NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The amphetamines are indirect acting sympathomimetic amines and powerful central nervous system stimulants which are used in the therapy of attention deficit disorder, hyperactivity and narcolepsy. Amphetamines also have a potential for abuse and illicit forms of amphetamines constitute some of the most dangerous, but widely used drugs of abuse. High doses of amphetamines can be associated with liver injury and distinctive forms of clinically apparent liver injury which has been most commonly associated with methylenedioxymethamphetamine (MDMA: “ecstasy”).
Background
The amphetamines (am fet' a meens) are potent central nervous system (CNS) stimulants that are believed to act by causing release of norepinephrine at CNS nerve terminals promoting neurotransmission. Oral therapy with amphetamine has been shown to increase cognitive abilities and improve psychological functioning and performance in children and adults with suspected attention deficit disorders. The amphetamines often have a paradoxical calming action in children with hyperactivity. Amphetamines are also used in the therapy of narcolepsy and formerly for the treatment of obesity. Several forms of amphetamine have been approved for use in the United States. Amphetamine is available in multiple forms for oral administration including capsules, tablets, oral solutions, and as extended-release and long-acting forms in concentrations varying from 2.5 to 54 mg in generic forms and under several brand names, including Adderall (dextroamphetamine and amphetamine), Dexedrine (dextroamphetamine), Vyvanse (lisdexamfetamine), Desoxyn (methamphetamine), and Benzedrine (amphetamine). Transdermal formulations are also available. The usual dose in adults is 10 mg two or three times daily and average maintenance dosage is 40 to 60 mg daily. The dosage in children varies by formulation. Amphetamine is a controlled substance (Schedule II) and has major abuse potential. Common side effects include headache, insomnia, irritability, elation, agitation, confusion, palpitations, tachycardia, nasal stuffiness, and decreased appetite.
Hepatotoxicity
In clinical trials, amphetamine has not been associated with serum aminotransferase elevations during therapy, but monitoring of serum enzymes during large scale, long term trials of therapy have not been reported.
More importantly, several amphetamines have been associated with clinically apparent and sometimes severe or even fatal instances of acute liver injury. These cases typically follow intravenous use, particularly when given in an excessive single dose. The synthetic amphetamine methylenedioxymethamphetamine (MDMA, but known familiarly as “ecstasy”) has been implicated in the largest number of cases, many of which were severe and led to acute liver failure and death. The onset of ecstasy associated hepatitis is generally 3 to 14 days after ingestion and the onset is typically abrupt with fatigue, weakness, jaundice and confusion. The pattern of serum enzyme elevations is typically hepatocellular and ALT and AST values may be markedly increased with similar increases in lactic dehydrogenase. Onset of symptoms within a day of exposure is most frequent with acute liver failure and prolongation of the prothrombin time and other organ injury often present (renal, cardiac, muscle) (Case 1). The clinical course and early histological features of ecstasy induced hepatitis are similar to the patterns that occur with ischemic hepatitis and liver injury due to hyperthermia. Other amphetamines (such as the "designer" amphetamine, methylone or "Explosion") can cause a similar pattern of hepatic injury, but usually only when given as an overdose.
A second pattern of clinically apparent liver injury linked to amphetamines is more acute viral hepatitis-like, presenting with onset of fatigue, nausea and jaundice 1 to 2 weeks after an exposure or daily use (Case 2). These cases also can be severe, but are usually self-limiting. Immunoallergic features are uncommon and autoantibodies are usually not present. Recovery occurs with 1 to 3 months. Recurrence on reexposure has not been reported. However, a difficulty with analysis of cases of amphetamine induced liver injury is that the agent is often used illegally and patients are usually unwilling to return for long-term follow up (Cases 1 and 2).
Likelihood score: A[HD] (well established cause of liver injury, but severe cases occur only with high doses).
Mechanism of Injury
The mechanism by which the amphetamines cause liver injury is unknown, but amphetamines undergo extensive hepatic metabolism largely by the hepatic P450 system (CYP 2D6) and generation of a toxic metabolite may be the cause of hepatic injury with high doses of amphetamines. In cases of hyperacute liver failure due to ecstasy and to intravenous amphetamine, hyperthermia, shock and ischemia may account for the early liver injury.
Outcome and Management
The liver injury due to the amphetamines is usually self-limited and resolves rapidly. However, the acute liver injury can be severe and may require emergency liver transplantation within days of presentation. Several cases of chronic hepatitis apparently due to ecstasy have been described, usually in patients who continue to abuse MDMA. In these cases, liver biopsy shows features of chronic hepatitis with fibrosis, but cirrhosis has yet to be described and the co-occurrence of chronic hepatitis C among drug users may complicate the clinical picture. Patients with ecstasy induced hepatitis should be cautioned against its continued use, but the frequency of recurrence upon rechallenge is unclear. As with other amphetamines, the liver injury appears to be partially dose related.
Central nervous system stimulants used for attention deficit disorder, narcolepsy or excessive sleepiness include methylphenidate, atomoxetine, modafinil, armodafinil and the amphetamines. Stimulants that are no longer used for medical conditions but that are abused include cocaine and ecstasy or methylenedioxymethamphetamine.
Drug Class: Central Nervous System Stimulants
CASE REPORTS
Case 1. Acute hepatic necrosis after methylenedioxymethamphetamine (Ecstasy) use.(1)
A 32 year old woman developed hallucinations and confusion a few hours after ingesting 100 to 150 mg of methylenedioxymethamphetamine (MDMA) and was brought to the emergency room with agitation, disorientation and high fever. On examination, she had tachycardia (150/min), hypotension (90/50 mm Hg), tachypnea (36/min) and fever (41.6 oC). She had dilated pupils, nystagmus and hyperreflexia. Laboratory tests showed normal blood counts and liver tests with mild dehydration (creatinine 1.9 mg/dL), and serious hypoxemia (pO2 44 mm). She was admitted to an intensive care unit for ventilator support for 24 hours. Thereafter, she had weakness, anorexia and nausea, but improved over the next week and was discharged 10 days after admission. She had no previous history of liver disease or jaundice and took no other medications. She had used MDMA previously without complications. She denied injection drug use. During the course of her hospitalization, she developed elevations in serum enzymes and jaundice (Table). The prothrombin time became abnormal, but she remained stable and liver test abnormalities were markedly improved over the 10 day hospitalization. Analysis of the recovered powder that was taken demonstrated that it was 99% pure MDMA.
