NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Nitrofurantoin is an oral antibiotic widely used either short term to treat acute urinary tract infections or long term as chronic prophylaxis against recurrent infections. Nitrofurantoin is one of the most common causes of drug induced liver disease and can cause either an acute or a chronic hepatitis-like syndrome that can be severe and lead to liver failure or cirrhosis.
Background
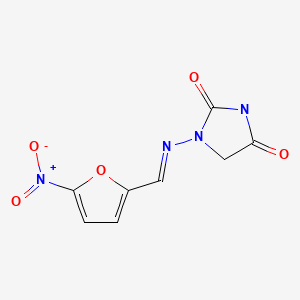
Structurally, nitrofurantoin (nye" troe fure an' toyn) is a nitrated 5-member furan ring with a side chain of hydantoin. Nitrofurantoin inhibits several bacterial enzyme systems and has broad antibacterial activity. Its precise mechanism of action is not known. Importantly, antibacterial resistance to nitrofurantoin is rare, which makes it an attractive choice for long term treatment. In addition, nitrofurantoin is well absorbed orally and is rapidly excreted in the urine so that drug levels in urine are high while serum levels are minimal, which makes it an appropriate agent to treat urinary tract but not systemic infections. Nitrofurantoin was first approved for use in the United States in 1953 and is still in wide use with more than 3 million prescriptions filled yearly. Current indications are treatment of acute and prophylaxis against chronic or recurrent urinary tract infections due to susceptible organisms. For treatment of acute infections, the recommended regimen is 50 to 100 mg orally four times daily for one week. For prophylaxis against chronic or recurrent infections, the recommended dose is 50 to 100 mg daily long term. Generic formulations are available (25, 50, and 100 mg); specific commercial names include Macrodantin, Macrobid and Furadantin, among others. Common side effects include nausea, diarrhea, dyspepsia, dizziness, drowsiness and rash. Nitrofurantoin has multiple rare, but potentially severe side effects that arise particularly with long term use and include interstitial pneumonitis, peripheral neuropathy, exfoliative dermatitis, hemolytic anemia, lupus-like syndromes and hepatotoxicity.
Hepatotoxicity
Nitrofurantoin is currently one of the most common causes of drug induced liver injury. Liver injury from nitrofurantoin can cause either an acute or chronic hepatitis-like syndrome. The acute form is typically associated with a 1 or 2 week course of treatment with nitrofurantoin and is rare (~0.3 cases per 100,000 prescriptions). Acute liver injury typically presents within a few weeks of starting nitrofurantoin and can arise up to a few weeks after stopping a defined course of treatment. The pattern of liver injury is usually hepatocellular with or without jaundice, and typically is accompanied by symptoms of fever and rash. The acute injury due to nitrofurantoin usually resolves rapidly once the medication is stopped, but severe and fatal instances have been reported. In some instances, autoimmune features are present, but these are more common with the chronic presentation of nitrofurantoin hepatotoxicity. The course and outcome of acute nitrofurantoin hepatotoxicity is variable, severe forms with acute liver failure can occur, and nitrofurantoin is regularly listed as one of the major causes of acute liver failure due to medications.
The chronic form of nitrofurantoin hepatotoxicity is more common than the acute form and typically presents months to years after initiation of long term prophylactic therapy. The estimated incidence of liver injury from nitrofurantoin is approximately 1 per 1500 persons exposed. The presentation is usually insidious and marked initially by fatigue and weakness followed by dark urine and jaundice. The clinical pattern and laboratory features can mimic autoimmune hepatitis with marked elevations in serum ALT levels, increases in gamma globulin levels, and the presence of antinuclear and anti-smooth muscle antibodies. In some instances, the onset is abrupt and resembles acute hepatitis. However, immunoallergic features of fever and rash are less common than with the acute form of nitrofurantoin hepatotoxicity. Liver histology typically demonstrates features of chronic hepatitis with inflammation, interface hepatitis, focal or centrilobular bridging necrosis and variable degrees of fibrosis. Cirrhosis as a result of nitrofurantoin hepatotoxicity has been reported and, if not recognized as due to the medication, can progress to end stage liver disease. There is a female preponderance and the risk of injury appears to increase with age particularly the chronic forms.
Likelihood score: A (well known cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of nitrofurantoin hepatotoxicity is not well known. Its nitro-reductive metabolism produces injurious oxidative free radicals which can damage hepatocytes. Many cases demonstrate evidence an autoimmune etiology and some studies have shown a linkage with HLA-DR6 and DR2.
Outcome and Management
The severity of nitrofurantoin induced liver injury ranges from mildly symptomatic elevations in serum aminotransferase levels (Cases 1 and 2), hepatitis with jaundice (Case 3) to fulminant liver failure and death (Case 4). Complete recovery is expected after stopping the drug, but recovery may be slow (2 to 6 months). In rare instances, evidence of chronic liver injury persists. Because of the autoimmune features of many cases of nitrofurantoin hepatotoxicity, corticosteroids are often used particularly in cases that are severe or slow to resolve. In many instances, corticosteroid therapy appears to lead to improvement. However, the ultimate benefit of immunosuppressive therapy remains to be proven and corticosteroids should be used cautiously and withdrawn as soon as possible, and with careful follow up to document lack of relapse after stopping. Rechallenge leads to recurrence and should be avoided. There does not appear to be cross reactivity to hepatic injury between nitrofurantoin and other commonly used antibiotics.
Drug Class: Antiinfective Agents
CASE REPORT
Case 1. Chronic hepatitis with hepatocellular pattern of serum enzyme elevations due to nitrofurantoin.(1)
A 68 year old woman was given nitrofurantoin (50 mg daily) for prophylaxis against recurrent urinary tract infections. She continued on therapy for 3 years at which time serum enzymes were found to be elevated. She had no symptoms that could be attributed to liver disease. Nitrofurantoin was stopped, but restarted one month later because of recurrence of urinary tract infections. Serum aminotransferase levels remained mildly elevated (2 to 3 times the upper limit of normal). Six months later she had the insidious onset of nausea, fatigue and weakness. Serum aminotransferase levels were still elevated, but alkaline phosphatase and bilirubin values were normal. Anti-smooth muscle antibody was present (1:80), but other autoantibodies were not detected. Tests for hepatitis A, B and C were negative. A liver biopsy showed chronic active hepatitis with moderate to severe inflammatory infiltrates and moderate fibrosis. The symptoms persisted for several months and the medication was discontinued again. Two months after stopping nitrofurantoin the patient had returned to her usual state of health and serum aminotransferase levels returned to normal.
Key Points
| Medication: | Nitrofurantoin, 50 mg daily |
|---|---|
| Pattern: | Hepatocellular (aminotransferase elevations only) |
| Severity: | 1+ (never jaundiced, never hospitalized) |
| Latency: | Several years, 6 months after restarting |
| Recovery: | Complete within 5 months |
| Other medications: | Sumatriptan, alendronate, clopidogrel, estrogen, verapamil |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Nitrofurantoin started | |||||
| 1 month | 79 | 62 | 0.4 | ||
| 3 months | 115 | ||||
| 4 months | 88 | Nausea, fatigue | |||
| 5 months | 255 | Liver biopsy | |||
| 6 months | 0 | 176 | 99 | 0.5 | SMA 1:80 |
| Nitrofurantoin stopped | |||||
| 9 months | 3 months | 52 | 101 | 0.7 | |
| 11 months | 5 months | 30 | 102 | 0.7 | |
| Normal Values | <42 | <115 | <1.2 | ||
Comment
Initially, the liver injury was believed to be caused by chronic hepatitis unrelated to medications. Because of appearance of symptoms and findings on liver biopsy, nitrofurantoin was subsequently suspected and discontinued. The resolution of liver test abnormalities and symptoms of fatigue after stopping nitrofurantoin supported the diagnosis of drug induced liver disease. While the clinical pattern resembles idiopathic chronic autoimmune hepatitis, the disease generally resolves once the medication is stopped with or without corticosteroid therapy. In any case, long term follow up to document resolution of hepatitis is warranted, particularly if corticosteroids were used initially.
