NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Decitabine is a cytosine analogue and an intravenously administered antineoplastic agent used in the therapy of myelodysplastic syndromes. Decitabine is associated with a low rate of transient serum enzyme elevations during therapy, but has not been implicated in causing clinically apparent liver injury with jaundice.
Background
Decitabine (dee sye' ta been) is a pyrimidine analogue (5-aza-deoxy-cytidine) which is the deoxyribose form of 5-azacitidine. Decitabine is converted intracellularly to a triphosphate which becomes incorporated into DNA and appears to inhibit DNA methylation, resulting in increased expression of silenced genes including tumor suppressor genes. Studies done in vitro and in vivo have shown that decitabine induces differentiation of bone marrow cells and results in normalization of bone marrow in a proportion of patients with myelodysplasia. Decitabine was approved for use in the United States in 2006 and the current single indication is for therapy of myelodysplastic syndromes (MDS). Decitabine is available as a powder or solution for injection in 50 mg vials generically and under the trade name of Dacogen. The usual dosage regimen in adults is 15 to 20 mg/m2 of body surface area given intravenously (iv) in several day regimens, with repeat cycles every 4 or 6 weeks. A minimum of 4 courses is recommended. Common side effects include bone marrow suppression, nausea, vomiting, diarrhea, stomatitis, bruising, abdominal pain, myalgias, headache, dizziness, fatigue, fever, rash and pruritus.
Decitabine is poorly absorbed orally due to its metabolism by cytidine deaminase found in the intestine and liver. In 2020, cedazuridine, an oral inhibitor of cytidine deaminase, was approved for use with a fixed dose of oral decitabine as treatment of adults with MDS. This oral combination therapy yielded similar plasma concentrations as iv administered decitabine and had similar effects on DNA methylation and clinical responses. The adverse event rates to the oral combination therapy were similar to those of iv decitabine, although direct comparisons were limited to single initial cycles of therapy. The oral form of decitabine is available only as a fixed combination with cedazuridine as tablets of 35 mg of decitabine and 100 mg of cedazuridine under the brand name Inqovi. The oral administration of decitabine avoids the inconvenience and difficulties of its iv administration on multiple days of each course of therapy.
Hepatotoxicity
In early clinical trials using high doses of decitabine, serum enzyme elevations occurred in up to 16% of patients with underlying liver disease or liver metastases, but rarely in persons without hepatic illness. In subsequent studies, serum ALT elevations were reported in 5% to 15% of treated patients, but all were self-limited and no clinically apparent liver injury was reported. Recent studies have reported elevations in serum bilirubin levels in 7% to 12% of treated patients, but the elevations resolved rapidly and were not associated with other clinical or laboratory evidence of liver injury. Monitoring of serum enzyme levels during treatment is recommended only in patients with concurrent liver disease.
The oral combination therapy of decitabine with cedazuridine appears to have a similar frequency and pattern of adverse events as iv decitabine alone. In several prospective clinical trials, single cycles of oral vs iv decitabine had similar rates of aminotransferase elevations and long term, multi-course regimens of the oral fixed dose combination therapy resulted in serum aminotransferase elevations in 20% to 37% of patients, which were above 5 times the upper limit of normal (ULN) in 2% to 3% with no cases of clinically apparent liver injury attributable to the chemotherapeutic agent.
Thus, despite widescale use as therapy of MDS, decitabine has not been convincingly linked to cases of clinically apparent liver injury. Nevertheless, the frequency of serum enzyme elevations with therapy make it difficult to say that decitabine is totally without potential for causing drug induced liver injury.
Likelihood score: E* (unproven but suspected, rare cause of clinically apparent liver injury).
Mechanism of Injury
Hepatotoxicity from decitabine appears to be rare and confined mostly to asymptomatic elevations in serum enzymes in patients receiving the highest doses or with underlying liver disease. Thus, the liver injury is likely due to direct toxicity which is generally minimal or mild except in susceptible patients.
Outcome and Management
The severity of the liver injury linked to decitabine therapy is usually mild in severity and without accompanying symptoms or jaundice. Decitabine has not been linked to cases of severe acute hepatitis, acute liver failure, chronic hepatitis or vanishing bile duct syndrome. There is no information on cross sensitivity to hepatic injury between decitabine and other cytidine analogues including azacitidine.