Key Points
| Medication: | Methylenedioxymethamphetamine (MDMA: “Ecstasy”) |
|---|---|
| Pattern: | Hepatocellular (R=~100) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 3 days to jaundice |
| Recovery: | Rapid, not completely documented |
| Other medications: | None mentioned |
Laboratory Values
| Time After Stopping | AST (U/L) | Alk P (U/L) | INR | Bilirubin* (mg/dL) | Other |
|---|---|---|---|---|---|
| Single Ingestion of MDMA | |||||
| 0 | 30 | 50 | 13.6 | 0.4 | Admission |
| 1 day | 385 | 65 | 33.2 | 1.2 | Extubation |
| 2 days | 37.9 | ||||
| 3 days | 1820 | 107 | 18.9 | 6.8 | |
| 4 days | 1435 | 90 | 13.8 | 7.6 | |
| 5 days | 1680 | 145 | 12.8 | 5.6 | |
| 10 days | 237 | 68 | 1.6 | Discharge | |
| Normal | <42 | <115 | <14.5 | <1.2 | |
Comment
Abuse of MDMA, an amphetamine derivative used orally as a “social” drug, often during “rave” parties, has been associated with severe toxic reactions with severe hyperthermia, shock, respiratory failure and hepatitis. This case demonstrates the timing of the “hyperacute” hepatic failure that can occur with MDMA use, with early and immediate presentation and with the acute adverse effects of amphetamines (sympathomimetic reactions of tachycardia, papillary dilation and confusion). The liver injury arises 1 to 3 days later, the prothrombin time becoming abnormal immediately followed by rise in ALT and AST and jaundice. The clinical pattern is “acute hepatic necrosis” as occurs with toxic reactions, most typically with acetaminophen overdose. The cause of the hyperacute hepatic failure due to MDMA may actually be hyperthermia as the early hepatic histology resembles hyperthermia induced liver injury (fat accumulation and central coagulative necrosis). Why a person who has been regularly abusing MDMA should suddenly have such an overwhelming toxic reaction is unclear, but it probably represents drug overdose and administration of more than claimed dose of the illegally (and unreliably) prepared substance, or else an underlying change in susceptibility (perhaps dehydration from participation in a “rave” or a concurrent illness). Differing slightly from this acute toxic hepatitis are cases of a more protracted course of an acute viral hepatitis-like outcome from MDMA use that typically have a longer latency and longer course (Case 2). Cases of liver injury due to amphetamine have resembled this toxic course and have usually arisen after an acute overdose, usually from intravenous amphetamine use.
Case 2. Acute hepatitis after methylenedioxymethamphetamine (Ecstasy) use.(2)
A 17 year old male who had been taking MDMA on weekends for 2 to 3 months developed fatigue followed by dark urine and jaundice. He had no previous history of liver disease or jaundice and took no other medications. He denied injection drug use. On examination, he was jaundiced but had no rash or fever. Laboratory testing showed elevations in serum bilirubin (5.6 mg/dL) and aminotransferase levels (Table). His prothrombin index was 58%. Tests for hepatitis A, B and C and for Epstein-Barr virus infection were negative as were autoantibodies. A liver biopsy showed acute hepatitis with occasional giant hepatocytes. His serum bilirubin continued to rise for the next 4 weeks, peaking at 28.1 mg/dL. Thereafter, he improved and all liver tests were normal 4 months later.
Key Points
| Medication: | Methylenedioxymethamphetamine (MDMA: “Ecstasy”) |
|---|---|
| Pattern: | Hepatocellular (R=~5.5) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 2 weeks to jaundice |
| Recovery: | Slow, almost complete by 4 months |
| Other medications: | None |
Laboratory Values
| Time After Stopping | ALT* (U/L) | Alk P* (U/L) | Bilirubin* (mg/dL) | Other |
|---|---|---|---|---|
| 2 days | 1118 | 5.6 | Prothrombin index 58% | |
| 4 days | 750 | 280 | 21.6 | |
| 5 days | 320 | 280 | 20.5 | |
| 7 days | 1090 | 480 | 27.8 | |
| 8 days | 850 | 240 | 22.2 | |
| 10 days | 800 | 21.6 | Liver biopsy: giant cells | |
| 11 days | 750 | 8.7 | ||
| 13 days | 300 | 240 | 3.2 | |
| 17 days | 50 | 200 | 2.4 | |
| 3 weeks | 50 | 200 | 2.4 | |
| Normal | <65 | <136 | <1.2 |
- *
Estimated from Figure 3.
Comment
Abuse of MDMA is frequently not mentioned by the patient, but should be considered in any young person with an unexplained acute hepatitis. This patient had an acute viral hepatitis-like presentation with a prolonged course starting a few weeks after last taking MDMA. There was no history of an acute episode of agitation, hypotension and hyperthermia and the pattern of liver enzyme elevations was more typical of hepatitis, remaining elevated for several weeks. The finding of giant cells on liver biopsy is largely nonspecific and does not help with the diagnosis.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Amphetamine – Generic, Benzedrine®
DRUG CLASS
Central Nervous System Stimulants
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
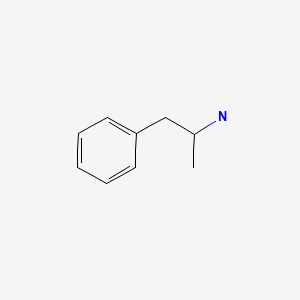
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Amphetamine | 300-62-9 | C9-H13-N |
|
CITED REFERENCES
- 1.
- Brown C, Osterloh J. Multiple severe complications from recreational ingestion of MDMA ('Ecstasy'). JAMA. 1987;258:780–1. [PubMed: 2886672]
- 2.
- Muñoz P, Drobinska A, Bianchi L, Züger C, Pirovino M. [Acute giant cell hepatitis in a 17-year old man]. Praxis (Bern 1994) 2004; 93: 2109-12. German. [PubMed: 15646679]
ANNOTATED BIBLIOGRAPHY
References updated: 25 August 2021
Abbreviations: MDEA (Eve), methylenedioxyethylamphetamine; MDMA (Ecstasy), methylenedioxymethamphetamine.