Case 2. Mild chronic hepatitis with hepatocellular pattern of enzyme elevations.(1)
A 76 year old woman with a history of recurrent bladder and kidney infections was treated with nitrofurantoin 100 mg daily. After a year, the dose was reduced to 50 mg daily because of serum aminotransferase elevations (Table). One month later, she complained of fatigue and a 10 lb. weight loss. Her serum ALT levels were markedly elevated, but alkaline phosphatase and bilirubin levels were normal. Antinuclear antibody was present (1:80) and globulin levels were mildly elevated. Tests for hepatitis A, B and C were negative. Liver ultrasound was normal. A liver biopsy showed chronic hepatitis, with mild activity and slight fibrosis. This patient had rapid clinical and biochemical improvement once nitrofurantoin was stopped.
Key Points
| Medication: | Nitrofurantoin |
|---|---|
| Pattern: | Hepatocellular (R=29) |
| Severity: | 1+ (never jaundiced, never hospitalized) |
| Latency: | 1 year |
| Recovery: | 6 weeks after stopping |
| Other medications: | Amlodipine, enalapril, simvastatin, ezetimibe |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | 19 | 64 | 1.1 | ||
| Nitrofurantoin started | |||||
| 11 months | 66 | 90 | 0.2 | ||
| 12 months | 83 | ||||
| 13 months | 154 | 10 lb. weight loss | |||
| 13.5 months | 741 | ||||
| 14 months | 0 | 1478 | 131 | 1.4 | |
| Nitrofurantoin stopped | |||||
| 15 months | 3 weeks | 292 | 81 | 0.6 | Liver biopsy |
| 17 months | 3 months | 35 | 67 | 0.3 | |
| Normal Values | <42 | <115 | <1.2 | ||
Comment
The prolonged incubation period to onset of liver injury due to nitrofurantoin frequently leads to mistaken or delayed diagnosis. The clinical pattern is more similar to chronic than acute hepatitis and recovery may be delayed. The current case is very typical and the patient was fortunate to have therapy stopped before the onset of jaundice. The injury is frequently accompanied by autoantibodies (ANA or SMA) which may persist after withdrawal of nitrofurantoin, but generally in low titer.
Case 3. Severe chronic active hepatitis with autoimmune features and pseudotumor due to nitrofurantoin hepatotoxicity.(1)
An 84 year old woman was treated with nitrofurantoin 50 mg once daily for seven months. Because of recurrence of urinary tract infections, it was restarted one year later. After another 14 months of taking nitrofurantoin, she was found to have jaundice and peripheral edema. She was hospitalized for investigation. Serum aminotransferase, alkaline phosphatase, and bilirubin levels were elevated (Table). MRI suggested an infiltrating tumor in the liver, but endoscopic retrograde cholangio-pancreatography was unrevealing and biopsy of the mass showed no evidence of tumor, but rather chronic inflammation and submassive hepatic necrosis. Tests for hepatitis A, B and C were negative. Antinuclear antibody and smooth muscle antibody titers were strongly positive. Symptoms of abnormal liver tests improved slowly after nitrofurantoin was stopped. At follow up 7 months later, the patient was asymptomatic, and liver enzyme levels were in reference range.
Key Points
| Medication: | Nitrofurantoin |
|---|---|
| Pattern: | Chronic hepatitis, cholestatic pattern of enzymes (R=1.7) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | Three years after starting (intermittent use) |
| Recovery: | Protracted but complete |
| Other medications: | Losartan, warfarin, atorvastatin, diltiazem |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| 0 | Nitrofurantoin started | ||||
| 8 months | Nitrofurantoin stopped | ||||
| Pre-Restarting | 16 | ||||
| Nitrofurantoin restarted | |||||
| 7 months | 52 | ||||
| 9 months | 115 | ||||
| 10 months | 127 | 109 | 0.9 | ||
| 12 months | 190 | 175 | 1.5 | ||
| 13 months | 197 | 359 | 3.8 | Edema | |
| 14 months | 0 | 307 | 503 | 5 | Jaundice |
| Nitrofurantoin stopped again | |||||
| 15 months | 1 month | 89 | 301 | 5.8 | Albumin 2.6, ANA 1:320 |
| 17 months | 3 months | 45 | 145 | 1.1 | |
| 21 months | 7 months | 39 | 0.9 | Albumin 3.7 | |
| Normal Values | <42 | <130 | <1.2 | ||
Comment
This elderly lady presented with a clinical picture suggestive of liver cancer after more than a year of intermittent therapy with nitrofurantoin. A liver biopsy demonstrated a histologic picture of severe chronic active hepatitis. Autoimmune markers were present. The resolution of the injury after withdrawal of drug supports the diagnosis of a drug induced chronic hepatitis with autoimmune features.
Case 4. Acute liver failure due to nitrofurantoin.(1)
A 46 year old woman with history of spina bifida and neurogenic bladder was treated with nitrofurantoin in a dose of 50 mg per day for recurrent urinary tract infections. Therapy was effective in suppressing infections and she remained on the drug for 3 and a half years, when she was found to have jaundice, followed shortly thereafter by fatigue, nausea and vomiting. She was hospitalized for investigation. Serum aminotransferase and bilirubin levels were markedly elevated. Tests for hepatitis A, B and C were negative. Anti-nuclear and anti-smooth muscle antibody titers were elevated. CT of the abdomen showed no evidence of biliary obstruction or hepatic masses. Because of worsening hepatic failure, she was transferred to a liver transplant center, but she rapidly developed mental obtundation, coma and respiratory failure, followed by intractable cerebral edema and death five days later. Autopsy showed a small, shrunken liver weighing ~600 g.
Key Points
| Medication: | Nitrofurantoin |
|---|---|
| Pattern: | Hepatocellular (R=16) |
| Severity: | 5+ (death) |
| Latency: | 3.5 years |
| Recovery: | None |
| Other medications: | None |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comments |
|---|---|---|---|---|---|
| Pre | Pre | 23 | 152 | 0.7 | |
| 0 | Nitrofurantoin started | ||||
| 3.5 years | Jaundice, fatigue, nausea | ||||
| -2 days | 1988 | 338 | 28.9 | Hospitalized: INR = 4.0 | |
| -1 day | 1676 | 296 | 27.7 | INR = 5.0 | |
| 0 | Nitrofurantoin stopped | ||||
| 3.5 years | 1 day | 1525 | 285 | 31.5 | INR = 6.0 |
| 2 days | 1325 | 260 | 26.6 | INR = 11.0 | |
| 3 days | 907 | 150 | 27.3 | Transferred | |
| 4 days | 538 | 134 | 28 | Respiratory failure, | |
| 5 days | 491 | 184 | 28.4 | Cerebral edema, Death | |
| Normal Values | <42 | <115 | <1.2 | ||
Comment
The clinical pattern indicated an acute hepatitis-like picture, but the timing and gradual onset was more representative of chronic hepatitis with a severe unrelenting course. Nevertheless, autopsy revealed massive hepatic necrosis suggesting an acute process. In hepatocellular forms of drug induced liver disease, jaundice indicates severe injury with a mortality rate that is greater than 10%, a finding stressed by the late Dr. Hyman J. Zimmerman and familiarly known as “Hy’s law”.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Nitrofurantoin – Generic (Various), Furadantin®, Macrodantin®
DRUG CLASS
Antiinfective Agents, Urinary (Nitrofuran Derivative)
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Nitrofurantoin | 67-20-9 17140-81-7 (Monohydrate) 54-87-5 (Sodium) | C8-H6-N4-O5 C8-H6-N4-O5.H2-O C8-H6-N4-O5.Na |
|
CITED REFERENCE
- 1.