Drug Class: Antineoplastic Agents
Other drugs for myelodysplastic syndromes: Azacitidine, Cedazuridine, Luspatercept
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Decitabine – Generic, Dacogen®
Decitabine and Cedazuridine – Inqovi®
DRUG CLASS
Antineoplastic Agents
COMPLETE LABELING (Decitabine)
COMPLETE LABELING (Decitabine and Cedazuridine)
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Decitabine | 2353-33-5 | 2353-33-5 |
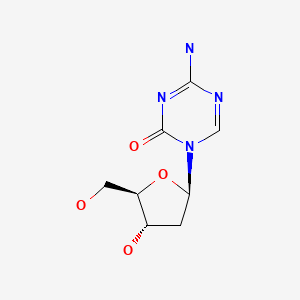
|
ANNOTATED BIBLIOGRAPHY
References updated: 27 July 2023
Abbreviations: AML, acute myelogenous leukemia; iv, intravenous; MDS, myelodysplastic syndrome; sc, subcutaneously.
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999; decitabine is not discussed).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 541-67.(Review of hepatotoxicity of cancer chemotherapeutic agents; decitabine is not discussed).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Inhibitors of histone deacetylase. Pathway targeted therapies: monoclonal antibodies, protein kinase inhibitors, and various small molecules. In, Brunton LL Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, p. 1203-36.(Textbook of pharmacology and therapeutics).
- Wijermans PW, Lü M, Verhoef G, Klimek V, Bosly A. An epigenetic approach to the treatment of advanced MDS; the experience with the DNA demethylating agent 5-aza-2'-deoxycytidine (decitabine) in 177 patients. Ann Hematol 2005; 84: 9-17. [PubMed: 16211386](Pooled results of 3 European trials of decitabine in 177 patients with myelodysplasia; response rate was 49%, liver enzyme elevations occurred in 16% of patients in one study and "liver toxicity" was reported in 1% of patients in another, but no case of clinically apparent liver injury or death from liver disease was reported).
- Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, Klimek V, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 2006; 106: 1794-803. [PubMed: 16532500](Among 170 patients with myelodysplastic syndromes [MDS] treated with decitabine vs standard of care, hyperbilirubinemia occurred in 7% on decitabine vs none receiving standard care; no mention of ALT elevations).
- Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O'Brien S, Cortes J, Faderl S, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 2007; 109: 52-7. [PubMed: 16882708](95 patients with severe myelodysplasia or chronic leukemia were randomized to 3 regimens of decitabine [iv or sc for 5 days, or iv at half dose for 10 days]; ALT elevations occurred in 14 patients [15%] and were above 5 times ULN in 4 [4%], but all resolved and none required dose modification or discontinuation).
- Aribi A, Borthakur G, Ravandi F, Shan J, Davisson J, Cortes J, Kantarjian H. Activity of decitabine, a hypomethylating agent, in chronic myelomonocytic leukemia. Cancer 2007; 109: 713-7. [PubMed: 17219444](Among 19 patients with chronic leukemia treated with decitabine intravenously [iv] or subcutaneously for 5-10 days every 4 weeks, one had ALT elevations above 5 times ULN, but "non-hematologic side effects were minimal").
- Jabbour E, Issa JP, Garcia-Manero G, Kantarjian H. Evolution of decitabine development: accomplishments, ongoing investigations, and future strategies. Cancer 2008; 112: 2341-51. [PMC free article: PMC4784235] [PubMed: 18398832](Review of structure, mechanism of action, pharmacokinetics, efficacy in various conditions and safety of decitabine; hyperbilirubinemia occurred in up to 12% of patients receiving decitabine, but ALT elevations and cause of bilirubin rise were not discussed).
- Steensma DP, Baer MR, Slack JL, Buckstein R, Godley LA, Garcia-Manero G, Albitar M, et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial. J Clin Oncol 2009; 27: 3842-8. [PMC free article: PMC4879689] [PubMed: 19528372](Among 99 patients with MDS treated with decitabine [20 mg/m2] daily for 5 days every 28 days, one patient died of hepatic failure, but no details given).
- Garcia JS, Jain N, Godley LA. An update on the safety and efficacy of decitabine in the treatment of myelodysplastic syndromes. Onco Targets Ther 2010; 3: 1-13. [PMC free article: PMC2895778] [PubMed: 20616953](Systematic review of safety and efficacy of decitabine in MDS; liver dysfunction was reported in 1-11% of patients, but details not provided or discussed).
- Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, Gau JP, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012; 30: 2670-7. [PMC free article: PMC4874148] [PubMed: 22689805](Among 485 patients with AML treated with decitabine, cytarabine or supportive care, adverse events were similar with decitabine and cytarabine; no discussion of ALT elevations or liver injury).
