.1 Medications recommended for moderate to severe pain include analgesics and opioids.3 However, some medications have been linked to abuse and adverse events.3 For severe cases of chronic pain, a combination of psychological, behavioural, and physical interventions, such as cognitive behavioural therapy, are recommended with or without pharmaceutical interventions.1
Cognitive behavioural therapy programs include cognitive and behavioural strategies to reduce pain and improve quality of life.4 Cognitive approaches include hypnosis, cognitive coping skills, stress management, and guided imagery.4 Behavioural approaches include biofeedback, relaxation training, and behavioural management programmes.4 The elements of these two types of approaches can be adopted for a cognitive behavioural therapy program.4 In Canada, publicly funded cognitive behavioural therapy is rare4 and the effectiveness of cognitive behavioural therapy programs remains unclear.5 This report aims to review the clinical effectiveness of cognitive behavioural therapy for chronic non-cancer pain.
Research Question
What is the clinical effectiveness of cognitive behavioural therapy for chronic non-cancer pain?
Key Findings
There were five systematic reviews included in this report, four of which were Cochrane reviews. Four of the five included systematic reviews had one weakness in the AMSTAR critical domains. The clinical effectiveness of cognitive behavioural therapy was assessed in several populations. Cognitive behavioural therapy was associated with a reduction in pain or pain frequency in children with chronic headache, patients with chronic low back pain, patients with chronic neck pain, and patients with spinal cord injury and chronic pain, compared with wait-list control, no treatment, or standard care. In addition, cognitive behavioural therapy was also associated with a reduction in physical impairment in children with chronic headache, a reduction in disability and an improvement in quality of life in patients with chronic neck pain, and a reduction in kinesiophobia in patients with chronic neck pain. However, a significant pain reduction was not observed in children with chronic headache in smaller trials (fewer than 20 patients per arm) and in patients with spinal cord injury three months after intervention, compared with the control groups. Despite the inclusion of multiple systematic reviews, this report had limitations, such as the limited number of relevant primary studies identified within those systematic reviews, short durations of follow-up, and unclear definitions of chronic pain or cognitive behavioural therapy in certain studies. Further research on cognitive behavioural therapy in Canada may help reduce uncertainty.
Methods
Literature Search Methods
A limited literature search was conducted by an information specialist on key resources including Medline and PsycINFO via Ovid, the Cochrane Library, the University of York Centre for Reviews and Dissemination (CRD) databases, the websites of Canadian and major international health technology agencies, as well as a focused Internet search. The search strategy was comprised of both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. The main search concepts were cognitive behavioural therapy and chronic pain. Search filters were applied to limit retrieval to health technology assessments, systematic reviews, meta-analyses, or network meta-analyses, and randomized controlled trials or controlled clinical trials. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 1, 2014 and August 9, 2019.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , they were duplicate publications, or were published prior to 2014.
Critical Appraisal of Individual Studies
The included systematic reviews were critically appraised by one reviewer using the A Measurement Tool to Assess Systematic Reviews (AMSTAR) 2 checklist,6 and randomized studies were critically appraised using the Downs and Black checklist.7 Summary scores were not calculated for the included studies; rather, a review of the strengths and limitations of each included study was described narratively.
Summary of Evidence
Quantity of Research Available
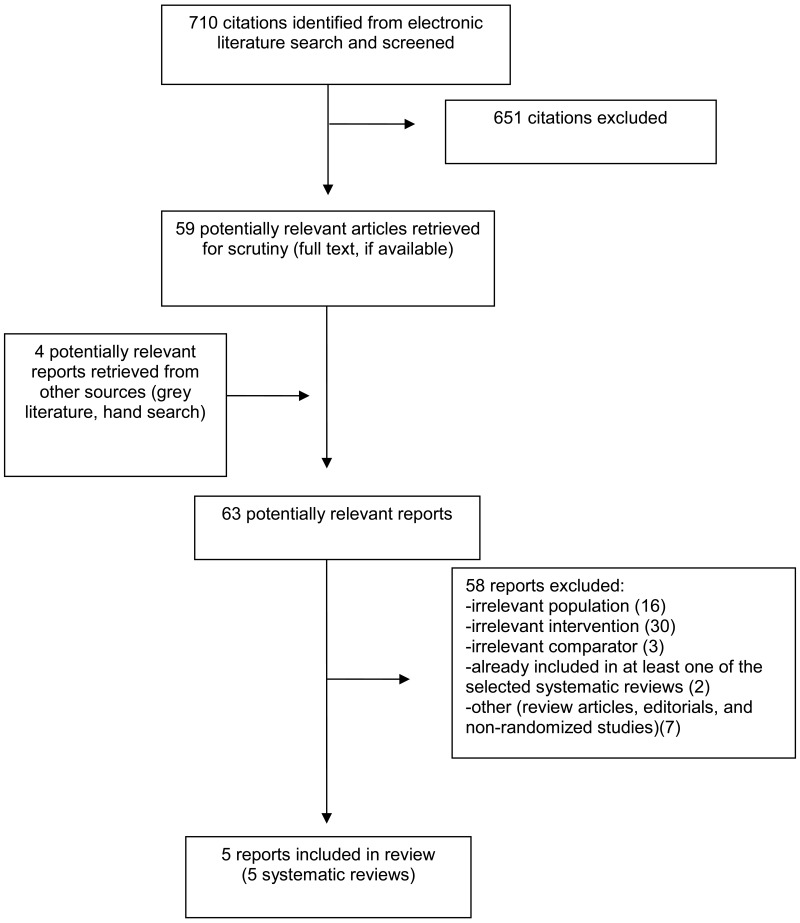
A total of 710 citations were identified in the literature search. Following screening of titles and abstracts, 651 citations were excluded and 59 potentially relevant reports from the electronic search were retrieved for full-text review. Four potentially relevant publications were retrieved from the grey literature search for full text review. Of these potentially relevant articles, 58 publications were excluded for various reasons, and five publications met the inclusion criteria and were included in this report. These comprised five systematic reviews (SRs). Appendix 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)8 flowchart of the study selection.
Additional references of potential interest are provided in Appendix 5.