- de Boer YS, Kosinski AS, Urban TJ, Zhao Z, Long N, Chalasani N, Kleiner DE, et al; Drug-Induced Liver Injury Network. Features of autoimmune hepatitis in patients with drug-induced liver injury. Clin Gastroenterol Hepatol 2017; 15: 103-112.e2. 27311619. [PMC free article: PMC5370577] [PubMed: 27311619]
ANNOTATED BIBLIOGRAPHY
References updated: 01 May 2020
Abbreviations: SMA, smooth muscle antibody; ANA, antinuclear antibody.
- Zimmerman HJ. Nitrofurantoin. In, Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd Ed. Philadelphia: Lippincott, 1999. p. 603-5.(Expert review of nitrofurantoin hepatotoxicity published in 1999; while there were no reports of liver injury during the first decade of its use, nitrofurantoin later became a frequent cause of hepatotoxicity presenting either acutely with cholestatic or hepatocellular jaundice or chronically with a chronic hepatitis-like syndrome with latency varying from 1 month to many years, typically with autoimmune features and sometimes with granulomas).
- Moseley RH. Nitrofurantoin. Hepatotoxicity of antimicrobial and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd Edition. Amsterdam: Elsevier, 2013. p. 469-70.(Expert review of nitrofurantoin induced liver injury mentions that a wide variety of forms of injury have been described including cholestatic hepatitis, hepatocellular injury, granulomatous hepatitis, chronic hepatitis and cirrhosis; clinical features often resemble autoimmune hepatitis and incidence increases with age and is higher in women).
- MacDougall C. Sulfonamides, trimethoprim-sulfamethoxazole, quinolones, and agents for urinary tract infections. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1011-21.(Textbook of pharmacology and therapeutics).
- Ernaelsteen D, Williams R. Jaundice due to nitrofurantoin. Gastroenterology. 1961;41:590–3.(54 year old woman who had developed fever, rash and malaise 5 days after starting a second course of nitrofurantoin, redeveloped fever and rash one day after starting a third course [bilirubin 2.1 rising to 6.1 mg/dL, ALT 72 U/L, Alk P 3.5 times ULN, eosinophils 1,600], resolving within 6 weeks of stopping).
- Cook GC, Sherlock S. Jaundice and its relation to therapeutic agents. Lancet. 1965;1(7378):175–9. [PubMed: 14238042](Among 20 cases of drug induced cholestatic jaundice, 18 had taken phenothiazines and 1 nitrofurantoin, a 54 year old woman who developed jaundice within 8 days of starting nitrofurantoin [bilirubin 2.0 mg/dL, Alk P 3.5 times ULN], resolving within 50 days; probably same case as in Ernaelsteen [1961]).
- Jokela S. Liver disease due to nitrofurantoin. Gastroenterology. 1967;53:306–11.(41 year old woman developed nausea within 2 weeks of starting nitrofurantoin [bilirubin 13 mg/dL, ALT 380 U/L, Alk P 2 times ULN, 5% eosinophils], resolving within 1 month on corticosteroids, redeveloping jaundice [bilirubin 13 mg/dL, ALT 900, Alk P 3 times ULN] within 2 weeks of restarting).
- Jowers LV, Shannon SR. Jaundice due to nitrofurantoin. J S C Med Assoc. 1967;63:357–8. [PubMed: 5234064]
- Wasowska T, Krus S. Jaundice induced by furadantin treatment: case description. Pol Med J. 1968;7:322–7. [PubMed: 5658046](50 year old woman developed fever, jaundice, acidosis and coma 10 days after starting a second course of nitrofurantoin [bilirubin 16 mg/dL, ALT 22 U/L, Alk P 2 times ULN, prothrombin index 68%], jaundice improving, but patient dying 11 days later of renal failure due to pyelonephritis).
- Murphy KJ, Innis MD. Hepatic disorder and severe bleeding diathesis following nitrofurantoin ingestion. JAMA. 1968;204:396–7. [PubMed: 5694455](65 year old man developed excessive bleeding [protime 58 sec] 1 month after starting nitrofurantoin [bilirubin 0.9 rising to 5.4 mg/dL, ALT not provided, Alk P 2.5 times ULN] requiring plasma infusions, ICU care and tracheostomy, but resolving within 2 weeks of stopping).
- Bhagwat AG, Warren RE. Hepatic reaction to nitrofurantoin. Lancet. 1969;2(7634):1369. [PubMed: 4188135](57 year old woman developed rash and arthralgias 2 weeks after starting nitrofurantoin [bilirubin 0.5 mg/dL, AST 98 U/L, Alk P 3 times ULN], resolving within 7 days of stopping).
- Sotaniemi E, Hokkanen O, Kaipainen WJ. Hepatic injury and multiple drug treatment. Ann Clin Res. 1971;3:220–5. [PubMed: 5093153](Among 55 patients admitted for drug induced liver injury to one hospital in Finland during a 5 year period [0.3% of total admissions], the most commonly implicated agents were sulfonamides [n=23], nitrofurantoin [11: 20%], oral contraceptives [11], hydralazine [8], and chloramphenicol [6]; 39 [79%] were taking more than one agent).
- Lamberger B, von Schenck H. Lakartidningen. 1973;70:2655. [Nitrofurantoin induced icterus] Swedish. [PubMed: 4787340](48 year old woman developed jaundice 4 years after starting daily nitrofurantoin [bilirubin 17 mg/dL, ALT 1275 U/L, GGT 4 times ULN], resolving upon stopping and recurring twice 2 weeks [bilirubin normal and ALT 825 U/L] and then 1 week after restarting [bilirubin 3.2 mg/dL and ALT 1225 U/L]).
- Goldstein LI, Ishak KG, Burns W. Hepatic injury associated with nitrofurantoin therapy. Am J Dig Dis. 1974;19:987–98. [PubMed: 4424634](Two cases: 44 year old man developed liver test abnormalities 5 months after starting nitrofurantoin [bilirubin 2.5 mg/dL, ALT 100 U/L, Alk P 35 U/L], resolving within 2 weeks of stopping and recurring within days of restarting; 50 year old woman developed rash, fever and arthralgias within a week of starting nitrofurantoin [bilirubin normal, ALT 100 U/L, Alk P normal], resolving within 2 months).
- Selroos O, Edgren J. Lupus-like syndrome associated with pulmonary reaction to nitrofurantoin. Report of three cases. Acta Med Scand. 1975;197:125–9. [PubMed: 1079107](Three patients who developed pulmonary toxicity and a lupus-like syndrome 12-38 months after starting nitrofurantoin included a 73 year old woman with liver injury [bilirubin normal, ALT 76 U/L, Alk P 9 times ULN, ANA positive] and biopsy showing chronic active hepatitis, whose liver test abnormalities resolved within 4 months of stopping and whose repeat liver biopsy was normal).
- Klemola H, Penttilä O, Runeberg L, Tallqvist G. Anicteric liver damage during nitrofurantoin medication. Scand J Gastroenterol. 1975;10:501–5. [PubMed: 1153946](Four women, ages 60 to 85 years, developed symptoms and liver test abnormalities without jaundice 10 weeks to 1.5 years after starting nitrofurantoin [ALT 161, 93, 69 and 466 U/L], with hyperglobulinemia and liver biopsy showing chronic hepatitis [3 with cirrhosis], but rapid resolution [2-6 weeks] upon stopping nitrofurantoin).
- Engel JJ, Vogt TR, Wilson DE. Cholestatic hepatitis after administration of furan derivatives. Arch Intern Med. 1975;135:733–5. [PubMed: 150825](42 year old woman developed fever, rash and nausea 5 days after starting nitrofurantoin [bilirubin 3.4 mg/dL, ALT 256 U/L, Alk P 350 U/L, atypical lymphocytosis], with recurrence of enzyme elevations within a week of starting furazolidone [a furan structurally related to nitrofurantoin] suppositories for vaginitis).
