NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Albendazole is an anthelmintic agent used predominantly in treatment of echinococcosis, a parasitic worm that causes cysts in liver and lung. Albendazole therapy is commonly associated with mild and transient serum enzyme elevations and rarely can lead to clinically apparent acute liver injury.
Background
Albendazole (al ben' da zole) is a benzimidazole anthelmintic agent similar in structure and mechanism of action to thiabendazole and mebendazole and the veterinary agent fenbendazole. The benzimidazoles act by selective binding to beta-tubulin of parasitic worms, causing their immobilization and death. Albendazole has proven efficacy against several parasitic worms and was approved for use in the United States in 1996. Current indications for albendazole include echinococcosis, cysticercosis and strongyloidiasis against which it is more effective than mebendazole (largely because it is better absorbed). Albendazole is also used for hookworm, whipworm and pinworm infestations. Albendazole is available in tablets of 200 mg generically and under the trade name of Albenza. The dose and duration of albendazole therapy varies by indication, being a single dose of 400 mg orally for minor infestations (which can be repeated a few weeks later), and 400 mg twice daily for 1 to 2 weeks or longer for more serious systemic infections. Albendazole is generally well tolerated; side effects include gastrointestinal upset, dizziness, headaches and hair loss. Uncommon, but potentially severe adverse reactions include bone marrow suppression, pancytopenia, granulocytopenia, sudden worsening of neurocysticercosis, hypersensitivity reactions, rash, erythema multiforme and Stevens Johnson syndrome.
Hepatotoxicity
Albendazole therapy has been associated with transient and asymptomatic elevations in serum aminotransferase levels in up to 50% of patients treated for more than a few weeks. These abnormalities rapidly improve with stopping therapy which is rarely required (~4%). Albendazole has also been associated with rare instances of clinically apparent liver injury. The onset of injury has been within a few days to as long as 2 months of starting therapy or more rapidly with multiple courses of treatment. The injury can also arise 1 to 2 weeks after a short course of albendazole (1 to 3 days). The pattern of serum enzyme elevations is typically hepatocellular or mixed. Allergic features (rash, fever, eosinophilia) may be present but are not prominent. Most cases have been mild and recovery is distinctively rapid once the drug is stopped. Rapid recurrence with rechallenge has been reported but with similar severity. Cases with acute liver failure leading to emergency liver transplantation or death have also occurred.
Likelihood score: B (highly likely cause of clinically apparent liver injury).
Mechanism of Injury
Albendazole acts by binding tubulin in parasitic worms which it does with greater avidity than the tubulin in mammalian cells, but some of the toxicity of the benzimidazoles may be related to this tubulin-binding activity. In most instances of clinically apparent liver injury, hypersensitivity appears to be the cause. A similar cholestatic or mixed hepatitis has been described in dogs receiving fenbendazole.
Outcome and Management
Albendazole is usually well tolerated and the liver injury reported with its use has been mild and self-limited in course. Nevertheless, routine monitoring of serum enzymes is recommended before therapy and every 2 weeks with extended courses of treatment. Patients with acute liver injury attributed to albendazole should avoid repeat exposure as recurrence is common. It is unknown whether there is cross sensitivity with other benzimidazoles (such as mebendazole), but there probably is and switching to another class of anthelmintic agents is appropriate if therapy is still needed.
Drug Class: Anthelmintic Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Albendazole – Generic, Albenza®
DRUG CLASS
Anthelmintic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
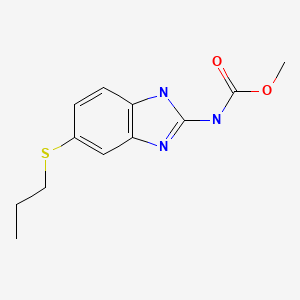
| Albendazole | 54965-21-8 | C12-H15-N3-O2-S |
|
ANNOTATED BIBLIOGRAPHY
References updated: 18 September 2021
- Zimmerman HJ. Anthelminthics. Hepatic injury from antimicrobial agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 626-8.(Expert review of hepatotoxicity of anthelmintics written in 1999; albendazole is associated with serum aminotransferase elevations in 20% of patients and has been linked to rare instances of acute liver injury).