- Zimmerman HJ. Amphetamines. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 711.(Expert review of hepatotoxicity published in 1999; many instances of acute hepatitis from amphetamine use [“hippie hepatitis”] have been published; “the amphetamine most involved in hepatic injury goes by the street name of ‘ecstasy’”).
- Larrey D, Ripault MP. Illegal and recretational compounts. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 456-7.(Review of hepatotoxicity of amphetamine and their derivatives mentions that the liver injury may be mostly due to hyperthermia).
- Westfall TC, Macarthur H, Westfall DP. Adrenergic agonists and antagonists. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 191-224.(Textbook of pharmacology and therapeutics).
- Harvey JK, Todd CW, Howard JW. Fatality associated with benzedrine ingestion; a case report. Del Med J. 1949;21:111–5. [PubMed: 18133760](35 year old man developed confusion and hallucinations a few hours after taking 250 mg of amphetamine with alcohol and 3 days later developed jaundice, agitation, fever [104oF] followed by circulatory collapse: autopsy showed centrilobular fat and hepatic necrosis).
- Ginsberg MD, Hertzmann M, Schmidt-Nowara WW. Amphetamine intoxication with coagulopathy, hyperthermia and reversible renal failure. Ann Intern Med. 1970;73:81–5. [PubMed: 5433281](21 year old man took 2.2 g of amphetamine and developed agitation, delirium and fever to 108oF with subsequent coagulopathy, delirium and acute renal failure, but eventual recovery).
- Gastpar M, Ladewig D, Richter R, Weidmann M. Schweiz Med Wochenschr. 1974;104:1173–81. [Intravenous drug administration and hepatitis among young people in Basel] German. [PubMed: 4475482](Study of 61 drug abusing youths in Basel, Switzerland; a history of jaundice or hepatitis was frequent, but not associated with a specific drug; 97% were taking amphetamines).
- Rubel LR, Ishak KG. The liver in fatal exertional heatstroke. Liver. 1983;3:249–60. [PubMed: 6672506](Liver histology of 50 cases of fatal heatstroke arising during military training in 17 to 36 year old’s; initially fatty change, vacuolization and congestion followed by zone 3 coagulative necrosis, cholestasis and microvacuolar fatty change).
- Dowling GP, McDonough ET 3rd, Bost RO. 'Eve' and 'Ecstasy'. A report of five deaths associated with the use of MDEA and MDMA. JAMA. 1987;257:1615–7. [PubMed: 2881002](5 deaths due to acute toxicity of MDMA and MDEA ingestion, usually sudden death within hours of ingestion, cardiovascular collapse or suicide).
- Brown C, Osterloh J. Multiple severe complications from recreational ingestion of MDMA ('Ecstasy'). JAMA. 1987;258:780–1. [PubMed: 2886672](32 year old woman took 100-150 mg of MDMA and developed hallucinations, temperature to 41.6oC with subsequent jaundice [bilirubin peak 7.6 mg/dL on day 4, ALT 214 U/L, AST 1820 U/L, LDH 1225 U/L, Alk P 145 U/L], rhabdomyolysis, thrombocytopenia but rapid resolution [~1 week]: Case 1).
- Fahal IH, Sallomi DF, Yaqoob M, Bell GM. Acute renal failure after ecstasy. BMJ. 1992;305:29. [PMC free article: PMC1882520] [PubMed: 1353389](23 year old man took MDMA during rave party and presented with hyperthermia [40oC], jaundice [bilirubin 4.5 mg/dL, ALT 289 U/L] and renal failure, with slow recovery).
- Henry JA, Jeffreys KJ, Dawling S. Toxicity and deaths from 3,4-methylene-dioxymethamphetamine ("ecstasy"). Lancet. 1992;340:384–7. [PubMed: 1353554](Review of poison reports between 1990-92 from Guy's Hospital, London found 7 fatal cases of MDMA toxicity, 5 men and 2 women, ages 16 to 21 years, presenting with temperature >40oC, hypotension, cyanosis and death in 2 to 60 hours due to cardiac toxicity, rhabdomyolysis and toxic hepatitis [7 cases of liver injury with one death]).
- Randall T. Ecstasy-fueled 'rave' parties become dances of death for English youths. JAMA. 1992;268:1505–6. [PubMed: 1355567](News report on ecstasy toxicity and rave parties from the UK).
- Henry JA. Ecstasy and the dance of death. Severe reactions are unpredictable. BMJ. 1992;305:5–6. [PMC free article: PMC1882471] [PubMed: 1353390](Editorial on problem of use of ecstasy in rave parties and subsequent complications).
- Shearman JD, Chapman RW, Satsangi J, Ryley NG, Weatherhead S. Misuse of ecstasy. BMJ. 1992;305:309. [PMC free article: PMC1882752] [PubMed: 1356543](Letter in response to editorial by Henry [1992]; 27 year old woman with recurrent jaundice arising 8-15 days after taking MDMA [bilirubin 27.5 mg/dL, AST 1717 U/L, Alk P 439 U/L], biopsy showing florid hepatitis).
- Gorard DA, Davies SE, Clark ML. Misuse of ecstasy. BMJ. 1992;305:309. [PMC free article: PMC1882758] [PubMed: 1356542](Letter in response to editorial by Henry [1992]; 20 year old man developed jaundice 20 days after taking MDMA [bilirubin 7.7 mg/dL, AST 2050 U/L, Alk P 201 U/L], resolving within 4 weeks).
- Ijzermans JN, Tilanus HW, De Man RA, Metselaar HJ. "Ecstasy" and liver transplantation. Ann Med Interne (Paris). 1993;144:568. [PubMed: 8179247](3 cases: ages 24, 18 and 21 years; two cases with onset of jaundice after 4 and 6 months of intermittent use and one after initial use of ecstasy [bilirubin 26.0, 28.0 and 1.0 mg/dL, ALT 540, 1360 and 1100 U/L, protime 36%, 15% and 38%], one requiring liver transplantation, one with delayed and one with rapid recovery).
- de Man RA, Wilson JH, Tjen HS. Ned Tijdschr Geneeskd. 1993;137:727–9. [Acute liver failure caused by methylenedioxymethamphetamine ('ecstasy')] Dutch. [PubMed: 8097286](18 year old woman developed acute liver failure having used ecstasy on weekends for several months [bilirubin 28.2 mg/dL, ALT 1360 U/L, Alk P 30 U/L, protime 36%], recovering spontaneously over the ensuing 2 months).