- Fagrell B, Strandberg I, Wengle B. A nitrofurantoin-induced disorder simulating chronic active hepatitis. A case report. Acta Med Scand. 1976;199:237–9. [PubMed: 1258704](78 year old woman developed abdominal pain and anorexia 12 months after starting daily nitrofurantoin [peak bilirubin 7.8 mg/dL, AST 930 U/L, ANA >1:1600], resolving within 2 months of stopping, but ANA still weakly positive one year later [1:25]).
- Strömberg A, Wengle B. Letter: Chronic active hepatitis induced by nitrofurantoin. Br Med J. 1976;2(6028):174–5. [PMC free article: PMC1687416] [PubMed: 1276848](Among 23 patients diagnosed with chronic active hepatitis between 1969-74, 8 appeared to have drug induced disease, 3 from oxyphenisatin, 1 dihydralazine, 1 sulfonamide, and 3 nitrofurantoin, all resolving with drug discontinuation).
- Strohscheer H, Wegener HH. MMW Munch Med Wochenschr. 1977;119:1535–6. [Nitrofurantoin-induced granulomatous hepatitis] German. [PubMed: 414090](Two women, ages 59 and 58 years, developed fever within days of starting nitrofurantoin with minimal ALT elevations and liver biopsies showing granulomas; the fever resolved once nitrofurantoin was stopped).
- Mullick FG, Drake RM, Irey NS. Morphologic changes in adverse drug reactions in infants and children. Hum Pathol. 1977;8:361–78. [PubMed: 302237](Review of pathology of adverse drug reactions in children, including 5 month old boy who developed rash 3 days after starting nitrofurantoin who then became jaundiced [bilirubin 10.6 mg/dL, ALT 1260 U/L] and died of liver failure a few weeks later; autopsy showed massive hepatic necrosis).
- Lindberg J, Lindholm A, Iwarson S. Genetic factors in the development of chronic active hepatitis. Lancet. 1977;1(8002):67–8. [PubMed: 63711](HLA B8 and or B12 was present in 14 of 16 [88%] cases of cryptogenic autoimmune hepatitis, but in only 12 of 25 [48%] cases of viral or drug induced chronic active hepatitis, 2 being due to nitrofurantoin).
- Maddrey WC, Boitnott JK. Drug-induced chronic liver disease. Gastroenterology. 1977;72:1348–53. [PubMed: 323097](Review of drugs that cause chronic hepatitis including oxyphenisatin, isoniazid, methyldopa, and phenothiazines; nitrofurantoin mentioned as a cause in 4 published cases, all with complete recovery on stopping the drug, but several with cirrhosis).
- Rotermund HM. Med Klin. 1977;72:2139–45. [Drug-induced chronic aggressive hepatitis] German . [PubMed: 593197](Review of clinical and diagnostic features of drug induced chronic active hepatitis, common causes being oxyphenisatin and methyldopa and rare ones isoniazid, halothane, salicylates and sulfonamides).
- Hokkanen OT, Sotaniemi EA. Liver injury and multiple drug therapy. Arch Toxicol Suppl. 1978;(1):173–6. [PubMed: 277098](Among 100 patients admitted for drug induced liver injury to one hospital in Finland during a 10 year period, the most commonly implicated agents were sulfonamides [n=36], oral contraceptives [20], nitrofurantoin [16: 16%], methyldopa [10], hydralazine [8], and chlorpromazine [6]; 74% were taking more than one agent).
- Hatoff DE, Cohen M, Schweigert BF, Talbert WM. Nitrofurantoin: another cause of drug-induced chronic active hepatitis? A report of a patient with HLA-B8 antigen. Am J Med. 1979;67:117–21. [PubMed: 463905](27 year old woman developed fatigue and abdominal pain with AST elevations [~210 U/L] which remained elevated until nitrofurantoin was stopped 6 months later, biopsy showed cirrhosis; she was later reexposed and developed fever and AST elevation [~700 U/L], which resolved on prednisone therapy).
- Iwarson S, Lindberg J, Lundin P. Nitrofurantoin-induced chronic liver disease. Clinical course and outcome of five cases. Scand J Gastroenterol. 1979;14(4):497–502. [PubMed: 482863](Five women, ages 45 to 64 years, developed chronic hepatitis 1-3 years after starting daily nitrofurantoin therapy [ALT 588, 576, 390, 420 and 384 U/L, ANA 1:25 to 1:400, IgG 1.7-2.1 g/dL], resolving completely on stopping, but cirrhosis in follow up in one patient).
- Gleckman R, Alvarez S, Joubert DW. Drug therapy reviews: nitrofurantoin. Am J Hosp Pharm. 1979;36:342–51. [PubMed: 369367](Nitrofurantoin is bactericidal and has a low rate of bacterial resistance, but minimal serum levels are achieved as it is rapidly excreted in the urine which results in excellent urinary concentrations that is the basis of its use in urinary tract and bladder infections; hepatotoxicity is not dose related and can be severe with a multitude of clinical presentations including chronic active hepatitis).
- Wright R. Drug-induced chronic hepatitis. Springer Semin Immunopathol. 1980;3:331–8. [PubMed: 7022714](Review of drug induced chronic hepatitis focusing upon nitrofurantoin and methyldopa).
- Holmberg L, Boman G, Böttiger LE, Eriksson B, Spross R, Wessling A. Adverse reactions to nitrofurantoin. Analysis of 921 reports. Am J Med. 1980;69:733–8. [PubMed: 7435512](Between 1966 and 1976, 921 adverse events due to nitrofurantoin were reported to a Swedish registry; 50 [6%] of which were hepatic, including 1 fatality due to cirrhosis; mean age 59 years, 86% women, half with latency <1 month, but some with latency >1 year).
- Black M, Rabin L, Schatz N. Nitrofurantoin-induced chronic active hepatitis. Ann Intern Med. 1980;92:62–4. [PubMed: 7350873](Two cases: 48 year old woman developed fatigue 18 months after starting daily nitrofurantoin [bilirubin 1.3 mg/dL, ALT 209 U/L, Alk P 85 U/L, ANA >1: 1280], resolving within 2 months of stopping, but with persistence of ANA; 59 year old woman developed fatigue 2 years after starting daily nitrofurantoin [bilirubin 4.2 mg/dL, ALT 1328 U/L, Alk P 242 U/L, ANA >1:1280], resolving within 3-6 months of stopping, but with persistence of ANA).
- Sharp JR, Ishak KG, Zimmerman HJ. Chronic active hepatitis and severe hepatic necrosis associated with nitrofurantoin. Ann Intern Med. 1980;92:14–9. [PubMed: 7350868](5 women with chronic hepatitis due to nitrofurantoin, arising 1 month to 3 years after starting therapy [bilirubin 5.9-44 mg/dL, AST 435-1000 U/L, ANA positive in 2 of 3 tested], biopsies showing chronic active hepatitis with variable degrees of fibrosis, 2 died; review of literature found 20 cases, all in women, usually arising after 6 months, few with fever or rash, some with cirrhosis, slow resolution).
- Tolman KG. Nitrofurantoin and chronic active hepatitis. Ann Intern Med. 1980;92:119–20. [PubMed: 7350855](Editorial accompanying articles by Black [1980] and Sharp [1980] discussing nitrofurantoin induced chronic hepatitis and its pathogenesis).
- Sippel PJ, Agger WA. Nitrofurantoin-induced granulomatous hepatitis. Urology. 1981;18:177–8. [PubMed: 7269024](49 year old woman developed fever and fatigue 1 month after starting nitrofurantoin [bilirubin not given, ALT 100 U/L, Alk P 255 U/L], with biopsy showing "granulomatous hepatitis", resolving within 4 months of stopping).
- Spoelstra P, Janssens AR, Ruiter DJ, de Vries RR. Ned Tijdschr Geneeskd. 1981;125:61–3. [Chronic active hepatitis caused by nitrofurantonin] Dutch. [PubMed: 7464961](72 year old woman developed hepatitis several years after starting nitrofurantoin [bilirubin 10.8 mg/dL, ALT 177 U/L, Alk P 382 U/L, ANA positive], biopsy suggesting chronic hepatitis, but resolving within several months of stopping).
