NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Zanamivir is an inhibitor of the influenza neuraminidase enzyme and is given by inhalation as therapy and prophylaxis against influenza A and B. Zanamivir has not been associated with clinically apparent liver injury, at least when given by inhalation.
Background
Zanamivir (za nam' i vir) is a sialic acid analogue and a potent inhibitor of the neuraminidase of influenza viruses. Inhibition of this enzyme causes a decrease in viral replication, probably as a result of interference with particle formation and release. Zanamivir is active against both influenza A and B viruses, but has no activity against other common upper respiratory tract viruses. In addition, resistance to zanamivir can develop rapidly. Zanamivir is indicated for therapy or post-exposure prevention of influenza A or B. Zanamivir was approved in the United States in 1999 and is frequently used during influenza outbreaks. Zanamivir is available as a powder for inhalation (5 mg/blister pack) under the brand name of Relenza. The recommended dose for therapy in adults is 2 oral inhalations of 10 mg each, twice daily for 5 days; the usual prophylactic regimen is 10 mg once daily for 10 days, starting within 2 days of close contact with an infected person or for 28 days in a community setting. Side effects are uncommon and include mild nausea, dizziness, headache, cough, nasal and throat irritation and bronchospasm. Rare but potentially severe adverse reactions include hypersensitivity reactions, severe bronchospasm and neuropsychiatric syndromes including disorientation, hallucinations and mania.
Hepatotoxicity
In randomized controlled trials, 2% to 3% of zanamivir recipients developed ALT or AST elevations above twice the upper limit of the normal range, but a similar rate was found in placebo-treated patients. Despite widespread use, there is little evidence that zanamivir when used by inhalation causes liver injury, either in the form of asymptomatic serum enzyme elevations or clinically apparent liver disease. In pilot studies of intravenous zanamivir for severe influenza, serum enzyme elevations have been reported in ~10% of patients, occasionally with jaundice, but the role of zanamivir versus the underlying severe viral infection has not been defined.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Zanamivir has little hepatic metabolism and does not affect cytochrome P450 (CYP) activity. The typical course of zanamivir is for 5 to 10 days only, and the brief exposure and minimal hepatic metabolism may account for its absence of hepatotoxicity when given by inhalation.
Drug Class: Antiviral Agents
Other Drugs in the Class for Influenza: Amantadine, Baloxavir, Oseltamivir, Peramivir, Rimantadine
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Zanamivir – Relenza®
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
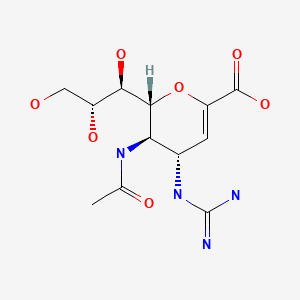
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Zanamivir | 139110-80-8 | C12-H20-N4-O7 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 22 June 2020
- Zimmerman HJ. Antiviral agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 621-3.(Expert review of antiviral agents and liver injury published in 1999; amantadine and rimantadine have not caused "overt hepatic injury" and oseltamivir and zanamivir are not mentioned).
- Núñez M. Influenza virus treatments. Hepatic toxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 513.(Review of hepatotoxicity of antiviral agents; zanamivir is not discussed).
- Acosta EP. Antiviral agents (nonretroviral). In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1105-18.(Textbook of pharmacology and therapeutics).
- Hayden FG, Osterhaus AD, Treanor JJ, Fleming DM, Aoki FY, Nicholson KG, Bohnen AM, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. GG167 Influenza Study Group. N Engl J Med. 1997;337:874–80. [PubMed: 9302301](Two controlled trials of 5 day course of zanamivir or placebo in 417 adults with an influenza-like illness; "No drug-related effects on blood counts, blood chemical values, or urinalysis results were found").
- Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. The MIST (Management of Influenza in the Southern Hemisphere Trialists) Study Group. Lancet. 1998;352:1877–81. [PubMed: 9863784](Controlled trial of 5 day course of zanamivir vs placebo in 455 patients with influenza-like symptoms; "Laboratory results and vital signs did not differ between the two groups").
- Freund B, Gravenstein S, Elliott M, Miller I. Zanamivir: a review of clinical safety. Drug Saf. 1999;21:267–81. [PubMed: 10514019](Extensive review of safety and tolerance of zanamivir given by inhalation; ALT and AST elevations above twice the upper limit of normal occurred in 2-3% of zanamivir and 2% of placebo recipients; no mention of clinically apparent liver injury).