- de Man RA. Ned Tijdschr Geneeskd. 1994 Sep 10;138:1850–5. [Morbidity and mortality caused by use of ecstasy] Dutch. [PubMed: 7935920]
- Oranje WA, von Pol P. vd Wurff A, Zeijen RN, Stockbrügger RW, Arends JW. XTC-induced hepatitis. Neth J Med. 1994;44:56–9. [PubMed: 7911561](25 year old woman developed fatigue and jaundice 1-2 weeks after her last use of MDMA [referred to as XTC] having used it on weekends for 2 months [bilirubin 19.2 mg/dL, ALT 1157 U/L, Alk P normal], biopsy showing an acute hepatitis).
- Jones AL, Jarvie DR, McDermid G, Proudfoot AT. Hepatocellular damage following amphetamine intoxication. J Toxicol Clin Toxicol. 1994;32:435–44. [PubMed: 8057403](Two cases; 21 year old man developed confusion, seizures and fever [41oC] 6 hours after amphetamine ingestion with subsequent jaundice [7.3 mg/dL, ALT 8371 U/L, Alk P 101 U/L on day 3], resolving over next 2 weeks; 18 year old woman fainted during a rave party and ecstasy use with fever [42oC] tachycardia and hypoxemia and subsequent jaundice [bilirubin 3.0 mg/dL, ALT 1240, Alk P 137 on day 4], resolving rapidly).
- Deltenre P, Henrion J, Jacques JM, Schapira M, Ghilain JM, Maisin JM, Heller FR. Acta Gastroenterol Belg. 1994;57:341–5. [Toxic hepatitis due to ecstasy administration: report of a possible case and literature review] French. [PubMed: 7709705](22 year old man taking ecstasy for at least 6 months developed jaundice [bilirubin 13.8 mg/dL, ALT 3390 U/L, Alk P 266 U/L], which resolved within 2 months of stopping).
- Huarte Muniesa MP, Pueyo Royo AM. Rev Esp Enferm Dig. 1995;87:681–3. [Acute hepatitis due to ingestion of Ecstasy] Spanish. [PubMed: 7577130](23 year old male sporadic user of ecstasy developed jaundice [bilirubin 10.6 mg/dL, ALT 700 U/L, Alk P 304 U/L], resolving in 6 weeks).
- Dykhuizen RS, Brunt PW, Atkinson P, Simpson JG, Smith CC. Ecstasy induced hepatitis mimicking viral hepatitis. Gut. 1995;36:939–41. [PMC free article: PMC1382638] [PubMed: 7615289](3 cases: 24 year old man with diabetes developed jaundice 3 days after having a beer spiked with MDMA [peak bilirubin 20.2 mg/dL, AST 950 U/L, Alk P 251 U/L], resolving over next 1-4 months; 22 year old man developed lethargy followed by jaundice 2-3 weeks after taking MDMA [peak bilirubin 21.7 mg/dL, AST 1410 U/L, Alk P 223 U/L] given short course of prednisolone, resolving in next 3 months; 23 year old man developed jaundice during intermittent use of MDMA [peak bilirubin 4.4 mg/dL, AST 639 U/L, Alk P 265 U/L], resolving over next 5 weeks).
- Khakoo SI, Coles CJ, Armstrong JS, Barry RE. Hepatotoxicity and accelerated fibrosis following 3,4-methylenedioxymetamphetamine ("ecstasy") usage. J Clin Gastroenterol. 1995;20:244–7. [PubMed: 7797836](22 year old woman developed jaundice 3 months after starting weekly MDMA abuse [bilirubin 3.1 mg/dL, AST 2314 U/L, Alk P 145 U/L, protime 16.6 seconds]; continued intermittent ecstasy use and 6 months later developed ascites and worsening jaundice [bilirubin 23.9 mg/dL, AST 2214 U/L, Alk P 253 U/L protime 24.1 seconds], biopsy showed fibrosis; partial response to prednisone).
- Fidler H, Dhillon A, Gertner D, Burroughs A. Chronic ecstasy (3,4-methylenedioxymetamphetamine) abuse: a recurrent and unpredictable cause of severe acute hepatitis. J Hepatol. 1996;25:563–6. [PubMed: 8912157](Two cases of acute hepatitis after MDMA, 19 year old man and 18 year old woman developed jaundice 3 and 5 months after starting MDMA use [bilirubin 7.7 and 5.6 mg/dL, ALT 745 AND 2435 U/L, Alk P 180 and 176 U/L], recurring in both after 2 months of restarting abuse, one patient had chronic liver enzyme elevations using chronic use).
- Milroy CM, Clark JC, Forrest AR. Pathology of deaths associated with "ecstasy" and "eve" misuse. J Clin Pathol. 1996;49:149–53. [PMC free article: PMC500349] [PubMed: 8655682](Autopsy results on 7 deaths from MDMA or MDEA use, all with zone 3 necrosis with acute inflammation surrounding it and fatty change; similar to hyperthermia).
- Ellis AJ, Wendon JA, Portmann B, Williams R. Acute liver damage and ecstasy ingestion. Gut. 1996;38:454–8. [PMC free article: PMC1383078] [PubMed: 8675102](Three patterns of liver injury in 8 patients after ecstasy use; first, acute toxicity with hyperthermia and collapse marked by hyperacute liver failure, histology showing microvesicular fat and small areas of collapse; second, typical acute liver failure arising 1-3 weeks after last use of MDMA, histology showing submassive necrosis; third, severe acute hepatitis like syndrome arising using long term use of MDMA with recovery in 2-3 months, histology showing marked inflammation and spotty necrosis).
- Chenard-Neu MP, Boudjema K, Bernuau J, Degott C, Belghiti J, Cherqui D, Costes V, et al. Auxiliary liver transplantation: regeneration of the native liver and outcome in 30 patients with fulminant hepatic failure – a multicenter European Study. Hepatology. 1996;23:1119–27. [PubMed: 8621143](Combined experience with 50 auxiliary liver transplants for acute liver failure from 12 European centers; 2 cases were attributed to ecstasy, both survived and had partial regeneration and fibrosis in the native liver).