- Anttinen H, Ahonen A, Leinonen A, Kallioinen M, Heikkinen ES. Diagnostic imaging of focal nodular hyperplasia of the liver developing during nitrofurantoin therapy. Acta Med Scand. 1982;211:227–32. [PubMed: 7080869](6 year old girl found to have focal nodular hyperplasia 7 months after starting daily nitrofurantoin therapy; all liver tests were normal and the lesion was successfully resected).
- Miller AR, Addis BJ, Clarke PD. Nitrofurantoin and chronic active hepatitis. Ann Intern Med. 1982;97:452. [PubMed: 7114646](65 year old man developed jaundice 12 months after starting daily nitrofurantoin [bilirubin 3.2 mg/dL, moderate ALT and Alk P elevations, ANA positivity], resolving with prednisone therapy within 4 months of stopping).
- Penn RG, Griffin JP. Adverse reactions to nitrofurantoin in the United Kingdom, Sweden, and Holland. Br Med J (Clin Res Ed). 1982;284(6327):1440–2. [PMC free article: PMC1498323] [PubMed: 6282377](Between 1964 and 1980, the UK drug regulatory authority received 18 reports of liver injury due to nitrofurantoin [1 fatal]; rates were similar to those from Sweden).
- Berry WR, Warren GH, Reichen J. Nitrofurantoin-induced cholestatic hepatitis from cow's milk in a teenaged boy. West J Med. 1984;140:278–80. [PMC free article: PMC1021624] [PubMed: 6730475](16 year old boy developed rash, fever and jaundice [bilirubin 15.4 mg/dL, ALT 68 U/L, Alk P 3 times ULN] without any drug exposure, except for recently drinking milk from cows who were being treated with parenteral nitrofurantoin).
- Baetens P, Ramboer C. Chronic active hepatitis due to hydroxymethyl-nitrofurantoin in a male patient. Acta Clin Belg. 1984;39:85–91. [PubMed: 6741394](66 year old man developed weight loss and fatigue, 4 years after starting daily nitrofurantoin [bilirubin 0.8 mg/dL, ALT 225 U/L, Alk P 152 U/L, IgG 2343 mg/dL, ANA positive], biopsy showing chronic hepatitis without fibrosis and laboratory abnormalities resolving within 3 months of stopping).
- Young TL, Achkar E, Tuthill R, Ferguson DR. Chronic active hepatitis induced by nitrofurantoin. Cleve Clin Q. 1985;52:253–6. [PubMed: 4028427](2 cases: 71 and 61 year old women developed chronic hepatitis 2 and 5 years after starting daily nitrofurantoin [bilirubin 16.0 and 10.5 mg/dL, AST 1828 and 1200 U/L, Alk P 580 and 400 U/L, ANA 1:320 and 1:160], both improving on stopping, but one requiring corticosteroids).
- Stricker BH, Blok AP, Claas FH, Van Parys GE. Desmet VJl. Hepatic injury associated with the use of nitrofurans: a clinicopathological study of 52 reported cases. Hepatology. 1988;8:599–606. [PubMed: 3371877](Analysis of 52 cases of nitrofurantoin hepatotoxicity reported to a Central Registry in the Netherlands; onset varied from less than a week to several years after starting nitrofurantoin; overall, 59% were jaundiced, 11% had rash and 20% eosinophilia, 45 were fatal, estimated to occur in 1:3000-1:5000 users; separated cases into "acute" vs "chronic" injury, chronic cases more likely to have ANA positivity and hepatocellular pattern of injury).
- Shah RR, Wade G. Reappraisal of the risk/benefit of nitrofurantoin: review of toxicity and efficacy. Adverse Drug React Acute Poisoning Rev. 1989;8:183–201. [PubMed: 2694823](Review of structure, pharmacokinetics, safety and efficacy of nitrofurantoin for urinary tract infections; nitrofurantoin is a nitrated furan 5-member ring with a hydantoin side chain, which is minimally metabolized by the liver and excreted rapidly in the urine).
- Coraggio MJ, Gross TP, Roscelli JD. Nitrofurantoin toxicity in children. Pediatr Infect Dis J. 1989;8:163–6. [PubMed: 2652087](Review of reports of severe adverse reactions to nitrofurantoin in children from literature and FDA reports found 40 reports, 11 had liver injury [2 fatal] yielding estimate of 1 case per million users).
- Kursbaum A, Rottenstreich E. Harefuah. 1990;119:427–8. [Acute hepatitis and pneumonitis associated with nitrofurantoin] Hebrew. [PubMed: 2074065](65 year old woman developed abdominal pain and cough 2 months after starting nitrofurantoin [AST 378 U/L, bilirubin and Alk P normal, ANA negative], resolving within 4 weeks of stopping).
- Mollison LC, Angus P, Richards M, Jones RMcL, Ireton J. Hepatitis due to nitrofurantoin. Med J Aust. 1992;156:347–9. [PubMed: 1588868](3 women, ages 33, 56 and 62 years, developed jaundice 4 years [2] and 6 weeks after starting daily nitrofurantoin therapy [bilirubin 27.1, 33.0 and 3.5 mg/dL, AST 1045, 839 and 94 U/L, Alk P 101, 247 and 322 U/L, ANA 1:160, 1:2560, and not provided], one had liver transplant, one died and one recovered).
- Paiva LA, Wright PJ, Koff RS. Long-term hepatic memory for hypersensitivity to nitrofurantoin. Am J Gastroenterol. 1992;87:891–3. [PubMed: 1615946](56 year old woman developed fever and jaundice 2.5 weeks after taking 2 doses of nitrofurantoin [bilirubin 11.4 rising to 32.4 mg/dL, ALT 3355 U/L, Alk P 215 U/L, protime 16 sec], and subsequent medical history identified a similar bout of hepatitis [bilirubin 21.8 mg/dL, ALT 470 U/L, Alk P 225 U/L] 3 months after starting nitrofurantoin 17 years earlier).
- Reinhart HH, Reinhart E, Korlipara P, Peleman R. Combined nitrofurantoin toxicity to liver and lung. Gastroenterology. 1992;102(4 Pt 1):1396–9. [PubMed: 1551546](67 year old woman developed cough, lung infiltrates and then jaundice 2 years after starting daily nitrofurantoin [bilirubin 7.6 rising to 14.6 mg/dL, ALT 1184 U/L, Alk P 203 U/L, ANA 1:640], resolving slowly upon stopping).
- Friis H, Andreasen PB. Drug-induced hepatic injury: an analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J Intern Med. 1992;232:133–8. [PubMed: 1506809](Adverse drug reaction reports between 1978 and 1987 in Denmark; nitrofurantoin accounted for 16 cases of liver injury [1.3%], 4 of which were fatal and ranking 4th as a cause of hepatotoxicity).
- Hebert MF, Roberts JP. Endstage liver disease associated with nitrofurantoin requiring liver transplantation. Ann Pharmacother. 1993;27:1193–4. [PubMed: 8251685](40 year old woman developed acute liver failure 4 weeks after starting nitrofurantoin [200 mg twice daily] [bilirubin 25.4 mg/dL, ALT 1182 U/L, Alk P 246 U/L, ANA negative], with progressive stupor and undergoing emergency liver transplantation).
- Mulberg AE, Bell LM. Fatal cholestatic hepatitis and multisystem failure associated with nitrofurantoin. J Pediatr Gastroenterol Nutr. 1993;17:307–9. [PubMed: 8271132](16 year old girl developed erythema multiforme, respiratory failure and jaundice [bilirubin 3.8 mg/dL, ALT 267 U/L, Alk P 610 U/L, GGT 331 U/L, eosinophils 29%], dying of respiratory failure despite improvement in liver injury upon stopping).