- Keiser J, McCarthy J, Hotez PJ. Chemotherapy of helminth infections. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1001-7.(Textbook of pharmacology and therapeutics).
- Morris DL, Smith PG. Albendazole in hydatid disease—hepatocellular toxicity. Trans R Soc Trop Med Hyg. 1987;81:343–4. [PubMed: 3617201](Among 40 patients treated with albendazole for echinococcosis, 6 had ALT elevations [62-970 U/L, Alk P ~360 U/L] and 1 jaundice [bilirubin 5.7 mg/dL], with rapid resolution on stopping; 2 had positive rechallenge).
- Choudhuri G, Prasad RN. Jaundice due to albendazole. Indian J Gastroenterol. 1988;7:245–6. [PubMed: 3182029](30 year old man with echinococcal cyst developed jaundice 2.5 months after starting albendazole [bilirubin 5.6 mg/dL, ALT 320 U/L, Alk P 1.8 times ULN], resolving within 10 days of stopping; positive rechallenge with appearance of jaundice within two days).
- Jagota SC. Jaundice due to albendazole. Indian J Gastroenterol. 1989;8:58. [PubMed: 2914724](Letter in response to Choudhuri and Prasad recommending limiting dose of albendazole to 400 mg twice daily in cycles of 28 days. Reply by authors stresses the elements of hypersensitivity in the case report).
- el-Mufti M, Kamag A, Ibrahim H, Taktuk S, Swaisi I, Zaidan A, Sameen A, et al. Albendazole therapy of hydatid disease: 2-year follow-up of 40 cases. Ann Trop Med Parasitol. 1993;87:241–6. [PubMed: 8257234](Among 40 Egyptian patients with echinococcal cysts treated with albendazole [usually in three 28 day cycles], hepatotoxicity arose in 2 patients [5%], both resolving on discontinuation; details not given, but Alk P said to be unreliable as a marker of liver injury because it is often raised before therapy).
- Gil-Grande LA, Rodriguez-Cabeiro F, Prieto JG, Sanchez-Ruano JJ, Brasa C, Agular L, Garcia-Hoz F, et al. Randomised controlled trial of efficacy of albendazole in intra-abdominal hydatid disease. Lancet. 1993;342:1269–72. [PubMed: 7901585](Among 43 patients treated with albendazole [10 mg/kg/day] for 10-111 days, 27 had ALT elevations during therapy, which were > twice ULN in 21 and >200 U/L in 4; liver biopsies showed “toxic hepatitis” and changes correlated with higher drug levels in liver; no jaundice or clinically apparent injury and all resolved).
- Klion AD, Massougbodji A, Horton J, Ekoué S, Lanmasso T, Ahouissou NL, Nutman TB. Albendazole in human loiasis: results of a double-blind, placebo-controlled trial. J Infect Dis. 1993;168:202–6. [PubMed: 8515109](23 patients with loiasis were treated with albendazole [n=11: 200 mg twice daily] or placebo [n=12] for 21 days; none developed ALT elevations or evidence of liver injury).
- Misra PK, Kumar A, Agarwal V, Jagota SC. A comparative clinical trial of albendazole versus metronidazole in children with giardiasis. Indian Pediatr. 1995;32:779–82. [PubMed: 8617554](Randomized trial of albendazole [400 mg/day for 5 days] vs metronidazole [7.5 mg/kg thrice daily for 5 days] in 64 children with giardiasis; all children were cured and side effects were similar, no differences in biochemical test results).
- Horton RJ. Albendazole in treatment of human cystic echinococcosis: 12 years of experience. Acta Trop. 1997;64:79–93. [PubMed: 9095290](Current dose of albendazole for therapy of echinococcosis is 800 mg daily for ~3 months; serum enzyme elevations occurred in 10-20% of patients, requiring discontinuation in 4%, all cases were reversible, some possibly due to underlying liver cyst disease).