The descriptions of primary studies included in each of the five identified SRs were assessed for eligibility for this report according to the CADTH PICO elements. The subset of primary studies that reported relevant comparisons to this report is as follows. In the SR by Amatya et al., there were no primary studies that were eligible for this report.9 In the SR by Fisher et al., seven of the 47 included RCTs met the inclusion criteria of this report.4 Chou et al. included 114 publications, one of which was an SR (Henschke 2010; 30 RCTs included, five relevant to this review)10 that was eligible for this report.11 Monticone et al. included 10 RCTs, three of which were eligible for this report.12 One of the 16 RCTs included by Boldt et al. was eligible for this report.13 There was no overlap in the primary studies included in the SRs that met the PICO criteria for this CADTH report.
Summary of Study Characteristics
Study Design
The five identified SRs by Amatya et al., Fisher et al., Chou et al., Monticone et al., and Boldt et al. were Cochrane reviews published in 2019, 2018, 2017, 2015, and 2014 respectively.4,9,11–13 The SR by Chou et al. published in 2017 was sponsored by the Agency for Healthcare Research and Quality11 and was a concise version of the SR by Chou et al. published in 2016.14 The search timeframes were specified in all SRs.4,9,11–13
Country of Origin
The first authors of the SRs by Amatya et al., Fisher et al., Chou et al., Monticone et al., and Boldt et al. were based in Australia, the UK, the US, Italy, and Switzerland respectively.4,9,11–13
Patient Population
Amatya et al. included studies that recruited individuals aged 18 years and over with chronic pain and multiple sclerosis.9 The minimum levels of pain on visual analogue scale was three (maximum of the scale: 10) and pain lasted for more than three months.9 Fisher et al. analyzed data from children or adolescents with chronic or recurrent pain.4 Chronic pain was defined as pain that recurred or persisted for more than three months.4 Chou et al. analyzed data from patients with low back pain undergoing psychological therapies.11 Pain was categorized as acute, subacute, chronic, radical or non-radical.11 One SR (Henschke 2010)10 included in the SR by Chou et al. focused on patients with chronic pain.11 Monticone et al. included studies that enrolled patients with subacute (one to three months) or chronic (longer than 3 months) neck pain.12 Neck pain included pain, muscle tension, or stiffness localized in the neck and might have originated from several sources, such as the spine or soft tissues.12 Boldt et al. included studies that followed up patients with chronic pain and spinal cord injury.13 Chronic pain was defined as persistent pain lasting longer than expected time of healing and presenting continuously or intermittently for at least three months.13
Interventions and Comparators
Amatya et al. aimed to include non-pharmacological interventions and eligible comparators were other non-pharmacological interventions, no treatment, sham, or usual care.9 However, cognitive behavioural therapy was not assessed in the primary studies in the SR.9 Fisher et al. planned to include any psychological interventions, compared with active treatment, usual care, or waitlist control.4 Cognitive behavioural therapy programs were evaluated in seven of the 47 RCTs in this SR and compared with standard medical care (not explained), wait-list control, or patient education.4 Chou et al. compared non-pharmacological options with sham treatment, wait-list control, or usual care.11 One SR of the 114 publications (Henschke 201010) were eligible for this report comparing cognitive behavioural therapy with wait-list control.11 Monticone et al. assessed cognitive behavioural therapy and compared wait-list control and patient education.12 Cognitive behavioural therapy included cognitive reconditioning and behavioural modifications in order to reduce the impact of pain and disability.12 Three of the ten included RCTs met the inclusion criteria of this report.12 Boldt et al. compared non-pharmacological interventions with controls, such as pharmacological or non-pharmacological treatments.13 One of the sixteen included RCTs compared cognitive behavioural therapy with wait-list control and was eligible for this report.13
Outcomes
Amatya et al. assessed pain reduction measured by validated scales, such as a visual analogue scale or Likert scale.9 The duration of follow-up in the RCTs ranged from one to 12 months.9 The primary outcomes in the SR by Fisher et al. were pain intensity and pain-related disability (scales not specified) and the secondary outcomes were depression, anxiety, and adverse events.4 Chou et al. evaluated long- or short-term pain, function, return to work, and harms in the primary studies and the duration of follow-up in primary studies ranged from one to 12 months.11 The primary outcomes evaluated by Monticone et al. were pain measured by a visual analogue or a numeric rating scale and the secondary outcomes included disability, psychological indicators (including anxiety, depression and fear of pain), global improvement, and adverse events.12 The duration of follow-up in the primary studies ranged from two to 12 months.12 The primary outcomes in the SR by Boldt et al. were pain measures, such as numeric rating scales and pain questionnaires.13 The secondary outcomes included anxiety, depression, quality of life, and adverse events.13 The duration of follow-up ranged from three to 12 months.13