- Berson A, Freneaux E, Larrey D, Lepage V, Douay C, Mallet C, Fromenty B, Benhamou JP, Pessayre D. Possible role of HLA in hepatotoxicity. An exploratory study in 71 patients with drug-induced idiosyncratic hepatitis. J Hepatol. 1994;20:336–42. [PubMed: 8014443](HLA A, B, DR and DQ typing done on 71 patients with hepatotoxicity showed slight increase in A11 [23% vs 12% in controls] mostly with antidepressants [50%] and diclofenac [75%: 3/4], but otherwise without prominent correlations; no cases of nitrofurantoin were tested).
- Westphal JF, Vetter D, Brogard JM. Hepatic side-effects of antibiotics. J Antimicrob Chemother. 1994;33:387–401. [PubMed: 8040106](Review of hepatotoxicity of antibiotics including nitrofurantoin).
- Hautekeete ML. Hepatotoxicity of antibiotics. Acta Gastroenterol Belg. 1995;58:290–6. [PubMed: 7491842](Review of antibiotic hepatotoxicity including nitrofurantoin which can cause both an acute and chronic hepatitis, either hepatocellular or cholestatic, the incidence estimated to be 2 per 10,000 patient years).
- Reddy KR, Schiff ER. Hepatotoxicity of antimicrobial, antifungal, and antiparasitic agents. Gastroenterol Clin North Am. 1995;24:923–36. [PubMed: 8749905](Review of hepatotoxicity of antimicrobials, nitrofurantoin being mentioned as the prototype example of drug induced chronic hepatitis, arising after 4 weeks to 11 years of therapy, usually in older patients and accompanied by autoantibodies).
- Burgert SJ, Burke JP, Box TD. Reversible nitrofurantoin-induced chronic active hepatitis and hepatic cirrhosis in a patient awaiting liver transplantation. Transplantation. 1995;59:448–9. [PubMed: 7871583](51 year old woman developed jaundice 12 years after starting daily nitrofurantoin [bilirubin 3.8 mg/dL, ALT 470 U/L, Alk P 168 U/L, ANA negative, globulins 5.9 g/dL], which worsened over 6 month period, but then resolved upon stopping and with corticosteroid therapy).
- George DK, Crawford DH. Antibacterial-induced hepatotoxicity. Incidence, prevention and management. Drug Saf. 1996;15:79–85. [PubMed: 8862966](Review of hepatotoxicity of antibiotics including nitrofurantoin).
- Pillans PI. Drug associated hepatic reactions in New Zealand: 21 years experience. N Z Med J. 1996;109:315–9. [PubMed: 8816722](Adverse drug reaction reports over 21 year period in New Zealand identified 943 cases of hepatotoxicity; nitrofurantoin accounted for 12 cases [1.3%] and ranked 20th overall).
- Yalçin S, Sahin A, Yalçin B, Altinok G. Nitrofurantoin toxicity to both liver and lungs. Liver. 1997;17:166–7. [PubMed: 9249732](62 year old woman developed cough and shortness of breath after taking nitrofurantoin daily for 5 years [bilirubin 0.9 mg/dL, ALT 178 U/L, Alk P 77 U/L, globulins 6.3 g/dL, ANA positive], resolving within 2 weeks of stopping).
- Kelly BD, Heneghan MA, Bennani F, Connolly CE, O'Gorman TA. Nitrofurantoin-induced hepatotoxicity mediated by CD8+ T cells. Am J Gastroenterol. 1998;93:819–21. [PubMed: 9625135](75 year old woman developed cough and fatigue 18 months after starting daily nitrofurantoin [bilirubin 0.8 mg/dL, ALT 238 U/L, Alk P 126 U/L], resolving within 6 months of stopping).
- Brumfitt W, Hamilton-Miller JM. Efficacy and safety profile of long-term nitrofurantoin in urinary infections: 18 years' experience. J Antimicrob Chemother. 1998;42:363–71. [PubMed: 9786476](Retrospective analysis of 219 women on long term nitrofurantoin prophylaxis of urinary tract infections, reported no serious adverse events or jaundice; nausea occurred in 46%, but often resolved with continued use or dose adjustment).
- Karmon Y, Edoute Y. Harefuah. 1999;136:406–8. [Hepatic injury associated with use of nitrofurantoin] Hebrew. [PubMed: 10914251](Review of liver injury of nitrofurantoin).
- Schattner A, Von der Walde J, Kozak N, Sokolovskaya A, Knobler H. Nitrofurantoin-induced immune-mediated lung and liver disease. Am J Med Sci. 1999;317:336–40. [PubMed: 10334121](60 year old woman developed cough and jaundice 3 years after starting daily nitrofurantoin [bilirubin 13.2 rising to 21.0 mg/dL, ALT 467 U/L, Alk P 352 U/L, ANA positive], who died suddenly of sepsis soon thereafter).
- Dam-Larsen S, Kromann-Andersen H. Ugeskr Laeger. 1999;161:6650–2. [Hepatic toxicity of nitrofurantoin. Cases reported to the Center for Monitoring Adverse Drug Reactions 1968-1998] Danish. [PubMed: 10643355](44 cases of nitrofurantoin hepatotoxicity were reported to a Danish registry between 1968 and 1998; 93% were women, median age 69 years, injury arising after 2 days to 7 years of use, 4 had acute liver failure, 5 had recurrence on reexposure).
- Edoute Y, Karmon Y, Roguin A, Ben-Ami H. Fatal liver necrosis associated with the use of nitrofurantoin. Isr Med Assoc J. 2001;3:382–3. [PubMed: 11411208](73 year old woman developed jaundice 14 months after starting daily nitrofurantoin [bilirubin 14.5 mg/dL, ALT 884 U/L, Alk P 190 U/L, ANA positive], treated with corticosteroids, but developed hepatic failure, ascites, renal failure and died 13 days after admission).
- Amit G, Cohen P, Ackerman Z. Nitrofurantoin-induced chronic active hepatitis. Isr Med Assoc J. 2002;4:184–6. [PubMed: 11908259](3 women, ages 64 to 76 years developed jaundice 1-5 years after starting nitrofurantoin [bilirubin 14.7, 15.3 and 2.2 mg/dL, ALT 1700, 515 and 850 U/L, Alk P 300, 390 and 110 U/L, all ANA positive], all treated with corticosteroids, 1 died).
- Liu ZX, Kaplowitz N. Immune-mediated drug-induced liver disease. Clin Liver Dis. 2002;6:755–74. [PubMed: 12362579](Review of role of immune system in drug induced liver disease; nitrofurantoin mentioned, but not specifically discussed).
- Thiim M, Friedman LS. Hepatotoxicity of antibiotics and antifungals. Clin Liver Dis. 2003;7:381–99. vi-vii. [PubMed: 12879990](Review of hepatotoxicity of antimicrobials including nitrofurantoin).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants done in the United States between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, 7 of which [5%] were attributed to nitrofurantoin, ranking 6th in frequency).
- Karpman E, Kurzrock EA. Adverse reactions of nitrofurantoin, trimethoprim and sulfamethoxazole in children. J Urol. 2004;172:448–53. [PubMed: 15247700](Review of side effects of nitrofurantoin in children, including cutaneous, gastrointestinal, hematologic, neurologic, pulmonary and liver adverse events; hepatic side effects are the most lethal complications, with 20 cases reported in children, 3 fatal).
- Linnebur SA, Parnes BL. Pulmonary and hepatic toxicity due to nitrofurantoin and fluconazole treatment. Ann Pharmacother. 2004;38:612–6. [PubMed: 14966256](73 year old man developed cough, shortness of breath and pulmonary infiltrates and found to have ALT elevations [223 U/L], with normal bilirubin 5 years after starting nitrofurantoin and 2 months after starting fluconazole, resolving within 4 weeks of stopping both medications).
- Galan MV, Potts JA, Silverman AL, Gordon SC. The burden of acute nonfulminant drug-induced hepatitis in a United States tertiary referral center. J Clin Gastroenterol. 2005;39:64–7. [PubMed: 15599214](Between 1993 and 2002, 96 patients were evaluated for acute liver disease at a single referral center; 32 were attributed to a medication among whom 44% were women, mean age 42 [range 16-80] years, and 3 were due to nitrofurantoin [10%], making it 4th in frequency).