- Pereira SP, McCarthy M, Ellis AJ, Wendon J, Portmann B, Rela M, Heaton N, et al. Auxiliary partial orthotopic liver transplantation for acute liver failure. J Hepatol. 1997;26:1010–7. [PubMed: 9186831](Experience with auxiliary partial liver transplants in 7 patients with acute liver failure; 3 patients died early due to sepsis [including single case due to ecstasy]; native liver regenerated in 2 of 3 surviving patients).
- Brauer RB, Heidecke CD, Nathrath W, Beckurts KT, Vorwald P, Zilker TR, Schweigart U, et al. Liver transplantation for the treatment of fulminant hepatic failure induced by the ingestion of Ecstasy. Transpl Int. 1997;10:229–33. [PubMed: 9163865](18 year old female who had used ecstasy over a two month period develop lethargy, nausea and jaundice [bilirubin 15.4 mg/dL, ALT 1530 U/L] and progressed to acute liver failure, underwent successful liver transplant 10 days later, graft showing submassive necrosis).
- Hellinger A, Rauen U, de Groot H, Erhard J. Dtsch Med Wochenschr. 1997;122:716–20. [Auxiliary liver transplantation for acute liver failure after intake of 3,4-methylenedioxymethamphetamine ("Ecstasy")] German. [PubMed: 9213536](18 year old female developed jaundice 8 days after taking ecstasy, after 8 months of abuse [bilirubin 7.3 mg/dL, ALT 1460 U/L, protime 47%], with progressive hepatic failure and successful auxiliary liver transplant 26 days later).
- Pascual Bartolomé S, Sarrión Martínez JV, García Herola A, Berenguer Lapuerta J. Med Clin (Barc). 1997;108:279. [Hepatitis and Ecstasy] Spanish. [PubMed: 9121201](17 year old developed jaundice without fever, after several months of ecstasy use on weekends [bilirubin 18.7 mg/dL, ALT 1485 U/L, Alk P 150 U/L], treated with prednisone and recovered within 2 months).
- Tillmann HL, van Pelt FN, Martz W, Luecke T, Welp H, Dörries F, Veuskens A, et al. Accidental intoxication with methylene dianiline p,p'-diaminodiphenylmethane: acute liver damage after presumed ecstasy consumption. J Toxicol Clin Toxicol. 1997;35:35–40. [PubMed: 9022650](Six patients took chemical agent that caused Epping jaundice thinking that it was MDMA, and all developed fever and acute jaundice [bilirubin 10.8 mg/dL, ALT 77 U/L, Alk P 344 U/L], all resolving rapidly).
- Shiloach E, Zecler E, Horowiz M, Scapa E. Harefuah. 1998;134:266–9, 335. [Acute liver damage after using ecstasy] [PubMed: 10909502]
- Indart Pérez A, Mendia Gorostidi A, Barrio Andrés J, Arens Miravé I. Gastroenterol Hepatol. 1998;21:499. [Acute hepatitis induced by Ecstasy] Spanish. [PubMed: 9927798](24 year old man developed agitation and seizures after taking ecstasy, developing jaundice 3 days later [bilirubin 20.1 mg/dL, ALT 2484 U/L, Alk P 420 U/L, INR 3.9], resolving spontaneously over next 2 months).
- Roques V, Perney P, Beaufort P, Hanslik B, Ramos J, Durand L, Le Bricquir Y, et al. Presse Med. 1998;27:468–70. [Acute hepatitis due to ecstasy] French. [PubMed: 9767974](27 year old man developed weight loss and jaundice without fever after 4 years of ecstasy use, immediately after increasing use for a 15 day period [bilirubin 14.5 mg/dL, ALT 60 times ULN, Alk P 1.5 times ULN, protime 43%], resolving within 2 months of stopping use).
- Giner Durán R, Flors H, Millán M, Manzanera R. Gastroenterol Hepatol. 1998;21(3):158. [Hepatitis from ecstasy] Spanish. [PubMed: 9607299](17 year old female developed abdominal pain and jaundice one week after taking ecstasy [bilirubin 9.4 mg/dL, ALT 4080 U/L, Alk P 154 U/L, protime 65%], with resolution in 6 weeks).
- Andreu V, Mas A, Bruguera M, Salmerón JM, Moreno V, Nogué S, Rodés J. Ecstasy: a common cause of severe acute hepatotoxicity. J Hepatol. 1998;29:394–7. [PubMed: 9764985](Among 62 cases of acute liver failure seen in Barcelona between 1994-1997, 5 [8%] were due to MDMA toxicity, all men, ages 17 to 20 years, with jaundice arising after 4-48 weeks of use, 7-15 days after last dose [bilirubin 21-38.9 mg/dL, ALT 2623-5282 U/L]).
- Jones AL, Simpson KJ. Review article: mechanisms and management of hepatotoxicity in ecstasy(MDMA) and amphetamine intoxications. Aliment Pharmacol Ther. 1999;13:129–33. [PubMed: 10102941](Review of clinical and histological features of MDMA hepatotoxicity and possible mechanisms).
- Schirren CA, Berghaus TM, Sackmann M. Thrombotic thrombocytopenic purpura after Ecstasy-induced acute liver failure. Ann Intern Med. 1999;130(2):163. [PubMed: 10068371](20 year old man developed fatigue and jaundice 1 week after ingestion of ecstasy, with subsequent acute liver failure and thrombotic thrombocytopenic purpura, ultimately responding to prednisone and plasma exchange).
- Beitia G, Cobreros A, Sainz L, Cenarruzabeitia E. Ecstasy-induced toxicity in rat liver. Liver. 2000;20:8–15. [PubMed: 10726956](Toxicity of MMDA in rats is mild, but appears to be characterized by centrilobular necrosis).
- Hoover-Stevens S, Kovacevic-Ristanovic R. Management of narcolepsy in pregnancy. Clin Neuropharmacol. 2000;23:175–81. [PubMed: 11020119](Review of the efficacy of medications for narcolepsy and their safety during pregnancy and breast feeding; animal studies suggest that amphetamine may be teratogenic, but there have been no adequate human studies of its safety in pregnancy).