Additional details regarding the characteristics of included publications are provided in Appendix 2.
Summary of Critical Appraisal
The description and explanation of the review methods were important to for the readers to understand the relevance and importance of the review objectives. Amatya et al., Fisher et al., Chou et al., Monticone et al., and Boldt et al. described the components of populations, interventions, comparators, and outcomes in the research questions or selection criteria.4,9,11,12 All review groups explained the selection of study designs.4,9,11–13 Only Fisher et al., and Chou et al. published the review protocols before conducting the reviews.4,11,15 The funding sources of the SRs were declared.4,9,11–13 The heterogeneity in the results of the review was discussed in all SRs.4,9,11–13 However, only Amatya et al. and Chou et al. reported the sources of funding for the primary studies.9,11
Systematic literature searches decreased the probability of omitting primary studies. Comprehensive literature searches were conducted in all SRs.4,9,11–13 Independent study selection and data extraction could minimize human errors. However, Fisher et al. did not report study selection in duplicate.4 Fisher et al. and Chou et al. did not extract data from the primary studies in duplicate.4,11
The provision of a list of excluded studies and the description of included studies demonstrated the rigour of study assessment. The excluded studies and the reasons for exclusion were reported in the SRs.4,9,11–13 The populations, interventions, comparators, and outcomes of the included studies were described in all SRs.4,9,11–13
The assessment of the risk of bias in the primary studies helped readers to understand the strengths and limitations of the evidence. The risk of bias in the primary studies was assessed in all SRs with published tools.4,9,11–13 The risk of bias in the primary studies were discussed in all SRs.4,9,11–13
Meta-analyses aggregated the results from multiple studies and the quality of meta-analyses could be assessed. Fisher et al., Monticone et al., and Boldt et al. conducted meta-analyses and used appropriate statistical methods.4,12 Monticone et al. was the only one to assess the potential impact of the risk of bias in the primary studies regarding the results of meta-analysis.12 Fisher et al. assessed the risk of publication bias in the primary studies, and detected a high probability of this bias.4
Additional details regarding the strengths and limitations of included publications are provided in Appendix 3.
Summary of Findings
Clinical Effectiveness of Cognitive Behavioural Therapy
There were no primary studies on cognitive behavioural therapy in the SR by Amatya et al.9
Pain
In the subgroup analysis in the SR by Fisher et al., primary studies of different sample sizes were meta-analyzed separately (more or fewer than 20 patients per arm).4 Based on two studies with more than 20 patients per arm, cognitive behavioural therapy was associated with reduction in headache frequency in children, compared to standard medical care, wait-list control.4 However, cognitive behavioural therapy was not associated with the reduction in headache frequency or depression based on two meta-analyses of the primary studies with fewer than 20 patients per arm.4 The exact reason for the difference was not reported.4 In the SR by Chou et al., different types of non-pharmacologic interventions were meta-analyzed separately and cognitive behavioural therapy was associated with reduction in chronic low back pain compared with wait-list control.11 In the SR by Monticone et al., cognitive behavioural therapy significantly reduced neck pain at short-term follow-up, compared with wait-list control or no treatment.12 In the SR by Boldt et al., there was one RCT eligible for this report among the included primary studies that recruited patients with chronic pain and spinal cord injury.13 Cognitive behavioural therapy was associated with the reduction in the Chronic Pain Grade intensity scores, compared with wait-list control three weeks after intervention.13 However, there was no significant difference in the same pain scores between the two groups at three-month follow-up.13
Psychological indicators including kinesiophobia and distress
In the SR by Monticone et al., cognitive behavioural therapy had no significant effect on psychological indicators, such as kinesiophobia and distress at short-term follow-up, compared with no treatment.12
Disability
In the SR by Monticone et al., cognitive behavioural therapy significantly reduced disability at short-term follow-up, compared with no treatment.12
Quality of life
In the SR by Monticone et al., cognitive behavioural therapy significantly significantly improved quality of life at short-term follow-up.12
Appendix 4 presents a table of the main study findings and authors’ conclusions.