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, et al. Spanish Group for the Study of Drug-Induced Liver Disease. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–21. [PubMed: 16083708](Reports to a Spanish network found 461 cases of drug induced liver disease, nitrofurantoin was not listed in the top 20 agents implicated [those with at least 5 cases]).
- Salle V, Lafon B, Smail A, Cévallos R, Chatelain D, Andréjak M, Ducroix JP. Rev Med Interne. 2006;27:344–6. [Nitrofurantoin-induced lupus-like syndrome associated with hepatitis] [PubMed: 16364504](71 year old woman developed polyarthralgias and liver test abnormalities after 4 years of therapy with nitrofurantoin [bilirubin not given, ALT 263 U/L, Alk P 310 U/L, GGT 801 U/L, ANA 1:2560], improving rapidly upon withdrawal and with prednisone therapy).
- Boelsterli UA, Ho HK, Zhou S, Leow KY. Bioactivation and hepatotoxicity of nitroaromatic drugs. Curr Drug Metab. 2006;7:715–27. [PubMed: 17073576](Review of metabolism and hepatic toxicity of nitroaromatic drugs, including nitrofurantoin which causes oxidative stress within hepatocytes which can deplete glutathione, produce oxidative free radicals, disrupt calcium homeostasis and increase labile iron pools).
- Koulaouzidis A, Bhat S, Moschos J, Tan C, De Ramon A. Nitrofurantoin-induced lung- and hepatotoxicity. Ann Hepatol. 2007;6:119–21. [PubMed: 17519837](57 year old woman developed cough, pulmonary infiltrates and abnormal liver tests 16 months after starting nitrofurantoin [bilirubin 0.5 mg/dL, ALT 424 U/L, Alk P 282 U/L, ANA negative], resolving within 4 months of stopping).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, nitrofurantoin was implicated in 14 cases and ranked second only to amoxicillin/clavulanate as a cause of liver injury).
- Aksamija A, Horvat G, Habek D, Zalac D, Jendris E. Nitrofurantoin-induced acute liver damage in pregnancy. Arh Hig Rada Toksikol. 2009;60:357–61. [PubMed: 19789166](31 year old woman in her 36th week of gestation was found to have serum enzyme elevations 2 weeks after starting nitrofurantoin [bilirubin 1.2 mg/dL, ALT 940 U/L, Alk P 273 U/L] at the time of induced delivery, which resolved rapidly upon stopping the medication).
- Beigel R, Perets R, Mouallem M. Acute kidney injury, hepatitis, and CPK elevation associated with nitrofurantoin therapy. Am J Med Sci. 2009;337:132–3. [PubMed: 19214031](57 year old woman developed fever within a day of starting nitrofurantoin and was found to have serum enzyme elevations when it was stopped after 5 days [bilirubin 1.1 mg/dL, ALT 192 U/L, Alk P 183 U/L, 11.7% eosinophils], resolving rapidly).
- Cetti RJ, Venn S, Woodhouse CR. The risks of long-term nitrofurantoin prophylaxis in patients with recurrent urinary tract infection: a recent medico-legal case. BJU Int. 2009;103:567–9. [PubMed: 18782308](Woman who developed pulmonary and hepatic complications after 2.5 years of nitrofurantoin therapy successfully sued her physicians for failing to follow recommendations of the British National Formulary).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, of which 12 were attributed to nitrofurantoin which ranked in the top 3 most common single causes).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India; none were attributed to nitrofurantoin].
- Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, Neuhauser M, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51:2040–8. [PubMed: 20512992](Retrospective analysis of 261 cases of autoimmune hepatitis, 24 [9%] of which were due to a medication; 11 nitrofurantoin and 11 minocycline; drug induced cases resembled idiopathic cases in all regards, except that all patients were able to stop corticosteroids without relapse).
- Appleyard S, Saraswati R, Gorard DA. Autoimmune hepatitis triggered by nitrofurantoin: a case series. J Med Case Rep. 2010;4:311. [PMC free article: PMC2955056] [PubMed: 20863377](Three women, ages 65, 42 and 74 years developed liver injury 2-6 years after starting nitrofurantoin [bilirubin 37.9, normal and 23 mg/dL, ALT 1322, 469 and 1423 U/L, Alk P 227, normal and 207 U/L, ANA 1:320 to 1: 640], all survived, but required corticosteroid therapy for extended periods).
- Ozaslan E. Drug-induced autoimmune hepatitis: an easily reversible type of liver fibrosis? Hepatology. 2011;53:370. [PubMed: 20848612](Letter in response to Björnsson [2010] discussing the reversibility of early fibrosis in cases of drug induced autoimmune hepatitis).
- Czaja AJ. Drug-induced autoimmune-like hepatitis. Dig Dis Sci. 2011;56:958–76. [PubMed: 21327704](Review of drug induced autoimmune hepatitis, the principal causes being minocycline and nitrofurantoin; other causes being methyldopa, hydralazine, statins, fibrates, diclofenac, anti-TNF agents, interferons, propylthiouracil and isoniazid).
- Sugimoto K, Ito T, Yamamoto N, Shiraki K. Seven cases of autoimmune hepatitis that developed after drug-induced liver injury. Hepatology. 2011;54:1892–3. [PubMed: 21725992](Letter describing 7 patients, one man and 6 women, ages 20 to 68 years, with autoimmune hepatitis attributed to medications, but with relapsing course despite stopping medications which included ofloxacin, a cephalosporin, benzbromarone, NSAIDs and herbals).
- Milić R, Plavec G, Tufegdzić I, Tomić I, Sarac S, Loncarević O. Nitrofurantoin-induced immune-mediated lung and liver disease. Vojnosanit Pregl. 2012 Jun;69(6):536–40. [PubMed: 22779302](55 year old woman developed lung and liver injury after long term use of nitrofurantoin, resolving with stopping therapy and corticosteroids).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144(7):1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 4 attributed to nitrofurantoin [ranking 3rd] for an estimated incidence of 1 per 1369 persons exposed).
- Sakaan SA, Twilla JD, Usery JB, Winton JC, Self TH. Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication. South Med J. 2014;107:107–13. [PubMed: 24926677](Review of the incidence, epidemiology, pathogenesis, clinical features, course and outcome, and management of nitrofurantoin induced liver injury).
- deLemos AS, Foureau DM, Jacobs C, Ahrens W, Russo MW, Bonkovsky HL. Drug-induced liver injury with autoimmune features. Semin Liver Dis. 2014;34:194–204. [PubMed: 24879983](Review of drug induced liver injury with autoimmune features including examples, one due to nitrofurantoin).
- Hydes T, Wright M, Jaynes E, Nash K. Nitrofurantoin immune-mediated drug-induced liver injury: a serious complication of a commonly prescribed medication. BMJ Case Rep. 2014;2014:bcr2013203136. pii. [PMC free article: PMC3947996] [PubMed: 24599428](2 women, ages 50 and 75, developed jaundice 12 months after starting chronic therapy with nitrofurantoin, both with ALT elevations known for 6 months [bilirubin 7.7 and 7.6 mg/dL, ALT 747 and 868 U/L, Alk P 294 and 132 U/L, INR normal and 1.5, ANA negative but IgG elevated in both], both improving rapidly with stopping nitrofurantoin and starting prednisone).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, 17 were attributed to nitrofurantoin [9.6%], ranking third).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 42 cases [5.8%] were attributed to nitrofurantoin, ranking third; mean age 64 years, all women, 62% hepatocellular, 52% ANA positive, mean initial ALT 932 U/L, Alk P 278 U/L, bilirubin 8.7 mg/dL, 9% fatal, 24% with evidence of chronic injury in follow up).
