NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Quinapril is an angiotensin-converting enzyme (ACE) inhibitor used in the therapy of hypertension and congestive heart failure. Quinapril is associated with a low rate of transient serum aminotransferase elevations, but has yet to be linked to instances of acute liver injury.
Background
Quinapril (kwin' a pril] is an ACE inhibitor which is approved for use alone and in combination with other agents in the therapy of hypertension. Like other ACE inhibitors, quinapril inhibits the conversion of angiotensin I, a relatively inactive molecule, to angiotensin II which is the major mediator of vasoconstriction and volume expansion induced by the renin-angiotensin system. Other host enzymes besides that which converts angiotensin I to II may be inhibited as well, which may account for some of the side effects of the ACE inhibitors. Quinapril was approved for use in the United States in 1991 and current indications are for therapy of hypertension and heart failure. Quinipril is available in 5, 10, 20 and 40 mg tablets in many generic forms and under the trade name Accupril. The typical daily dose in adults is 10 to 40 mg in one or two divided doses which is administered long term. Quinapril is also available in fixed dose combinations with hydrochlorothiazide (Accuretic). Common side effects include dizziness, fatigue, headache, cough, gastrointestinal upset and skin rash.
Hepatotoxicity
Quinapril, like other ACE inhibitors, has been associated with a low rate of serum aminotransferase elevations (<2%) that, in controlled trials, was no higher than with placebo therapy. These elevations were transient and rarely required dose modification. Clinically apparent cases of acute liver injury due to quinapril have yet to be published. However, most ACE inhibitors have been associated with rare instances of clinically apparent liver injury, which typically arises 2 to 12 weeks after starting therapy and is associated with a cholestatic pattern of injury which can be severe and prolonged. Immunoallergic manifestations (rash, fever, eosinophilia) are infrequent and most patients do not develop autoantibodies. Rare instances of severe acute hepatocellular injury, sometimes arising 1 to 4 years after starting ACE inhibitors, have been described. The product label for quinapril mentions the possibiltiy of drug induced liver injury.
Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the minor serum aminotransferase elevations associated ACE inhibitors including quinapril is not known. Quinapril is hydrolyzed in the liver to the active metabolite quinaprilat, but undergoes minimal further hepatic metabolism which may explain why it rarely causes clinically apparent hepatotoxicity.
Outcome and Management
There have been no instances of quinapril associated liver injury described to provide an overall description of its course and outcome. Most instances of acute liver injury reported with ACE inhibitors have been self limited, but there have been rare reports of acute liver failure due to captopril, enalapril, lisinopril and benazepril and several reports of cholestatic hepatitis due to ACE inhibitors leading to prolonged jaundice and vanishing bile duct syndrome. Patients with severe quinapril induced acute liver injury should avoid use of other ACE inhibitors, although cross sensitivity to liver injury among the members of this class of agents has not always been shown.
References to the safety and potential hepatotoxicity of quinapril are given in the Overview section on the Angiotensin-Converting Enzyme (ACE) Inhibitors.
Drug Class: Antihypertensive Agents, Angiotensin-Converting Enzyme Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Quinapril – Generic, Accupril®
DRUG CLASS
Angiotensin-Converting Enzyme Inhibitors
Product labeling at DailyMed, National Library of Medicine, NIH
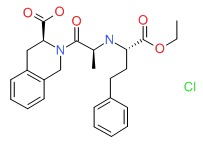
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Quinapril | 82586-55-8 | C25-H30-N2-O5.Cl-H |
 |
- PubChem SubstanceRelated PubChem Substances
- Review Quinapril. A reappraisal of its pharmacology and therapeutic efficacy in cardiovascular disorders.[Drugs. 1994]Review Quinapril. A reappraisal of its pharmacology and therapeutic efficacy in cardiovascular disorders.Plosker GL, Sorkin EM. Drugs. 1994 Aug; 48(2):227-52.
- Clinical pharmacology of quinapril in healthy volunteers and in patients with hypertension and congestive heart failure.[Angiology. 1989]Clinical pharmacology of quinapril in healthy volunteers and in patients with hypertension and congestive heart failure.Sedman AJ, Posvar E. Angiology. 1989 Apr; 40(4 Pt 2):360-9.
- Haemodynamic response and pharmacokinetics after the first dose of quinapril in patients with congestive heart failure.[Br J Clin Pharmacol. 1994]Haemodynamic response and pharmacokinetics after the first dose of quinapril in patients with congestive heart failure.Squire IB, Macfadyen RJ, Lees KR, Hillis WS, Reid JL. Br J Clin Pharmacol. 1994 Aug; 38(2):117-23.
- Review Benefit and risk evaluation of quinapril hydrochloride.[Expert Opin Drug Saf. 2023]Review Benefit and risk evaluation of quinapril hydrochloride.Barkhordarian M, Lawrence JA, Ulusan S, Erbay MI, Aronow WS, Gupta R. Expert Opin Drug Saf. 2023 Apr; 22(4):271-277. Epub 2023 Apr 17.
- Review Adverse effects of angiotensin-converting enzyme inhibitors in antihypertensive therapy with focus on quinapril.[Am J Cardiol. 1992]Review Adverse effects of angiotensin-converting enzyme inhibitors in antihypertensive therapy with focus on quinapril.Materson BJ. Am J Cardiol. 1992 Apr 2; 69(10):46C-53C.
- Quinapril - LiverToxQuinapril - LiverTox
- Obstructive Sleep-Disordered Breathing - StatPearlsObstructive Sleep-Disordered Breathing - StatPearls
- CARS1 cysteinyl-tRNA synthetase 1 [Homo sapiens]CARS1 cysteinyl-tRNA synthetase 1 [Homo sapiens]Gene ID:833Gene
- 833[uid] AND (alive[prop]) (1)Gene
- LOC105376713 [Homo sapiens]LOC105376713 [Homo sapiens]Gene ID:105376713Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...