- McNicholl IR, McNicholl JJ. Neuraminidase inhibitors: zanamivir and oseltamivir. Ann Pharmacother. 2001;35:57–70. [PubMed: 11197587](Review of efficacy and safety of both zanamivir and oseltamivir; no mention of hepatotoxicity or ALT elevations).
- Kaji M, Fukuda T, Tanaka M, Aizawa H. A side effect of neuraminidase inhibitor in a patient with liver cirrhosis. J Infect Chemother. 2005;11:41–3. [PubMed: 15729487](Two patients [a man and a woman, ages 66 and 70] with cirrhosis and hepatocellular carcinoma received zanamivir after exposure to influenza and developed rash and fever 1-2 days later, but without any changes in the already elevated serum enzymes and bilirubin).
- Jefferson T, Demicheli V, Rivetti D, Jones M, Di Pietrantonj C, Rivetti A. Antivirals for influenza in healthy adults: systematic review. Lancet. 2006;367:303–13. [PubMed: 16443037](Analysis of 51 reports of 52 controlled trials of antivirals for influenza; "Neuraminidase inhibitors are not associated with any adverse events when used as treatment as opposed to prophylaxis").
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the United States collected between 2004 and 2008, 8 were attributed to antiviral agents, but none to anti-influenza medications).
- Antiviral drugs for influenza. Med Lett Drugs Ther. 2009;51:89–92. [PubMed: 20220738](Review of status of antiviral agents for prevention and treatment of influenza A and B; no mention of hepatic toxicity of any of the anti-influenza agents).
- Antiviral drugs. Treat Guidel Med Lett. 2013;11(127):19–30. [PubMed: 23459414](Review of status of non-antiretroviral antiviral agents; no mention of hepatotoxicity in discussion of side effects of zanamivir).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to a drug used to treat influenza).
- Heneghan CJ, Onakpoya I, Thompson M, Spencer EA, Jones M, Jefferson T. Zanamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ. 2014;348:g2547. [PMC free article: PMC3981976] [PubMed: 24811412](Systematic review of 26 clinical trials of zanamivir for treatment and prevention of influenza found no evidence for increased risk of harm or adverse events).
- Marty FM, Man CY, van der Horst C, Francois B, Garot D, Mánez R, Thamlikitkul V, et al. Safety and pharmacokinetics of intravenous zanamivir treatment in hospitalized adults with influenza: an open-label, multicenter, single-arm, phase II study. J Infect Dis. 2014;209:542–50. [PMC free article: PMC4047294] [PubMed: 23983212](Among 130 adults with influenza treated with intravenous zanamivir for a median of 5 days, acute, but self-limited liver injury was reported in 17 patients [13%] and was considered severe in 3 [2%] based upon ALT levels above 3 times ULN and jaundice).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 12 cases [1.3%] were attributed to antiviral agents, but none to antivirals used to treat influenza).
- Chan-Tack KM, Kim C, Moruf A, Birnkrant DB. Clinical experience with intravenous zanamivir under an Emergency IND program in the United States (2011-2014). Antivir Ther. 2015;20:561–4. [PubMed: 25667992](Among 364 patients with severe, life-threatening influenza who received emergency authorization for zanamivir therapy between 2011 and 2014, 87% required mechanical ventilation and among 135 with outcome data, the mortality rate was 62% and the adverse event rate 43% [23/53 with data] including 7 patients with “increasing liver function tests”).
- Marty FM, Vidal-Puigserver J, Clark C, Gupta SK, Merino E, Garot D, Chapman MJ, et al. Intravenous zanamivir or oral oseltamivir for hospitalized patients with influenza: an international, randomised, double-blind, double-dummy, phase 3 trial. Lancet Respir Med. 2017;5:135–46. [PubMed: 28094141](Among 626 patients with symptoms of influenza [488 confirmed] treated with zanamivir [300 or 600 mg] or oseltamivir twice daily for 5-10 days, time to clinical improvement was similar in all three groups as were adverse events including respiratory failure and death; ALT elevations arose in 1.2% of zanamivir vs 2.0% of oseltamivir treated subjects).
- Bradley JS, Blumer JL, Romero JR, Michaels MG, Munoz FM, Kimberlin DW, Pahud B, et al. Intravenous zanamivir in hospitalized patients with influenza. Pediatrics. 2017;140:e20162727. [PubMed: 29051331](Among 71 children hospitalized for severe influenza treated with zanamivir twice daily for median of 5 days, clinical improvement occurred in most, 5 children died [7%] and treatment related adverse events were similar to those described in adults, and “no clinically significant trends were observed for ALT or total bilirubin levels”).