- Yeong TT, Lim KH, Goubet S, Parnell N, Verma S. Natural history and outcomes in drug-induced autoimmune hepatitis. Hepatol Res. 2016;46:E79–88. [PubMed: 25943838](Among 82 patients with autoimmune hepatitis, 11 were due to medication [nitrofurantoin 4, statins 4, herbal products in 2 and diclofenac in 2], clinical features were similar but those due to medications rarely relapsed on stopping and none died or required liver transplant).
- Stine JG, Northup PG. Autoimmune-like drug-induced liver injury: a review and update for the clinician. Expert Opin Drug Metab Toxicol. 2016;12:1291–301. [PubMed: 27402321](Review of drug induced liver injury with autoimmune features).
- Claussen K, Stocks E, Bhat D, Fish J, Rubin CD. How common are pulmonary and hepatic adverse effects in older adults prescribed nitrofurantoin? J Am Geriatr Soc. 2017;65:1316–20. [PMC free article: PMC5478436] [PubMed: 28306135](Among 3400 patients 65 years or older prescribed nitrofurantoin over a 7 year period, electronic medical records identified 641 with a possible adverse event, but on review of charts most were considered unrelated and only one case of hepatotoxicity was considered possibly related).
- Björnsson ES. Drug-induced liver injury due to antibiotics. Scand J Gastroenterol. 2017;52:617–23. [PubMed: 28276834](Review of the hepatotoxicity of antibiotics which are the single major drug class implicated in liver injury and nitrofurantoin often ranking among the top 3 antimicrobials, the estimated frequency being ~1:1500 users, cases usually having hepatocellular pattern of injury often with autoimmune features).
- Prieto de Paula JM, Martín-Luquero Ibáñez M, Franco Hidalgo S. Nitrofurantoin and non-autoimmune hepatitis. Med Clin (Barc). 2017;148:382–3. [PubMed: 28238334](47 year old woman with recurrent cystitis developed jaundice and abdominal pain 3 months after starting nitrofurantoin [bilirubin 6.4 mg/dL, ALT 1680 U/L, Alk P 220 U/L, SMA 1:40, ANA negative], resolving within 45 days of stopping).
- de Boer YS, Kosinski AS, Urban TJ, Zhao Z, Long N, Chalasani N, Kleiner DE, et al. Drug-Induced Liver Injury Network. Features of autoimmune hepatitis in patients with drug-induced liver injury. Clin Gastroenterol Hepatol. 2017;15:103–112.e2. [PMC free article: PMC5370577] [PubMed: 27311619](Among 42 cases of nitrofurantoin induced liver injury, all were women, median age 65 years, latency less than 1 week in 5 and greater than 1 year in 26 cases, initial median ALT 739 U/L, Alk P 190 U/L, bilirubin 4.7 mg/dL, with autoimmune features in 83%, severe course in 30%, fatal in 2 and liver transplant in 3; ANA levels declined during follow up after stopping drug).
- Ramadas P, Krishnan P, Frechette V. An unusual case of nitrofurantoin-associated hepatotoxicity. Am J Ther. 2018;25:e378–e379. [PubMed: 28207423](79 year old woman with recurrent cystitis on nitrofurantoin for 6 years developed fatigue and anorexia [bilirubin 2.8 mg/dL, ALT 246 U/L, Alk P 275 U/L, ANA positive], with cirrhosis and splenomegaly on CT scan and improvement on stopping drug with normal liver enzymes 3 months later).
- Martínez-Casas OY, Díaz-Ramírez GS, Marín-Zuluaga JI, Muñoz-Maya O, Santos O, Donado-Gómez JH, Restrepo-Gutiérrez JC. Differential characteristics in drug-induced autoimmune hepatitis. JGH Open. 2018;2:97–104. [PMC free article: PMC6207017] [PubMed: 30483571](Among 190 patients diagnosed with autoimmune hepatitis over a 7 year period, 12 were considered drug induced from nitrofurantoin [n=8], diclofenac [n=2], adalimumab [1] or propylthiouracil [1], but were similar in age, gender and autoantibody status to spontaneous autoimmune hepatitis, differing by ability to stop corticosteroids [92% vs 23%], and absence of cirrhosis and liver transplantation and death which occurred in 13%, 6% and 3% of spontaneous cases).
- Kapral N, Saxena R, Sule AA, Markle B. Nitrofurantoin: friend or foe? BMJ Case Rep 2018; 2018. pii: bcr-2018-225629. [PMC free article: PMC6101326] [PubMed: 30115718](69 year old man developed jaundice within 5 days of starting nitrofurantoin for cystitis [bilirubin 12.4 mg/dL, ALT 608 U/L, Alk P 2640 U/L, INR 1.35], improving on stopping and with corticosteroid therapy).
- Cano-Paniagua A, Amariles P, Angulo N, Restrepo-Garay M. Epidemiology of drug-induced liver injury in a university hospital from Colombia: updated RUCAM being used for prospective causality assessment. Ann Hepatol. 2019;18:501–7. [PubMed: 31053545](Among 18 cases of drug induced liver injury seen at a University hospital in Colombia during a 1 year period [2015-16], 10 [56%] were attributed to antimicrobial agents including 1 due to nitrofurantoin).
- Benyamine A, Granel B, Belenotti P, Masson E, Default A, Garcia S, Serratrice J, et al. Severe nitrofurantoin-induced adverse drug reactions: is there a benefit of sequential therapy? Therapie. 2019;74:553–6. [PubMed: 31027709](72 year old woman developed pneumonitis and abnormal liver tests 6 months after starting nitrofurantoin [bilirubin 0.8 mg/dL, ALT 362 U/L, Alk P 99 U/L], biopsy showing chronic hepatitis, resolving within 40 days of stopping).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- An Unusual Case of Drug-Induced Liver Injury Secondary to Nitrofurantoin Use.[Cureus. 2022]An Unusual Case of Drug-Induced Liver Injury Secondary to Nitrofurantoin Use.Wonnacott S, Gala D, Shah M, Kaul D, Kumar V. Cureus. 2022 Jul; 14(7):e26882. Epub 2022 Jul 15.
- Review Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication.[South Med J. 2014]Review Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication.Sakaan SA, Twilla JD, Usery JB, Winton JC, Self TH. South Med J. 2014 Feb; 107(2):107-13.
- Autoimmune hepatitis triggered by nitrofurantoin: a case series.[J Med Case Rep. 2010]Autoimmune hepatitis triggered by nitrofurantoin: a case series.Appleyard S, Saraswati R, Gorard DA. J Med Case Rep. 2010 Sep 23; 4:311. Epub 2010 Sep 23.
- Nitrofurantoin-induced immune-mediated lung and liver disease.[Vojnosanit Pregl. 2012]Nitrofurantoin-induced immune-mediated lung and liver disease.Milić R, Plavec G, Tufegdzić I, Tomić I, Sarac S, Loncarević O. Vojnosanit Pregl. 2012 Jun; 69(6):536-40.
- Review Nitrofurantoin vs other prophylactic agents in reducing recurrent urinary tract infections in adult women: a systematic review and meta-analysis.[Am J Obstet Gynecol. 2016]Review Nitrofurantoin vs other prophylactic agents in reducing recurrent urinary tract infections in adult women: a systematic review and meta-analysis.Price JR, Guran LA, Gregory WT, McDonagh MS. Am J Obstet Gynecol. 2016 Nov; 215(5):548-560. Epub 2016 Jul 22.
- Nitrofurantoin - LiverToxNitrofurantoin - LiverTox
- Physiology, Bohr Effect - StatPearlsPhysiology, Bohr Effect - StatPearls
- Uncomplicated Urinary Tract Infections - StatPearlsUncomplicated Urinary Tract Infections - StatPearls
- A Quality Assurance Tool for JATS/BITS with Schematron and HTML reporting - Jour...A Quality Assurance Tool for JATS/BITS with Schematron and HTML reporting - Journal Article Tag Suite Conference (JATS-Con) Proceedings 2016
- