NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Acyclovir is a nucleoside analogue and antiviral agent used in therapy of herpes and varicella-zoster virus infections. Acyclovir has not been associated with clinically apparent liver injury.
Background
Acyclovir (ay sye' kloe vir) is an acyclic purine nucleoside analogue (acycloguanosine) which has antiviral activity against many herpes viruses, including herpes simplex 1 and 2, cytomegalovirus, Ebstein-Barr virus and varicella-zoster. Acyclovir is phosphorylated intracellularly by viral kinases, and the resultant triphosphate competes with guanosine for incorporation into viral DNA blocking viral DNA polymerase activity. Acyclovir is indicated for therapy of localized as well as disseminated herpes simplex infections, both type 1 and 2. It is also used for varicella-zoster infections (chickenpox and shingles). Acyclovir was approved for use in herpes virus infections in the United States in 1982, and is still widely used in treatment and prophylaxis of genital and mucocutaneous herpes simplex infection with almost 5 million prescriptions filled yearly. Acyclovir is available as capsules of 200 mg, tablets of 400 and 800 mg, oral suspensions, creams, ointments, and parenteral preparations in several generic forms, as well as under the brand name of Zovirax. The typical recommended oral dose in adults for genital or oral herpes simplex is 200 to 800 mg three to five times daily for 5 to 10 days; the usual prophylactic dose is 400 mg twice daily. The typical intravenous doses for severe infections is 5 to 10 mg/kg every 8 hours for 5 to 10 days. Side effects are uncommon with oral formulations, but can include myalgias, rash, temors, lethargy and confusion. Rare side effects include bone marrow toxicity and Stevens Johnson syndrome.
Hepatotoxicity
Despite widespread use, there is little evidence that acyclovir when given orally causes significant liver injury. Serum enzyme levels generally do not change during oral acyclovir therapy. High dose intravenous administration of acyclovir is associated with renal dysfunction and thrombocytopenia, and occasionally with transient mild-to-moderate elevations in serum ALT levels, which have been asymptomatic and self-limited. There have rare instances of acute, clinically apparent liver injury reported that were attributed to acyclovir or valacyclovir (a prodrug of acyclovir with better oral absorption), but these have not been particularly convincing. Some degree of liver injury and even jaundice can occur during the course of herpes simplex or varicella zoster infection, and these complications could be mistaken for drug induced liver injury. Furthermore, in the reported cases, patients were receiving other medications and had other unlying comorbidities that may have been responsible for the liver injury.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
Acyclovir is metabolized intracellularly in viral infected cells and is minimally metabolized by the liver. Acyclovir is excreted largely unchanged by the kidneys, perhaps accounting for the absence or rarity of hepatic injury.
Drug Class: Antiviral Agents
Other Antiviral Agents for Herpes Virus Infections: Cidofovir, Famciclovir, Foscarnet, Ganciclovir, Letermovir, Valacyclovir, Valganciclovir
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Acyclovir – Zovirax®
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
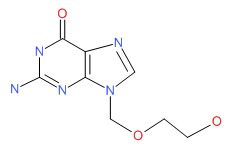
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Acyclovir | 59277-89-3 | C8-H11-N5-O3 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 04 February 2016
- Zimmerman HJ. Antiviral agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 621-3.(Expert review of antiviral agents and liver injury published in 1999; mentions that acyclovir has not caused "overt hepatic injury").
- Nunez M. Herpesviridae treatment. Hepatic toxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 512-3.(Review of hepatotoxicity of antiviral agents; mentions that there have been postmarketing reports of ALT elevaitons, hepatitis and jaundice due to acyclovir).
- Acosta EP, Flexner C. Antiviral agents(nonretroviral). In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1593-1622.(Textbook of pharmacology and therapeutics).
- Straus SE, Takiff HE, Seidlin M, Bachrach S, Lininger L, Di Giovanna JJ, Western KA, et al. Suppression of frequently recurring genital herpes. A placebo-controlled double-blind trial of oral acyclovir. N Engl J Med 1984; 310: 1545-50. [PubMed: 6328297](Placebo controlled trial of acyclovir in 35 patients with recurrent genital herpes; one patient had ALT elevations [88 U/L] that resolved even during continuation of therapy).
- O'Brien JJ, Campoli-Richards DM. Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1989; 37: 233-309. [PubMed: 2653790](Review of antiviral activity, mechanism of action, pharmacokinetics, clinical efficacy and adverse effects of acyclovir; side effects are usually mild, but high doses intravenously are associated with nausea, vomiting, lightheadedness, neurologic symptoms, and renal dysfunction; no mention of hepatotoxicity or ALT elevations).
- Tilson HH, Engle CR, Andrews EB. Safety of acyclovir: a summary of the first 10 years experience. J Med Virol 1993; Suppl 1: 67-73. [PubMed: 8245895](Between 10-15 million persons have been treated with acyclovir, but the sponsor received only 923 adverse event reports: 129 considered serious, but no specific mention of hepatotoxicity or ALT elevations).
- Styrt B, Freiman JP. Hepatotoxicity of antiviral agents. Gastroenterol Clin North Am 1995; 24: 839-52. [PubMed: 8749901](Review of liver toxicity of antiviral agents; acyclovir may be associated with minor liver test elevations, but there have been no published reports of it causing clinically apparent liver injury).
- Ormrod D, Scott LJ, Perrry CM. Valaciclovir: a review of its long term utility in the management of genital herpes simplex virus and cytomegalovirus infections. Drugs 2000; 59: 839-63. [PubMed: 10804039](Review of efficacy and safety of long term valaciclovir use; rates of side effects are similar in frequency to those in patients on placebo; a single case of hepatitis due to valaciclovir has been reported in abstract form).
- Bodsworth NJ, Crooks RJ, Borelli S, Vejlsgaard G, Paavonen J, Worm A-M, Uexkull N, et al., International Valaciclovir HSV Study Group. Genitourin Med 1997; 73: 110-6. [PMC free article: PMC1195783] [PubMed: 9215092](999 patients randomized at 48 sites to acyclovir or valacyclovir for 5 days for recurrent HSV infection; equivalent efficacy and "no clinically important changes from screening in any clinical chemistry variable").
- Renkes P, Trechot P, Blain H. Valaciclovir-induced hepatitis. Acta Clin Belg 1999; 54: 17-8. [PubMed: 10192972](71 year old woman with shingles developed abdominal pain 7 days after starting valacyclovir and 3 g/day of acetaminophen [bilirubin 3.3 mg/dL, ALT 376 U/L, Alk P 246 U/L], resolving within 2 weeks of stopping).
- Simpson D, Lyseng-Williamson KA. Famciclovir: a review of its use in herpes zoster and genital and orolabial herpes. Drugs 2006; 66: 2397-416. [PubMed: 17181386](Review of famciclovir, oral prodrug of penciclovir, used in herpes zoster and simplex virus infections for limited period as therapy and extended use for suppression; in suppression studies, ALT elevations above twice ULN occurred in 3.2% of famciclovir vs 1.5% of placebo recipients; no hepatic serious adverse events reported).
- Drugs for non-HIV viral infections. Treat Guidel Med Lett 2007; 5: 59-70. [PubMed: 17565338](Review of status of non-antiretroviral antiviral agents for prevention and treatment of herpes, varicella-zoster, cytomegalovirus, influenza A and B, and hepatitis B and C; no mention of liver related side effects for acyclovir).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 8 were attributed to antiviral agents including one attributed to valacyclovir, but none to acyclovir).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, 4 of which were due to antiretroviral agents, but none were attributed to an antiherpes virus agent).
- Antiviral drugs. Treat Guidel Med Lett 2013; 11 (127): 19-30. [PubMed: 23459414](Review of safety and efficacy of acyclovir treatment and prophylaxis against varicella and herpes zoster infections; mentions oral acyclovir has been linked to cases of Stevens Johnson syndrome, but does not specifically mention liver injury).