- Shibolet O, Kalish Y, Gillis S, Ilan Y. Harefuah. 2001;140:911–4, 991. [Hepatic and hematological complications following ecstasy usage] Hebrew. [PubMed: 11681122]
- Garbino J, Henry JA, Mentha G, Romand JA. Ecstasy ingestion and fulminant hepatic failure: liver transplantation to be considered as a last therapeutic option. Vet Hum Toxicol. 2001;43:99–102. [PubMed: 11308131](19 year old man took 1½ tablets of ecstasy with alcohol and developed fatigue followed by jaundice and worsening hepatic failure [ALT 336 rising to 1932 U/L, bilirubin rising to 38.2 mg/dL], followed by hepatic encephalopathy and emergency liver transplantation 31 days after the ingestion).
- Lawler LP, Abraham S, Fishman EK. 3,4-Methylenedioxymethamphetamine (ecstasy)-induced hepatotoxicity: multidetector CT and pathology findings. J Comput Assist Tomogr. 2001;25:649–52. [PubMed: 11473199](24 year old woman developed jaundice 1 week after using MDMA [bilirubin 14.9 mg/dL, ALT 2215 U/L, Alk P 113 U/L], CT scans showing multiple areas of arterial enhancement which returned to near normal in follow up).
- Jonas MM, Graeme-Cook FM. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 6-2001. A 17-year-old girl with marked jaundice and weight loss. N Engl J Med. 2001;344:591–9. [PubMed: 11207356](17 year old female developed jaundice after MDMA use [bilirubin 8.4 rising to 22.5 mg/dL, ALT 1236 U/L, Alk P 209 U/L], resolving in several weeks).
- De Carlis L, De Gasperi A, Slim AO, Giacomoni A, Corti A, Mazza E, Di Benedetto F, et al. Liver transplantation for ecstasy-induced fulminant hepatic failure. Transplant Proc. 2001;33:2743–4. [PubMed: 11498145](17 year old woman developed fatigue 6 days after taking MDMA over 10 day period [bilirubin 20 mg/dL, ALT 3040 U/L, INR 2.2], with worsening hepatic failure, undergoing liver transplantation 3 days later).
- Núñez O, Bañares R, Barrio J, Menchén L, Diego A, Salinero E, Clemente G. Gastroenterol Hepatol. 2002;25:497–500. [Variability of the clinical expression of Ecstasy-induced hepatotoxicity] Spanish. [PubMed: 12361531](Four cases of ecstasy induced liver disease: 2 women and 2 men, ages 17 to 28 years, developed jaundice 1-2 weeks after taking ecstasy [bilirubin 9.4-32 mg/dL, ALT 739-2303 U/L, Alk P 254-416 U/L, protime 15-62%], one undergoing liver transplantation 4 days after admission, the remaining 3 recovering within 2-3 months).
- Lange-Brock N, Berg T, Müller AR, Fliege H, Neuhaus P, Wiedenmann B, Klapp BF, et al. Z Gastroenterol. 2002;40:581–6. [Acute liver failure following the use of ecstasy (MDMA)] German. [PubMed: 12297982](17 year old female developed nausea and jaundice 2 days after using ecstasy, which had been used intermittently for 6 months [bilirubin 17.3 mg/dL, ALT 2136 U/L, protime 15%], progressive hepatic failure leading to liver transplantation within 4 days).
- Chaudier B, Oliver M, Coton T, Civatte M, Guisset M, Carré D, Debonne JM, et al. Gastroenterol Clin Biol. 2002;26:103–4. [Chronic hepatitis with an acute presentation due to Ecstasy] French. [PubMed: 11938056](18 year old female developed jaundice 3 weeks after taking 2 tablets of ecstasy [bilirubin 15.6 mg/dL, ALT 44 times ULN, GGT 1.4 times ULN, protime 96%], with subsequent worsening and liver biopsy showing chronic hepatitis with bridging fibrosis; one year later enzymes were normal, but repeat liver biopsy showed chronic hepatitis with mild fibrosis).
- Hwang I, Daniels AM, Holtzmuller KC. "Ecstasy"-induced hepatitis in an active duty soldier. Mil Med. 2002;167:155–6. [PubMed: 11873540](21 year old man developed nausea followed by jaundice 3 days after taking 3 tablets of ecstasy, after 3 months of intermittent use [bilirubin 25 mg/dL, ALT 1,347 U/L, Alk P 118 U/L, protime 18 seconds], ultimately with spontaneous recovery).
- Caballero F, Lopez-Navidad A, Cotorruelo J, Txoperena G. Ecstasy-induced brain death and acute hepatocellular failure: multiorgan donor and liver transplantation. Transplantation. 2002;74:532–7. [PubMed: 12352914](Outcome of organ transplants from two multiorgan transplant donors who died of brain death from MDMA overdose and hyponatremia; liver recipients had benign and uneventful outcomes; three cases of acute liver failure in 25, 27 and 16 year old’s with jaundice arising within days of taking ecstasy and undergoing successful emergency liver transplantation).
- Carrión JA, Escorsell A, Nogué S, Mas A. Med Clin (Barc). 2003;121:118–9. [Ecstasy-induced fulminant hepatic failure and emergency liver transplantation] Spanish. [PubMed: 12855141](23 year old man taking cocaine and MDMA for 6-8 months developed fatigue and jaundice 10 days after last dose [bilirubin 33.9 mg/dL, ALT 2900 U/L, Alk P 290 U/L, protime 15%], with progressive worsening and liver transplantation, liver showing submassive necrosis).
- Aknine X. Presse Med. 2004;33(18) Suppl:18–20. [Abnormalities of liver tests due to use of Ecstasy] French. [PubMed: 15617171](21 year old man with fatigue was found to have abnormal liver tests [bilirubin normal, ALT 5 times ULN, GGT normal] and history of weekend use of ecstasy for several years, enzymes becoming normal with stopping use).
- Muñoz P, Drobinska A, Bianchi L, Züger Ch, Pirovino M. [Acute giant cell hepatitis in a 17-year old man]. Praxis (Bern 1994) 2004; 93: 2109-12. German. [PubMed: 15646679](17 year old male developed jaundice after 2-3 months of intermittent ecstasy use [bilirubin 5.6 rising to 27.5 mg/dL, ALT 1118 U/L, Alk P 480 U/L] with protracted course, resolving by 4 months; a liver biopsy showed giant cells and focal necrosis).
- Salzmann J, Marie-Claire C, Noble F. Presse Med. 2004;33(18) Suppl:24–32. [Acute and long-term effects of ecstasy] French. [PubMed: 15617173](Review of the short- and long term toxicities of MDMA).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, 1 was attributed to cocaine; MDMA and ecstasy are not mentioned).
- Liechti ME, Kunz I, Kupferschmidt H. Acute medical problems due to Ecstasy use. Case-series of emergency department visits. Swiss Med Wkly. 2005;135:652–7. [PubMed: 16380853](Retrospective analysis of 52 cases of ecstasy intoxications during 3 year period in Switzerland; collapse [37%], palpitations [19%], dizziness [15%] and anxiety [14%] or panic [31% when used with cocaine], only six required intensive care including one with hepatic failure).
- Brncić N, Kraus I, Visković I, Mijandrusić-Sincić B, Vlahović-Palcevski V. 3,4-methylenedioxymethamphetamine (MDMA): an important cause of acute hepatitis. Med Sci Monit. 2006;12:CS107–9. [PubMed: 17072276](19 year old previously healthy man presented with jaundice 2 weeks after taking 2 tablets of ecstasy [bilirubin 7.1 rising to 15.2 mg/dL, ALT 3185 U/L, Alk P 247 U/L, GGT 135 U/L], resolving over the following 2 months).
- Kis L, Pfaffenberger M. [Painless jaundice after ecstasy and alcohol consumption]. Praxis (Bern 1994) 2007; 96: 1035-7. German. [PubMed: 17639967](21 year old male developed painless jaundice 5 days after taking ecstasy for the first time [bilirubin 16.5 mg/dL, ALT 50 times ULN, Alk P minimally elevated], resolving over subsequent 2 months).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J. Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 3 were attributed to atomoxetine and 1 to methylphenidate, but none to amphetamines or ecstasy).
- Hood B, Nowicki MJ. Eosinophilic hepatitis in an adolescent during lisdexamfetamine dimesylate treatment for ADHD. Pediatrics. 2010;125:e1510–3. [PubMed: 20457690](14 year old boy developed abdominal pain and jaundice 5 months after starting lisdexamfetamine [30 mg daily] for ADHD [bilirubin 9.2 mg/dL, ALT 2350 U/L], resolving within 2 months of stopping medication with prednisone therapy [later stopped]).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, including one attributed to cocaine and one to ecstasy [MDMA]).
- Colak Y, Tuncer I, Enc FY, Ozturk O, Kiziltas S, Ulasoglu C. Ecstasy induced fatal hepatic failure. J Gastrointestin Liver Dis. 2011;20:215–6. [PubMed: 21725523]
- (19 year old man developed nausea and abdominal pain 1 week after taking MDMA [bilirubin 7.8 rising to 26.0 mg/dL, ALT 375 rising to 4555 U/L, protime “infinite”], subsequently dying of progressive hepatic failure) .
- Fröhlich S, Lambe E, O'Dea J. Acute liver failure following recreational use of psychotropic "head shop" compounds. Ir J Med Sci. 2011;180:263–4. [PubMed: 21063803](28 year old man developed fever and a seizure after taking 12 tablets of a recreational amphetamine-like stimulant [butylone and methylenedioxypyrovalerone], rapidly developing shock, acute renal failure, rhabdomyolysis and liver failure [bilirubin 2.3 mg/dL, ALT 2500 U/L, CPK 112,000 U/L, INR 2.8 ], but ultimately recovering without transplantation).
- Pearson JM, Hargraves TL, Hair LS, Massucci CJ, Frazee CC 3rd, Garg U, Pietak BR. Three fatal intoxications due to methylone. J Anal Toxicol. 2012;36:444–51. [PubMed: 22589523](Three cases of fatal overdose of methylone, a synthetic amphetamine also called bk-MDMA or "explosion"; deaths were due to hyperthermia, seizures and respiratory depression, but two had metabolic acidosis and one had multiorgan failure with hepatic involvement).
- Kwon H, Lee SH, Kim SE, Lee JH, Jee YK, Kang HR, Park BJ, Park JW, Hong CS. Spontaneously reported hepatic adverse drug events in Korea: multicenter study. J Korean Med Sci. 2012;27:268–73. [PMC free article: PMC3286773] [PubMed: 22379337](Summary of 2 years of adverse event reporting in Korea; of 9360 reports, 567 were liver related, but none were attributed to amphetamines).
- Payancé A, Scotto B, Perarnau JM, de Muret A, Bacq Y. Severe chronic hepatitis secondary to prolonged use of ecstasy and cocaine. Clin Res Hepatol Gastroenterol. 2013;37:e109–13. [PubMed: 23910059](27 year old woman developed jaundice and ascites 9 months after starting regular use of cocaine and MDMA [bilirubin 4.5 mg/dL, ALT 220 U/L, Alk P 274 U/L], liver biopsy showing chronic hepatitis and cirrhosis, with gradual improvement upon stopping and with normal bilirubin after 2 months and normal enzymes after 10 months).
- McIntyre IM, Hamm CE, Aldridge L, Nelson CL. Acute methylone intoxication in an accidental drowning--a case report. Forensic Sci Int. 2013;231(1-3):e1–3. [PubMed: 23827713](19 year old woman found drowned, autopsy revealing high blood and liver methylone levels).
- Pateria P, de Boer B, MacQuillan G. Liver abnormalities in drug and substance abusers. Best Pract Res Clin Gastroenterol. 2013;27:577–96. [PubMed: 24090944](Review of the liver abnormalities among drug and substance abusers and hepatotoxicity of the major abused substances, including alcohol, acetaminophen, cannabis, amphetamines, cocaine, khat and other herbals).
- Vanga RR, Bal B, Olden KW. Adderall induced acute liver injury: a rare case and review of the literature. Case Rep Gastrointest Med. 2013;2013:902892. [PMC free article: PMC3706063] [PubMed: 23864967](55 year old woman developed acute liver injury with hepatic encephalopathy 9 months after starting amphetamine for ADHD and 5 days after increasing the dose [bilirubin 1.8 rising to 3.3 mg/dL, ALT ~5500 U/L], resolving rapidly, within 2 weeks of stopping).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, of which none were attributed to amphetamines or methylphenidate).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, but none were attributed to amphetamines).