- Lee YG, Kim I, Yoon SS, Park S, Cheong JW, Min YH, Lee JO, et al. Comparative analysis between azacitidine and decitabine for the treatment of myelodysplastic syndromes. Br J Haematol 2013; 161: 339-47. [PubMed: 23432512](Observational study comparing safety and efficacy of azacitidine vs decitabine in 300 Korean patients with MDS, found similar rates of efficacy and side effects; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 [5.5%] were attributed to antineoplastic agents, but none were linked to decitabine treatment).
- Salim O, Toptas T, Avsar E, Yucel OK, Ozturk E, Ferhanoglu B, Geduk A, et al. Azacitidine versus decitabine in patients with refractory anemia with excess blast-results of multicenter study. Leuk Res 2016; 45: 82-9. [PubMed: 27107658](Among 88 patients with refractory anemia treated with azacitidine or decitabine followed at 6 Turkish referral centers, overall survival, transfusion requirements and rates of adverse events and acute leukemia were similar in the two groups; no mention of ALT elevations or hepatotoxicity).
- Nieto M, Demolis P, Béhanzin E, Moreau A, Hudson I, Flores B, Stemplewski H, et al. The European Medicines Agency review of decitabine (Dacogen) for the treatment of adult patients with acute myeloid leukemia: summary of the scientific assessment of the Committee for Medicinal Products for Human Use. Oncologist 2016; 21: 692-700. [PMC free article: PMC4912358] [PubMed: 27091416](Review of the efficacy and safety of decitabine by a European expert panel concluded that its use should be limited to adults over the age of 65 with AML who are not candidates for standard induction therapy; and that adverse events are similar to those of low dose cytarabine, largely due to myelosuppression; no mention of ALT elevations or hepatotoxicity).
- Jabbour E, Short NJ, Montalban-Bravo G, Huang X, Bueso-Ramos C, Qiao W, Yang H, et al. Randomized phase 2 study of low-dose decitabine vs low-dose azacitidine in lower-risk MDS and MDS/MPN. Blood 2017; 130: 1514-22. [PMC free article: PMC5620419] [PubMed: 28774880](Among 113 patients with lower-risk MDS treated with low doses of azacitidine or decitabine, overall response rates favored decitabine [70% vs 49%], as did rates of transfusion independence and cytogenetic responses, and both therapies were "overall well tolerated"; no mention of ALT elevations or hepatotoxicity).
- Savona MR, Odenike O, Amrein PC, Steensma DP, DeZern AE, Michaelis LC, Faderl S, et al. An oral fixed-dose combination of decitabine and cedazuridine in myelodysplastic syndromes: a multicentre, open-label, dose-escalation, phase 1 study. Lancet Haematol. 2019;6:e194-e203. [PubMed: 30926081](Among 44 patients with various MDS treated with intravenous (iv) decitabine or various combinations of oral decitabine and cedazuridine, oral doses of 30 or 40 mg of decitabine and 100 mg of cedazuridine yielded 5-day area under the curve concentrations most similar to iv decitabine with similar adverse event rates and ALT elevations in 20% of subjects, all values being less than 3 times ULN).
- Zhou H, Qin P, Liu Q, Yuan C, Hao Y, Zhang H, Wang Z, et al. A prospective, multicenter study of low dose decitabine in adult patients with refractory immune thrombocytopenia. Am J Hematol. 2019;94:1374-1381. [PubMed: 31591739](Among 45 adults with refractory idiopathic thrombocytopenic purpura treated with low doses of decitabine [3.5 mg/m2 for 3 days in three 28-day cycles], 23 had a clinical response usually within 14-70 days, some requiring retreatment, with a 29% adverse event rate [none severe] including 2 patients with ALT elevations, both of which were transient and less than 3 times ULN ).
- Lee BH, Kang KW, Jeon MJ, Yu ES, Kim DS, Choi H, Lee SR, et al. Comparison between 5-day decitabine and 7-day azacitidine for lower-risk myelodysplastic syndromes with poor prognostic features: a retrospective multicentre cohort study. Sci Rep. 2020;10:39. [PMC free article: PMC6949213] [PubMed: 31913293](Among 111 adults with low- or intermediate-risk MDS treated with iv decitabine [20 mg/m2 for 5 days] or azacitidine [75 mg/m2 for 7 days], overall response rates were higher with decitabine [67% vs 44%] while adverse event rates were similar; no mention of ALT elevations or hepatotoxicity).