Limitations
Overall, there were various psychological interventions for chronic pain management and no primary studies focusing solely on the effectiveness of cognitive behavioural therapy were identified.4 One reason was that cognitive behavioural therapy was combined with other interventions to form complex interventional strategies, such as stepped care protocols, and case management strategies.16 Cognitive behavioural therapy included cognitive and behavioural interventions,4 but the types of cognitive or behavioural therapies were not reported.4,17 It was unclear which elements of cognitive behavioural therapy were effective. Furthermore, usual care or standard care was often unspecified in the identified SRs. The definition of chronic pain was also a limitation. Chronic pain was not explicitly defined in certain publications and it was unclear to which populations the results could be generalized.9 Lastly, the duration of follow-up was limited in the studies and the maximum was 30 months.4,13
Conclusions and Implications for Decision or Policy Making
There were five SRs identified for this report.4,9,11–13 Four SRs were Cochrane reviews4,9,12,13 and one SR was sponsored by the Agency for Healthcare Research and Quality.11 Except for the SR by Chou et al. that had no weakness in the AMSTAR critical domains, the others had one weakness in the critical domains. There was evidence to show the clinical effectiveness of cognitive behavioural therapy in patients with chronic pain. Cognitive behavioural therapy reduced the frequency of chronic headache in children based on the primary studies with more than 20 patients per arm, while this was not observed in smaller trials, compared with wait-list control, no treatment, or other pharmaceutical interventions.4 For patients with chronic low back pain, cognitive behavioural therapy was associated with pain reduction, compared with wait-list control .11 For patients with chronic neck pain, cognitive behavioural therapy significantly reduced pain and disability and improved quality of life at short-term follow-up, compared with no treatment.12 However, cognitive behavioural therapy was not associated with psychological indicators, such as kinesiophobia and anxiety in patients with chronic neck pain.12 For patients with spinal cord injury, cognitive behavioural therapy was associated with pain reduction initially compared to wait-list control, but there was no significant difference between the two groups three months later.13 The limitations to this report included no primary studies focusing solely on cognitive behavioural therapy and short durations of follow-up.9 Chronic pain and cognitive behavioural therapy were not well defined in several studies.4,13 Due to the lack of evidence in Canadian contexts and the unclear definitions of pain and cognitive behavioural therapy, further research addressing the application of cognitive behavioural therapy in Canada may help to reduce uncertainties.
References
- 1.
Jonesa
A. Chronic pain management and Canadian public health insurance: Do we need more comprehensive health care?
UBC Medical Journal. 2015;7(1).
- 2.
- 3.
- 4.
Fisher
E, Law
E, Dudeney
J, Palermo
TM, Stewart
G, Eccleston
C. Psychological therapies for the management of chronic and recurrent pain in children and adolescents.
Cochrane Database Syst Rev. 2018;9:CD003968. [
PMC free article: PMC6257251] [
PubMed: 30270423]
- 5.
Payne
KA, Myhr
G. Increasing access to Cognitive-Behavioural Therapy (CBT) for the treatment of mental illness in Canada: A research framework and call for action.
Healthc Policy. 2010;5(3):e173–e185. [
PMC free article: PMC2831741] [
PubMed: 21286263]
- 6.
- 7.
- 8.
Liberati
A, Altman
DG, Tetzlaff
J, Mulrow
C, Gotzsche
PC, Ioannidis
JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
J Clin Epidemiol. 2009;62(10):e1–e34. [
PubMed: 19631507]
- 9.
- 10.
- 11.
Chou
R, Deyo
R, Friedly
J, Skelly
A, Hashimoto
R, Weimer
M, et al. Nonpharmacologic therapies for low back pain: A systematic review for an American College of Physicians Clinical Practice Guideline.