- Gopal MG, Shannoma, Kumar B C S, M R, A S N, Manjunath NC. A comparative study to evaluate the efficacy and safety of acyclovir and famciclovir in the management of herpes zoster. J Clin Diagn Res 2013; 7: 2904-7. [PMC free article: PMC3919380] [PubMed: 24551671](Among 100 patients with herpes zoster treated for 7 days with either acyclovir or famciclovir, there was no "clincally significant difference" in serum biochemistry tests between the two groups).
- Pugi A, Bonaiuti R, Maggini V, Moschini M, Tuccori M, Leone R, Rossi M, et al. Safety profile of antiviral medications: a pharmacovigilance study using the Italian spontaneous-reporting database. Am J Health Syst Pharm 2013; 70: 1039-46. [PubMed: 23719881](Analysis of adverse drug events reported spontaneous during a 22 year period in Italy identified 863 reports involving antivirals, but liver related events were not among the 20 most reported reactions).
- Tachibana T, Nozaki A, Enaka M, Yamamoto E, Kawasaki R, Koharazawa H, Hagihara M, et al. Drug-induced liver injury after allogeneic bone marrow transplantation. Int J Hematol 2013; 98: 499-503. [PubMed: 24037455](23 year old woman with acute leukemia and allogeneic bone marrow transplant developed jaundice [bilirubin 14.0 mg/dL, ALT 1379 U/L, Alk P 1389 U/L] 180 days after transplant, while receiving oral acyclovir [for 104 days], voriconazole [60 days] and rebamipide [97 days], as well as tacrolimus and lansoprazole; a positive lymphocyte stimulation test was found for acyclovir, but not the other agents).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to acyclovir or any other antiviral agent).
- Kendrick JG, Ensom MH, Steer A, White CT, Kwan E, Carr RR. Standard-dose versus high-dose acyclovir in children treated empirically for encephalitis: a retrospective cohort study of its use and safety. Paediatr Drugs 2014; 16: 229-34. [PubMed: 24497110](Among 61 children with herpes simplex encephalitis treated with high or standard doses of intravenous acyclovir, "elevated liver enzymes" occurred in 4 [6.5%], 2 at each dose level; no mention of outcome, symptoms or jaundice).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-1352. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one case was attributed to acyclovir but its likelihood was judged to be only possible).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Valacyclovir.[LiverTox: Clinical and Researc...]Review Valacyclovir.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- In vitro susceptibility of varicella-zoster virus to acyclovir.[Antimicrob Agents Chemother. 1...]In vitro susceptibility of varicella-zoster virus to acyclovir.Biron KK, Elion GB. Antimicrob Agents Chemother. 1980 Sep; 18(3):443-7.
- Review Acyclovir-induced neurotoxicity with a positive cerebrospinal fluid varicella zoster PCR result creating a management dilemma: a case report.[J Med Case Rep. 2020]Review Acyclovir-induced neurotoxicity with a positive cerebrospinal fluid varicella zoster PCR result creating a management dilemma: a case report.Robertson KM, Harvey CL, Cunningham JM. J Med Case Rep. 2020 Sep 18; 14(1):156. Epub 2020 Sep 18.
- Review Herpes Simplex Virus and Varicella Zoster Virus Infections in Cancer Patients.[Viruses. 2023]Review Herpes Simplex Virus and Varicella Zoster Virus Infections in Cancer Patients.Tayyar R, Ho D. Viruses. 2023 Feb 5; 15(2). Epub 2023 Feb 5.
- Review Antiviral therapy for varicella and herpes zoster.[Semin Pediatr Infect Dis. 2002]Review Antiviral therapy for varicella and herpes zoster.Arvin AM. Semin Pediatr Infect Dis. 2002 Jan; 13(1):12-21.
- Acyclovir - LiverToxAcyclovir - LiverTox
- Diet, inflammation, and glycemic control in type 2 diabetes: an integrative revi...Diet, inflammation, and glycemic control in type 2 diabetes: an integrative review of the literature - Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews
- Oogenesis - Developmental BiologyOogenesis - Developmental Biology
- BAX BCL2 associated X, apoptosis regulator [Homo sapiens]BAX BCL2 associated X, apoptosis regulator [Homo sapiens]Gene ID:581Gene
- 581[uid] AND (alive[prop]) (1)Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...