- Cleary PR, Crofts J, Parry-Ford F, Chand M, Phin N. Characteristics and mortality of severe influenza cases treated with parenteral aqueous zanamivir, United Kingdom, October 2009 to January 2011. Influenza Other Respir Viruses. 2019;13:44–53. [PMC free article: PMC6304314] [PubMed: 30137684](Among 185 patient with laboratory confirmed severe influenza treated with intravenous zanamivir for up to 21 days during the 2009-2011 influenza seasons in the UK, 55% recovered, 10% had permanent sequelae and 34% died; “some adverse events were noted’ but it “was not possible to distinguish the effects of zanamivir from progression of disease”).
- Antiviral drugs for treatment and prophylaxis of seasonal influenza. Med Lett Drugs Ther. 2019;61(1563):1–4. [PubMed: 30681660](Concise review of the drug therapy of influenza mentions that there are no data suggesting the superiority of one drug over another, they are all approved for treatment of uncomplicated influenza, should be started as soon as possible, and have been shown to shorten the duration of symptoms by one day in adults; no mention of ALT elevations or hepatotoxicity of any of the neuraminidase inhibitors).
- Antiviral drugs for influenza. Med Lett Drugs Ther. 2020;62(1589):1–4. [PubMed: 31999661](Concise review of the drug therapy for treatment and prevention of influenza mentions that zanamivir is approved as inhalation therapy twice daily for 5 days for treatment and once daily for 7 days for prevention, and adverse events can include diarrhea, nausea, sinusitis, fever, arthralgia and bronchospasm; no mention of ALT elevations or hepatic adverse events).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group.[N Engl J Med. 1997]Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group.Hayden FG, Osterhaus AD, Treanor JJ, Fleming DM, Aoki FY, Nicholson KG, Bohnen AM, Hirst HM, Keene O, Wightman K. N Engl J Med. 1997 Sep 25; 337(13):874-80.
- Coadministration of orally inhaled zanamivir with inactivated trivalent influenza vaccine does not adversely affect the production of antihaemagglutinin antibodies in the serum of healthy volunteers.[Clin Pharmacokinet. 1999]Coadministration of orally inhaled zanamivir with inactivated trivalent influenza vaccine does not adversely affect the production of antihaemagglutinin antibodies in the serum of healthy volunteers.Webster A, Boyce M, Edmundson S, Miller I. Clin Pharmacokinet. 1999; 36 Suppl 1:51-8.
- Safety and efficacy of the neuraminidase inhibitor zanamivir in treating influenza virus infection in adults: results from Japan. GG167 Group.[Antivir Ther. 1999]Safety and efficacy of the neuraminidase inhibitor zanamivir in treating influenza virus infection in adults: results from Japan. GG167 Group.Matsumoto K, Ogawa N, Nerome K, Numazaki Y, Kawakami Y, Shirato K, Arakawa M, Kudoh S, Shimokata K, Nakajima S, et al. Antivir Ther. 1999; 4(2):61-8.
- Review Neuraminidase inhibitors for treatment and prophylaxis of influenza in children: systematic review and meta-analysis of randomised controlled trials.[BMJ. 2009]Review Neuraminidase inhibitors for treatment and prophylaxis of influenza in children: systematic review and meta-analysis of randomised controlled trials.Shun-Shin M, Thompson M, Heneghan C, Perera R, Harnden A, Mant D. BMJ. 2009 Aug 10; 339:b3172. Epub 2009 Aug 10.
- Review Neuraminidase inhibitors: zanamivir and oseltamivir.[Ann Pharmacother. 2001]Review Neuraminidase inhibitors: zanamivir and oseltamivir.McNicholl IR, McNicholl JJ. Ann Pharmacother. 2001 Jan; 35(1):57-70.
- Zanamivir - LiverToxZanamivir - LiverTox
- Nitrofurantoin - LiverToxNitrofurantoin - LiverTox
- Physiology, Bohr Effect - StatPearlsPhysiology, Bohr Effect - StatPearls
- Uncomplicated Urinary Tract Infections - StatPearlsUncomplicated Urinary Tract Infections - StatPearls
- A Quality Assurance Tool for JATS/BITS with Schematron and HTML reporting - Jour...A Quality Assurance Tool for JATS/BITS with Schematron and HTML reporting - Journal Article Tag Suite Conference (JATS-Con) Proceedings 2016
Your browsing activity is empty.
Activity recording is turned off.
See more...