Ann Intern Med. 2017;166(7):493–505. [
PubMed: 28192793]
- 12.
Monticone
M, Cedraschi
C, Ambrosini
E, Rocca
B, Fiorentini
R, Restelli
M, et al. Cognitive-behavioural treatment for subacute and chronic neck pain.
Cochrane Database Syst Rev. 2015(5):CD010664. [
PMC free article: PMC8922276] [
PubMed: 26006174]
- 13.
Boldt
I, Eriks-Hoogland
I, Brinkhof
MW, de Bie
R, Joggi
D, von Elm
E. Non-pharmacological interventions for chronic pain in people with spinal cord injury.
Cochrane Database Syst Rev. 2014(11):CD009177. [
PubMed: 25432061]
- 14.
Chou
R, Deyo
R, Friedly
J, Skelly
A, Hashimoto
R, Weimer
M, et al. AHRQ comparative effectiveness reviews.
Noninvasive treatments for low back pain
Rockville (MD):
Agency for Healthcare Research and Quality (US). 2016. [
PubMed: 26985522]
- 15.
Wang
L, Chang
Y, Kennedy
SA, Hong
PJ, Chow
N, Couban
RJ, et al. Perioperative psychotherapy for persistent post-surgical pain and physical impairment: A meta-analysis of randomised trials.
Br J Anaesth. 2018;120(6):1304–1314. [
PubMed: 29793597]
- 16.
Peterson
K, Anderson
J, Bourne
D, Mackey
K, Helfand
M. Evidence brief: Effectiveness of models used to deliver multimodal care for chronic musculoskeletal pain.
VA Evidence Synthesis Program Evidence Briefs. Washington (DC): Department of Veterans Affairs; 2017. [
PMC free article: PMC5902347] [
PubMed: 29633140]
- 17.
Barry
DT, Beitel
M, Cutter
CJ, Fiellin
DA, Kerns
RD, Moore
BA, et al. An evaluation of the feasibility, acceptability, and preliminary efficacy of cognitive-behavioral therapy for opioid use disorder and chronic pain.
Drug Alcohol Depend. 2019;194:460–467. [
PMC free article: PMC6312460] [
PubMed: 30508769]
- 18.
Eccleston
C, Palermo
TM, Williams
AC, Lewandowski Holley
A, Morley
S, Fisher
E, et al. Psychological therapies for the management of chronic and recurrent pain in children and adolescents.
Cochrane Database Syst Rev. 2014(5):CD003968. [
PMC free article: PMC5886855] [
PubMed: 24796681]
Appendix 1. Selection of Included Studies
Appendix 2. Characteristics of Included Publications
Table 2Characteristics of Included Systematic Reviews and Meta-Analyses
View in own window
| First Author, Publication Year, Country | Study Designs and Numbers of Primary Studies Included | Population Characteristics | Intervention and Comparator(s) | Clinical Outcomes, Length of Follow-Up |
|---|
| Amatya et al. 2018, Australia9 | 10 RCTs (no primary studies eligible for this report) RCTs or cross-over studies or clinical controlled trials eligible Multiple databases searched on 10 December 2017 Cochrane review | 565 persons aged 18 years and older with chronic pain and multiple sclerosis Minimum levels of pain on visual analogue scale of 3/10, more than 3 months | Non-pharmacological interventions eligible No RCT using cognitive behavioral therapy Interventions identified: transcutaneous electrical nerve stimulation, psychotherapy (telephone self-management, hypnosis and electroencephalogram biofeedback), transcranial random noise stimulation, transcranial direct stimulation, hydrotherapy (Ai Chi) and reflexology versus eligible comparators: above-mentioned interventions, no treatment, sham or usual care | Pain reduction measured by validated scales, such as a visual analogue scale or numerical rating scale, and Likert scale Follow-up: 1 to 12 months |
Fisher et al. 2018, the UK4 An update of the SR by Eccleston et al. 201418 | 47 RCTs (7 RCTs eligible for this report) Inclusion criteria: “RCTs with at least 10 participants in each arm post-treatment comparing psychological therapies with active treatment, treatment-as usual, or waiting-list control for children or adolescents with recurrent or chronic pain were eligible for inclusion” (p. 1) Internet-based interventions not eligible Multiple databases searched in May 2018 Cochrane review | 2,884 children and adolescents with chronic or recurrent pain Mean age: 12.65 years (SD = 2.21) Chronic pain: pain that recurs or persists for more than three months | Any psychological interventions versus active treatment, or treatment-as-usual, or waiting-list control Subset of eligible studies (7 RCTs): Cognitive behavioural therapy versus wait-list control (3 studies) or standard care that included analgesics (4 studies) Definitions: Behavioural strategies including “relaxation training, biofeedback, and behavioural management programmes (e.g. teaching parents strategies to reinforce adaptive behaviours such as school attendance)” (p. 6) Cognitive strategies including “hypnosis, stress management, guided imagery, and cognitive coping skills” (p. 6) “Cognitive-behavioural therapy programmes incorporate elements of both behavioural and cognitive strategies” (p. 6) | Primary outcomes: pain intensity and pain-related disability (measures not defined) Secondary outcomes: depression, anxiety, and adverse events Follow-up: 1 to 12 months |