- Molina JM, Chastang C, Goguel J, Michiels JF, Sarfati C, Desportes-Livage I, Horton J, et al. Albendazole for treatment and prophylaxis of microsporidiosis due to Encephalitozoon intestinalis in patients with AIDS: a randomized double-blind controlled trial. J Infect Dis. 1998;177:1373–7. [PubMed: 9593027](Controlled trial of albendazole vs placebo in 8 patients with HIV and E. intestinalis infection; ALT elevations occurred in 1 patient in each group).
- Inkatanuvat S, Suntharasamai P, Vutikes S, Riganti M. Changes of liver functions after albendazole treatment in human gnathostomiasis. J Med Assoc Thai. 1998;81:735–40. [PubMed: 9803063](98 patients with “gnathostomiasis” were given albendazole [800 mg daily] for 14 days; ALT elevations were present in 44% at day 14, 18% at day 28, peak values of 482 U/L, but all were asymptomatic and most returned to normal by 6 months).
- Dunyo SK, Nkrumah FK, Simonsen PE. A randomized double-blind placebo-controlled field trial of ivermectin and albendazole alone and in combination for the treatment of lymphatic filariasis in Ghana. Trans R Soc Trop Med Hyg. 2000;94:205–11. [PubMed: 10897370](Placebo controlled trial of single dose of albendazole, ivermectin or the combination of both in 1425 persons from filariasis-endemic villages; albendazole therapy was associated with a modest but delayed reduction in microfilarial levels; side effects were mild and self-limited and not different from placebo; no mention of jaundice or hepatitis).
- Shenoy RK, Suma TK, John A, Arun SR, Kumaraswami V, Fleckenstein LL, Na-Bangchang K. The pharmacokinetics, safety and tolerability of the co-administration of diethylcarbamazine and albendazole. Ann Trop Med Parasitol. 2002;96:603–14. [PubMed: 12396323](Pharmacokinetic studies of single oral doses of diethylcarbamazine and albendazole reported no drug-drug interactions and no adverse events).
- Matthaiou DK, Panos G, Adamidi ES, Falagas ME. Albendazole versus praziquantel in the treatment of neurocysticercosis: a meta-analysis of comparative trials. PLoS Negl Trop Dis. 2008;2:e194. [PMC free article: PMC2265431] [PubMed: 18335068](Systematic review of efficacy of albendazole versus praziquantel; no differences in rates of adverse events, but no details given).
- Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937–48. [PubMed: 18430913](Systematic review of efficacy and safety of albendazole, mebendazole and pyrantel pamoate as therapy of A. Lumbricoides, hookworm and T. Tichiura; albendazole was well tolerated in most studies, no significant adverse events were reported).
- Choi GY, Yang HW, Cho SH, Kang DW, Go H, Lee WC, Lee YJ, et al. Acute drug-induced hepatitis caused by albendazole. J Korean Med Sci. 2008;23:903–5. [PMC free article: PMC2580005] [PubMed: 18955802](47 year old man developed fever and rash 2 days after single dose of albendazole and 2 days later was jaundiced [bilirubin 2.8 mg/dL, ALT 4,622 U/L, Alk P 40 U/L, INR 1.44]; rapid recovery).
- Jevtić M, Mikić D, Arsić-Komljenović G, Stanković N, Ristanović E, Sjenicić G, Janićijević-Hudomal S. Vojnosanit Pregl. 2008;65:539–44. [Adverse effects of longterm, continual administration of high doses of albendazole in the treatment of echinococcal disease] Serbian. [PubMed: 18700464](Among 42 patients with echinococcosis treated with albendazole for 4 to 6 months, ALT elevations occurred in 36% and led to discontinuation in 7%, subsequently resolving in all).
- Mikić D, Jevtić M, Arsić-Komljenović G, Ristanović E, Stanković N, Sjenicić G, Janićijević-Hudomal S. Vojnosanit Pregl. 2009;66:833–9. [Impossibility of the treatment of inoperable liver multicystic echinococcosis due to adverse reactions to antihelminitics] Serbian. [PubMed: 19938764](27 year old with echinococcal cysts developed ALT elevations after 2 months of albendazole therapy [800 mg daily] and also did not tolerate praziquantel [rash] leaving no options).