- Dhillon S. Decitabine/Cedazuridine: first approval. Drugs. 2020;80:1373-1378. [PMC free article: PMC7708383] [PubMed: 32860582](Review of the mechanism of action, history of development, pharmacokinetics, clinical efficacy, and safety of the combination of oral decitabine and cedazuridine shortly after its approval for use in MDS in the US, discusses adverse events of myelosuppression and gastrointestinal upset but does not mention ALT elevations or hepatotoxicity).
- Garcia-Manero G, Griffiths EA, Steensma DP, Roboz GJ, Wells R, McCloskey J, Odenike O, et al. Oral cedazuridine/decitabine for MDS and CMML: a phase 2 pharmacokinetic/pharmacodynamic randomized crossover study. Blood. 2020;136:674-683. [PMC free article: PMC7414597] [PubMed: 32285126](Among 80 patients with MDS or chronic myelomonocytic leukemia treated with oral cedazuridine/decitabine or iv decitabine alone for 5 days in cycle one with cross over to the other regimen in cycle 2, area-under-the-curve plasma concentrations were similar in both groups as were demethylation rates and the clinical response rate with continued cycles of combination therapy was 60%; common adverse events were neutropenia and thrombocytopenia; no mention of ALT elevations or hepatotoxicity).
- Sekeres MA, Taylor J. Diagnosis and treatment of myelodysplastic syndromes: a review. JAMA. 2022;328:872-880. [PubMed: 36066514](Concise review of clinical features, natural history, diagnosis, and management of MDS; adverse events are listed in tables but without mention or discussion of ALT elevations or hepatotoxicity).
- Kim N, Norsworthy KJ, Subramaniam S, Chen H, Manning ML, Kitabi E, Earp J, et al. FDA approval summary: decitabine and cedazuridine tablets for myelodysplastic syndromes. Clin Cancer Res. 2022;28:3411-3416. [PMC free article: PMC9378483] [PubMed: 35435961](Summary of the basis for the FDA approval of an oral fixed dose combination of decitabine and cedazuridine with efficiency and safety results from a phase 3 open-label trial in 133 patients which demonstrated complete remissions in 18-21% of subjects and with an adverse event profile similar to that of iv decitabine; ALT elevations arose in 37% of patients but were above 5 times ULN in only 2%; no mention of serious hepatic adverse events).
- Liu H, Jiang H, Tong H, Xia R, Yang L, Zhao H, Ouyang J, et al. Decitabine in patients with myelodysplastic syndromes: A multi-center, open-label, dose comparison trial. Cancer Med. 2023;12:13885-13893. [PMC free article: PMC10358210] [PubMed: 37350499](Among 191 adults with intermediate- or high-risk MDS treated with decitabine in a standard [20 mg/m2 for 5 days] or extended regimen [12 mg/m2 for 8 days] per each of 4 cycles, overall response rates were similar [42% vs 38%] as were adverse event rates, including for grade 3 or above “hepatobiliary” events which arose in 3.2% vs 5.2%).
- Garcia-Manero G. Myelodysplastic syndromes: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98:1307-1325. [PubMed: 37288607](Review of the classification of MDS and risk categorization with an update on management including discussion of hypomethylation agents and luspatercept; no mention of ALT elevations or hepatotoxicity).
- Randall MP, DeZern AE. The management of low-risk myelodysplastic syndromes-current standards and recent advances. Cancer J. 2023;29:152-159. [PubMed: 37195771](Review of treatment of low-risk MDS including erythropoietin, iron chelation, lenalidomide, luspatercept and low dose hypomethylating agents; no discussion of hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Azacitidine.[LiverTox: Clinical and Researc...]Review Azacitidine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Cytarabine.[LiverTox: Clinical and Researc...]Review Cytarabine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Cedazuridine.[LiverTox: Clinical and Researc...]Review Cedazuridine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Gemcitabine.[LiverTox: Clinical and Researc...]Review Gemcitabine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Clofarabine.[LiverTox: Clinical and Researc...]Review Clofarabine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Decitabine - LiverToxDecitabine - LiverTox
- Baclofen - StatPearlsBaclofen - StatPearls
- HNRNPK heterogeneous nuclear ribonucleoprotein K [Homo sapiens]HNRNPK heterogeneous nuclear ribonucleoprotein K [Homo sapiens]Gene ID:3190Gene
- 3190[uid] AND (alive[prop]) (1)Gene
- Human papillomavirus type 9 genomic DNAHuman papillomavirus type 9 genomic DNAgi|397068|emb|X74464.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...