Chou et al. 2017, the US11 A concise version of the SR by Chou et al. published in 201614 | 114 publications (5 of the 30 RCTs included in a SR by Henschke et al. published in 2010 were eligible for this report)10 Multiple databases searched in February 2016 Funded by the Agency for Healthcare Research and Quality PROSPERO: CRD42014014735 | Total not reported 4,066 patients with low back pain undergoing psychological therapies (3,090 in the SR by Henschke et al. in 2010;10 976 in 4 trials) Pain categories: “acute (<4 weeks), subacute (4 to 12 weeks), or chronic (≥12 weeks) nonradicular or radicular low back pain” (p. 494) | Non-pharmacologic options versus sham treatment, wait list, or usual care, or 1 nonpharmacologic option Subset of eligible studies (5 RCTs from the SR by Henschke et al.10): Cognitive behavioural therapy versus waitlist control | “Long-term (≥1 year) or short-term (≤6 months) pain, function, return to work, and harms” (p. 494) Follow-up: 1 to 12 months if reported |
| Monticone et al. 2015, Italy12 | 10 RCTs (3 RCTs eligible for this report) Multiple databases searched in November 2014 Cochrane review | 836 patients with neck pain, subacute (1 to 3 months) or chronic (> 3 months) Neck pain: “pain, muscle tension, or stiffness localized in the neck and may originate from many structures, including the spine or soft tissues” (p. 2) | Cognitive behavioural therapy: “including cognitive reconditioning and behavioural modifications of specific activities with the aim of modifying or reducing the impact of pain and physical and psychosocial disability” (p. 7) versus various comparators including placebo, no treatment, waiting list controls, other types of interventions, or mixed interventions Subset of eligible studies (3 RCTs): Cognitive behavioural therapy versus wait-list control (2 studies) or medication (unspecified, 1 study) | Primary outcome: pain measured by a visual analogue scale or a numerical rating scale Secondary outcomes including disability, psychological indicators (including anxiety, depression, and fear of pain), quality of life, return to work, and adverse events Follow-up: 2 to 12 months |
| Boldt et al. 2014, Switzerland13 | 16 RCTs (1 RCT eligible for this report) Multiple databases searched in November 2014 Cochrane review | 616 patients with chronic pain and spinal cord injury Patient characteristics not reported Chronic pain: “pain that persists past the normal time of healing and is present continuously or intermittently for at least three to six months” (p. 3) | Any non-pharmacological intervention not involving intake of medication or other active substances to treat chronic pain in people with spinal cord injury versus “Control interventions included active pharmacological or nonpharmacological treatments, placebo and sham interventions or waiting list groups” (p. 3) Subset of eligible studies (1 RCT): Cognitive behavioural therapy versus wait-list control | Primary outcome: pain measures, such as numerical rating scales, visual analogue scales, and pain questionnaires Secondary outcome: anxiety, depression or quality of life, and adverse events Follow-up: 3 to 12 months |
CES = cranial electrotherapy stimulation; PROSPERO = International Prospective Register of Systematic Reviews; RCT = randomized controlled trial; rTMS = repetitive transcranial magnetic stimulation; SD = standard deviation; SR = systematic review; tDCS = transcranial direct current stimulation; TENS = transcutaneous electrical nerve stimulation
Appendix 3. Critical Appraisal of Included Publications
Table 3Strengths and Limitations of Systematic Reviews and Meta-Analyses using the AMSTAR 2 checklist6
View in own window
| Strengths | Limitations |
|---|
| Amatya et al., 20189 |
|---|
- -
PICO components included in the research questions and inclusion criteria - -
The selection of study designs described, not justified - -
A comprehensive literature search conducted - -
Study selection conducted in duplicate - -
Data extraction in duplicate - -
A list of excluded studies provided and justified - -
Included studies described - -
Risk of bias in the included studies assessed with published tools - -
Sources of funding for the included studies reported - -
Risk of bias in the included studies considered while interpreting the results - -
Heterogeneity discussed - -
Review authors’ competing interests declared
| - -
Review protocol not published a priori
|
| Fisher et al., 20184 |
|---|
- -
PICO components included in the research questions and inclusion criteria - -
The selection of study designs described, not justified - -
A comprehensive literature search conducted - -
A list of excluded studies provided and justified - -
Included studies described - -
Risk of bias in the included studies assessed with published tools - -
Risk of bias in the included studies considered while interpreting the results - -
Heterogeneity discussed - -
Review authors’ competing interests declared - -
Review protocol published a priori - -
Appropriate statistical methods used for meta-analysis - -
Risk of bias in the included studies considered in the meta-analysis - -
Publication bias assessed
| - -
Study selection not conducted in duplicate - -
Data extraction not in duplicate - -
Sources of funding for the included studies not reported
|
| Chou et al., 201711 |
|---|
- -
PICO components included in the research questions and inclusion criteria - -
The selection of study designs described, not justified - -
A comprehensive literature search conducted - -
Study selection conducted in duplicate - -
A list of excluded studies provided and justified (in the other publication)14 - -
Included studies described (in the other publication)14 - -
Risk of bias in the included studies assessed with published tools - -
Sources of funding for the included studies reported - -
Risk of bias in the included studies considered while interpreting the results - -
Heterogeneity discussed - -
Review authors’ competing interests declared - -
Review protocol published a priori
| - -
Data extraction not in duplicate
|
| Monticone et al., 201512 |
|---|
- -
PICO components included in the research questions and inclusion criteria - -
The selection of study designs described, not justified - -
A comprehensive literature search conducted - -
Study selection conducted in duplicate - -
Data extraction in duplicate - -
A list of excluded studies provided and justified - -
Included studies described - -
Risk of bias in the included studies assessed with published tools - -
Risk of bias in the included studies considered while interpreting the results - -
Heterogeneity discussed - -
Review authors’ competing interests declared - -
Appropriate statistical methods used for meta-analysis - -
Risk of bias in the included studies considered in the meta-analysis
| - -
Review protocol not published a priori - -
Sources of funding for the included studies not reported - -
Publication bias not assessed
|
| Boldt et al., 201413 |
|---|
- -
PICO components included in the research questions and inclusion criteria - -
The selection of study designs described (but not justified) - -
A comprehensive literature search conducted - -
Study selection conducted in duplicate - -
Data extraction in duplicate - -
A listof excluded studies provided and justified - -
Included studies described - -
Risk of bias in the included studies assessed with published tools - -
Risk of bias in the included studies considered while interpreting the results - -
Heterogeneity discussed - -
Review authors’ competing interests declared - -
Appropriate statistical methods used for meta-analysis - -
Risk of bias in the included studies considered in the meta-analysis
| - -
Review protocol not published a priori - -
Sources of funding for the included studies not reported - -
Publication bias not assessed
|
AMSTAR = A Measurement Tool to Assess Systematic Reviews, PICO = population, comparator, intervention, and outcomes
Appendix 4. Main Study Findings and Authors’ Conclusions
Table 4Summary of Findings Included Systematic Reviews and Meta-Analyses
View in own window
| Main Study Findings | Authors’ Conclusion |
|---|
| Amatya et al., 20189 |
|---|
Non-pharmacological interventions versus other non-pharmacological interventions or control No primary studies included in the SR eligible for this report | There were no trials using cognitive behavioural therapy for chronic pain in patients with multiple sclerosis |
| Fisher et al., 20184 |
|---|
Psychological interventions versus control Subgroup analysis (CBT studies eligible for this report’s criteria)
- -
2 RCTs with more than 20 participants per arm: “beneficial effect on reducing headache frequency (RR 1.88, 95% CI 1.36 to 2.58, P < 0.01; NNTB = 3.58).” (p. 17) - -
2 RCTs with fewer than 20 patients per arm: “not find a beneficial effect of psychological treatment (SMD 0.04, 95% CI −0.47 to 0.54, P = 0.88)” (p. 17) - -
3 RCTs with fewer than 20 patients per arm: “not find a beneficial effect of therapy on depression (SMD − 0.16, 95% CI −0.68 to 0.35, P = 0.53)” (p. 18)
| - -
“Psychological treatments delivered predominantly face-to-face might be effective for reducing pain outcomes for children and adolescents with headache or other chronic pain conditions post-treatment. However, there were no effects at follow-up” (p. 2) - -
“Psychological therapies were also beneficial for reducing disability in children with mixed chronic pain conditions at post-treatment and follow-up, and for children with headache at follow-up” (p. 2) - -
“We found no beneficial effect of therapies for improving depression or anxiety” (p. 2) - -