- Amoruso C, Fuoti M, Miceli V, Zito E, Celano MR, De Giorgi A, Nebbia G. Pediatr Med Chir. 2009;31:176–8. [Acute hepatitis as a side effect of albendazole: a pediatric case] Italian. [PubMed: 19839402](7 year old girl with pinworms developed jaundice during third course of albendazole [bilirubin 6.4 mg/dL, ALT 2200 U/L, GGT 58 U/L, Alk P 899 U/L], resolving rapidly upon stopping).
- Drugs for parasitic infections. Treat Guidel Med Lett. 2013;11 Suppl:e1–31.(Brief description of drugs for parasitic infections in adults and children as well as a table of their major side effects: albendazole is the drug of choice for ascariasis, cutaneous larva migrans, pinworm, hookworm, whipworm, trichinellosis, and several less common parasitic infections; side effects include ALT elevations).
- Namwanje H, Kabatereine N, Olsen A. A randomised controlled clinical trial on the safety of co-administration of albendazole, ivermectin and praziquantel in infected schoolchildren in Uganda. Trans R Soc Trop Med Hyg. 2011;105:181–8. [PubMed: 21353271](Trial comparing a 7 day course of the combination of albendazole, ivermectin and praziquantel to standard therapy in 235 children with lymphatic filariasis, schistosomiasis or helminthiasis found no differences in efficacy in decreasing egg counts or microfilariae and no change in liver tests).
- Marin Zuluaga JI, Marin Castro AE, Perez Cadavid JC, Restrepo Gutierrez JC. Albendazole-induced granulomatous hepatitis: a case report. J Med Case Rep. 2013;7:201. [PMC free article: PMC3750323] [PubMed: 23889970](25 year old woman developed jaundice 2 weeks after starting albendazole [bilirubin 13.8 mg/dL, ALT 1649 U/L, Alk P 145 U/L], biopsy showing epithelioid granulomas, resolving within 2 months).
- Gözüküçük R. Albendazole-induced toxic hepatitis: A case report. Turk J Gastroenterol. 2013;24:82–4. [PubMed: 23794356](28 year old man developed abnormal liver tests 20 days after starting albendazole for hydatid cyst disease [bilirubin not given, ALT 968 U/L, Alk P 209 U/L], resolving rapidly upon stopping and recurring within 3 days of restarting albendazole).
- Akhan O, Yildiz AE, Akinci D, Yildiz BD, Ciftci T. Is the adjuvant albendazole treatment really needed with PAIR in the management of liver hydatid cysts? A prospective, randomized trial with short-term follow-up results. Cardiovasc Intervent Radiol. 2014;37(6):1568–74. [PubMed: 24464258](Among 39 patients with hydatid cysts treated with "puncture, aspiration, injection and reaspiration" [PAIR] with or without albendazole, initial success rates were 100%, but recurrence occurred only in those who did not receive albendazole; side effects occurred in 24% of albendazole treated subjects but their nature was not disclosed).
- Sıvgın S, Eser B, Kaynar L, Kurnaz F, Sıvgın H, Yazar S, Cetin M, et al. Encephalitozoon intestinalis: a rare cause of diarrhea in an allogeneic hematopoietic stem cell transplantation (HSCT) recipient complicated by albendazole-related hepatotoxicity. Turk J Haematol. 2013;30:204–8. [PMC free article: PMC3878461] [PubMed: 24385787](50 year old man with acute leukemia and hematopoietic cell transplant developed liver test abnormalities 5 days after starting albendazole for E. intestinalis [peak bilirubin 0.9 mg/dL, ALT 519 U/L, Alk P 255 U/L], resolving within 2 weeks of stopping).
- Shah C, Mahapatra A, Shukla A, Bhatia S. Recurrent acute hepatitis caused by albendazole. Trop Gastroenterol. 2013;34:38–9. [PubMed: 23923374](7 year old boy had 4 episodes of jaundice, each arising shortly after a single dose of albendazole [bilirubin 2.3-5.5 mg/dL, ALT 1208-3005 U/L, Alk P 312-314 U/L], resolving within 10 days on each occasion).
- Nandi M, Sarkar S. Albendazole-induced recurrent hepatitis. Indian Pediatr. 2013;50:1064. [PubMed: 24382909](5 year old boy had 4 episodes of jaundice after receiving 2 to 3 days of albendazole [4th episode: bilirubin 11.5 mg/dL, ALT 2720 U/L, Alk P 1247 U/L, INR 1.6], resolving within 2 months of stopping).
- Ben Fredj N, Chaabane A, Chadly Z, Ben Fadhel N, Boughattas NA, Aouam K. Albendazole-induced associated acute hepatitis and bicytopenia. Scand J Infect Dis. 2014;46:149–51. [PubMed: 24423162](26 year old man with hydatic cyst developed jaundice 1 month after starting a second course of albendazole [bilirubin 20 mg/dL, ALT 2454 U/L, Alk P 198 U/L] with bone marrow suppression, resolving within 2 weeks of stopping).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin American published between 1996 and 2012, one case [non-fatal] was attributed to mebendazole, but none to albendazole).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to albendazole or any other anthelmintic agent).
- Amado-Diago CA, Gutiérrez-Cuadra M, Armiñanzas C, Arnaíz de Las Revillas F, Gómez-Fleitas M, Fariñas MC. Echinococcosis: A 15-year epidemiological, clinical and outcome overview. Rev Clin Esp (Barc). 2015;215(7):380–4. [PubMed: 26119089](Among 76 cases of hydatidosis seen at a Spanish referral hospital over a 15 year period, 94% had liver involvement, 58% men, ages 19 to 91 years, 37% received albendazole [usually with surgery or drainage], no mention of hepatotoxicity).
- Gomez I. Review of the treatment of liver hydatid cysts. World J Gastroenterol. 2015;21(1):124–31. Gavara C, López-Andújar R, Belda Ibáñez T, Ramia Ángel JM, Moya Herraiz Á, Orbis Castellanos F, Pareja Ibars E, et al. [PMC free article: PMC4284328] [PubMed: 25574085](Systematic review of the literature on therapy of hydatid cysts concludes that radical surgery with pre- and postoperative albendazole is the "best treatment option"; no discussion of adverse side effects).
- Verdugo Thomas F, Tapia Mingo A, Ramírez Montes D, Oporto Uribe S. Hepatitis tóxica por albendazol. Gastroenterol Hepatol. 2015;38:436–8. [Albendazole-induced toxic hepatitis] [PubMed: 25179365](15 year old female was treated with albendazole [400 mg twice daily for 7 days] 4 times over several years, the last time developing jaundice within 10 days of stopping [19.4 mg/dL, ALT 3741 U/L, Alk P 214 U/L, INR 1.9], resolving on no therapy over the next month).
- Bilgic Y, Yilmaz C, Cagin YF, Atayan Y, Karadag N, Harputluoglu MMM. Albendazole induced recurrent acute toxic hepatitis: a case report. Acta Gastroenterol Belg. 2017;80:309–311. [PubMed: 29560698](47 year old woman developed jaundice 10 days after receiving a single dose of albendazole for suspected parasitosis [bilirubin 4.3 mg/dL, ALT 1332 U/L, Alk P 159 U/L, INR 1.2], recovering within 2 months and reporting similar liver injury with jaundice after albendazole therapy 4 years previously).
- Aasen TD, Nasrollah L, Seetharam A. Drug-induced liver failure requiring liver transplant: report and review of the role of albendazole in managing echinococcal infection. Exp Clin Transplant. 2018;16:344–347. [PubMed: 27228108](38 year old Iraqi woman developed jaundice 6 weeks after starting albendazole [400 mg twice daily] for hydatid cyst disease [bilirubin 5.0 rising to 12.6 mg/dL, ALT 2118 U/L, INR 1.3 rising to 1.7], with progressive worsening hepatic failure and liver transplantation on hospital day 10).
- Moon SY, Baek YH, Lee SW. Korean J Gastroenterol. 2019;73:360–364. [Drug induced liver injury by prophylactic administration of albendazole] [PubMed: 31234627](30 year old Korean woman developed jaundice shortly after starting albendazole [bilirubin 7.2 mg/dL, ALT 2296 U/L, Alk P 162 U/L], resolving within a month of stopping, having had a similar episode with albendazole therapy 8 years previously).
- Asenov Y, Akin M, Ibiş C, Tekant Y, Özden I. Observed or predicted albendazole hepatotoxicity as an indication for a resection procedure in hepatic hydatid disease – a short series of cases. Chirurgia (Bucur). 2019;114:522–527. [PubMed: 31511139](Four patients from Turkey with hepatic hydatid disease were treated surgically and did not need anthelmintic therapy; 3 had developed ALT elevations without jaundice when therapy was attempted [initial ALT values 677, 642 and 482 U/L] and the 4 had autoimmune hepatitis diagnosed before therapy).
- Piloiu C, Dumitrascu DL. Albendazole-induced liver injury. Am J Ther. 2021;28:e335–e340. [PubMed: 33590990](Two cases from Romania; 22 year old woman developed jaundice within 10 days of starting albendazole [bilirubin 5.2 mg/dL, ALT 1757 U/L, Alk P 150, INR normal] and a 42 year old woman developing jaundice within a few days of starting albendazole [bilirubin 4.1, ALT 2489 U/L, Alk P 146 U/L, INR 2.35], both cases due to self-administration and both resolving within 1-2 months of stopping).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Clinical tolerance and efficacy of anti-parasitic treatment with albendazole in patients with alveolar echinococcosis: long-term follow-up observation in 117 patients.[Parasitol Res. 2021]Clinical tolerance and efficacy of anti-parasitic treatment with albendazole in patients with alveolar echinococcosis: long-term follow-up observation in 117 patients.Zavoikin VD, Zelya OP, Tumolskaya NI. Parasitol Res. 2021 Oct; 120(10):3603-3610. Epub 2021 Aug 25.
- Post-treatment follow-up study of abdominal cystic echinococcosis in tibetan communities of northwest Sichuan Province, China.[PLoS Negl Trop Dis. 2011]Post-treatment follow-up study of abdominal cystic echinococcosis in tibetan communities of northwest Sichuan Province, China.Li T, Ito A, Pengcuo R, Sako Y, Chen X, Qiu D, Xiao N, Craig PS. PLoS Negl Trop Dis. 2011 Oct; 5(10):e1364. Epub 2011 Oct 25.
- Albendazole-Induced Liver Injury.[Am J Ther. 2021]Albendazole-Induced Liver Injury.Piloiu C, Dumitrascu DL. Am J Ther. 2021 Feb 3; 28(3):e335-e340. Epub 2021 Feb 3.
- Review Anthelmintic Agents.[LiverTox: Clinical and Researc...]Review Anthelmintic Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Mebendazole.[LiverTox: Clinical and Researc...]Review Mebendazole.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Albendazole - LiverToxAlbendazole - LiverTox
- Inflammation and Immune Function - Antioxidants in Sport NutritionInflammation and Immune Function - Antioxidants in Sport Nutrition
- Compression Therapy in Diabetic Foot Ulcer Management: A Review of Clinical Effe...Compression Therapy in Diabetic Foot Ulcer Management: A Review of Clinical Effectiveness, Cost-effectiveness and Guidelines
- SMARCD1 SWI/SNF related BAF chromatin remodeling complex subunit D1 [Homo sapien...SMARCD1 SWI/SNF related BAF chromatin remodeling complex subunit D1 [Homo sapiens]Gene ID:6602Gene
- 6602[uid] AND (alive[prop]) (1)Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...