- Allen SB, Cross KP. Out of the frying pan, into the fire: a case of heat shock and its fatal complications. Pediatr Emerg Care. 2014;30:904–10. [PubMed: 25469604](15 year old male football player who was taking Adderall for ADHD collapsed during practice and on hospital admission had hyperthermia [107.3oF] and upon cooling and resuscitation developed hepatic, renal and respiratory failure [bilirubin not given, ALT 12 rising to 7839 U/L, Alk P 184 U/L, LDH 360 rising to 7839 U/L], dying within 4 days of multiorgan failure).
- Cohen PA. Hazards of hindsight--monitoring the safety of nutritional supplements. N Engl J Med. 2014;370:1277–80. [PubMed: 24693886](Commentary on the rise of reports of liver injury after use of poorly regulated nutritional supplements, some of which contain undisclosed synthetic amphetamine analogues meant to promote weight loss and boost energy levels).
- Wöllner K, Stockhausen S, Mußhoff F, Madea B. Arch Kriminol. 2015;235:53–61. [Death after the intake of amphetamine/ecstasy: two case reports] German. [PubMed: 26419092](Two fatal cases of suspected MDMA overdose: 19 year old man with rapidly fatal acute hepatic failure and a 39 year old man who collapsed at work were found to have elevated, post-mortem blood amphetamine levels).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to ecstasy [MDMA] or other amphetamines).
- Atayan Y, Çağın YF, Erdoğan MA, Harputluoglu MM, Bilgic Y. Ecstasy induced acute hepatic failure. Case reports. Acta Gastroenterol Belg. 2015;78(1):53–5. [PubMed: 26118578](Two Turkish men, ages 24 and 18, ingested ecstasy and presented 1 week later with jaundice [bilirubin 14 and 23 mg/dL, ALT 1237 and 2154 U/L, INR 1.3 and 2.5], one developing hepatic failure and requiring transplantation and the other recovering with conservative therapy).
- Maranella E, Mareri A, Nardi V, Di Natale C, Di Luca L, Conte E, Pannone V, et al. Severe neurologic and hepatic toxicity in a newborn prenatally exposed to methamphetamine. A case report. Brain Dev. 2019;41:191–4. [PubMed: 30213441](A full-term Italian newborn male with low birth weight, respiratory distress, seizures, neurologic signs and hepatomegaly had ALT 295 U/L, GGT 125 U/L, bilirubin 9.9 mg/dL and platelets of 29,000, and urine from both the child and mother tested positive for methamphetamine).
- Drugs for ADHD. Med Lett Drugs Ther. 2020;62(1590):9–15. [PubMed: 31999670](Concise review of drugs for attention deficit/hyperactivity disorder mentions that methylphenidate and the CNS stimulants are the first line agents for school age children and adolescents and that adverse events can include decreased appetite, abdominal pain, headache and insomnia, but does not mention ALT elevations or hepatotoxicity).
- Zhao T, Zhai C, Song H, Wu Y, Ge C, Zhang Y, Xu H, et al. Methamphetamine-Induced Cognitive Deficits and Psychiatric Symptoms Are Associated with Serum Markers of Liver Damage. Neurotox Res. 2020;37:67–76. [PubMed: 31691188](Cognitive deficits were greater among 106 Chinese male methamphetamine-addicts at the “Compulsory Isolated Drug Rehabilitation Centre” than among 76 healthy staff members, while serum ALT levels were similar and AST levels were lower which the authors suggested was due to a “phased characteristic of liver injury”).
- Patel H, Kumar K, Essrani RK, Niazi M, Makker J, Nayudu SK. Acute Hepatitis in a Yemeni Immigrant Associated with Khat: A "Biological Amphetamine" Carried in Cultures. Clin Pract. 2021;11:167–73. [PMC free article: PMC8006146] [PubMed: 33800126](21 year old Yemeni man developed right upper quadrant pain and liver test abnormalities [bilirubin 0.7/2.0 mg/dL, ALT 328 U/L, Alk P 78 U/L] and admitted to Khat use 5-6 times per month since moving to the US, resolving within a few months of stopping).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Teratogen update: Amphetamines.[Birth Defects Res. 2020]Teratogen update: Amphetamines.Garey JD, Lusskin SI, Scialli AR. Birth Defects Res. 2020 Sep; 112(15):1171-1182. Epub 2020 Aug 4.
- Review The neurotoxicity of amphetamines during the adolescent period.[Int J Dev Neurosci. 2015]Review The neurotoxicity of amphetamines during the adolescent period.Teixeira-Gomes A, Costa VM, Feio-Azevedo R, Bastos Mde L, Carvalho F, Capela JP. Int J Dev Neurosci. 2015 Apr; 41:44-62. Epub 2014 Dec 4.
- Review Central Nervous System (CNS) Stimulants.[LiverTox: Clinical and Researc...]Review Central Nervous System (CNS) Stimulants.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Attention Deficit/Hyperactivity Disorder (ADHD) Agents.[LiverTox: Clinical and Researc...]Review Attention Deficit/Hyperactivity Disorder (ADHD) Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Enduring deficits in sustained visual attention during withdrawal of intravenous methylenedioxymethamphetamine self-administration in rats: results from a comparative study with d-amphetamine and methamphetamine.[Neuropsychopharmacology. 2007]Enduring deficits in sustained visual attention during withdrawal of intravenous methylenedioxymethamphetamine self-administration in rats: results from a comparative study with d-amphetamine and methamphetamine.Dalley JW, Lääne K, Theobald DE, Peña Y, Bruce CC, Huszar AC, Wojcieszek M, Everitt BJ, Robbins TW. Neuropsychopharmacology. 2007 May; 32(5):1195-206. Epub 2006 Oct 11.
- Amphetamines - LiverToxAmphetamines - LiverTox
- Liver Function Tests - StatPearlsLiver Function Tests - StatPearls
- Child Abuse and Neglect - StatPearlsChild Abuse and Neglect - StatPearls
- PIAS2 protein inhibitor of activated STAT 2 [Homo sapiens]PIAS2 protein inhibitor of activated STAT 2 [Homo sapiens]Gene ID:9063Gene
- 9063[uid] AND (alive[prop]) (1)Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...