“The conclusions of this update replicate and add to those of a previous version of the review which found that psychological therapies were effective in reducing pain frequency/intensity for children with headache and mixed chronic pain conditions post-treatment” (p. 2)
|
| Chou et al., 201711 |
|---|
Non-pharmacologic options versus control Low back pain
- -
“Evidence continues to support the effectiveness of exercise, psychological therapies, multidisciplinary rehabilitation, spinal manipulation, massage, and acupuncture for chronic low back pain (SOE, low to moderate)” (p. 493) - -
“The systematic review found that compared with wait-list control or no psychological therapy,…cognitive behavioral therapy (5 trials: SMD, −0.60 [CI, −0.97 to −0.22]) resulted in lower posttreatment pain intensity (48)” (p. 497)
| - -
“Several nonpharmacologic therapies for primarily chronic low back pain are associated with small to moderate, usually short-term effects on pain; findings include new evidence on mind–body interventions” (p. 493)
|
| Monticone et al., 201512 |
|---|
CBT versus wait-list control or no treatment Neck pain
- -
“There is low quality evidence that CBT is better than no treatment at improving pain in the short term (SMD −0.58, 95% CI −1.01 to −0.16, I2 = 0%, p-value = 0.007; see Analysis 2.1; Figure 5).” (p. 16) - -
“Two of these RCTs (N = 46) also measured disability and psychological indicators, such as kinesiophobia, distress, and quality of life. There is low quality evidence that CBT had a significant positive benefit for disability (SMD−0.61, 95%CI −1.21 to −0.01, I2 = 0%, p-value = 0.05; see Analysis 2.2; Figure 6), and quality of life (SMD−0.93, 95%CI −1.54 to −0.31, I2 = 0%, p-value = 0.003; see Analysis 2.5). Finally, CBT compared to no treatment had no effect on kinesiophobia (measured on the Tampa Scale for Kinesiophobia: possible range 17 to 68, random-effects, MD −6.69, 95% CI −13.91 to 0.53, I2 = 72%, p-value = 0.07; see Analysis 2.3; very low quality), and distress (SMD −0.41, 95% CI −0.99 to 0.18, I2 = 0%, p-value = 0.17; see Analysis 2.4; low quality).” (p. 16) - -
“None of the included studies reported on adverse effects” (p. 2)
| - -
“With regard to chronic neck pain, CBT was found to be statistically significantly more effective for short-term pain reduction only when compared to no treatment, but these effects could not be considered clinically meaningful” (p. 2)
|
| Boldt et al., 201413 |
|---|
Multi-disciplinary cognitive behavioural programme versus wait list control (1 RCT included by Boldt et al.13) Short-term and long-term outcomes
- -
“The mean difference in CPG pain intensity scores between experimental and waiting list control group was −2.0 (95% CI −9.26 to 5.26; P value 0.59). After three months (long-term outcomes), the mean difference in CPG pain intensity scores was 0.40 (95% CI −7.3 to 8.1; P value 0.92). The study was fraught with high overall risk of bias. In particular, the comparison with a waiting list as the control intervention likely leads to overestimation of the effectiveness of the experimental intervention. Consequently, no conclusion can be drawn on the effectiveness of this multi-disciplinary cognitive behavioural programme” (p. 16)
Secondary outcome measures
- -
“Mean differences in HADS anxiety scores were - 0.10 (95% CI −1.86 to 1.66; P value 0.91) and 0.30 (95% CI - 1.51 to 2.11; P value 0.74), respectively, for short-term and longterm outcomes” (p. 18)
| - -
“Evidence is insufficient to suggest that non-pharmacological treatments are effective in reducing chronic pain in people living with SCI” (p. 2) - -
“The benefits and harms of commonly used non-pharmacological pain treatments should be investigated in randomised controlled trials with adequate sample size and study methodology” (p. 2)
|
CBT = cognitive behavioural therapy; CI = confidence interval, CPG = Chronic Pain Grade, HADS = Hospital Anxiety and Depression Scale; MD = mean difference; NNTB = number needed to treat for an additional beneficial outcome; NP = neck pain; RD = risk difference; RR = risk ratio; SCI = spinal cord injury; SMD = standardized mean difference; SOE = strength of evidence; VAS = visual analogue scale; WMD = weighted mean difference
Appendix 5. Additional References of Potential Interest
Reviews without systematic literature searches
Non-randomized studies
Blake
C, Cunningham
J, Power
CK, Horan
S, Spencer
O, Fullen
BM. The Impact of a Cognitive Behavioral Pain Management Program on Sleep in Patients with Chronic Pain: Results of a Pilot Study.
Pain Med. 2016;17(2):360–369. [
PubMed: 26352702]
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Cognitive Behavioural Therapy for Chronic Non-Cancer Pain: A Review of Clinical Effectiveness. Ottawa: CADTH; 2019 Sep. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.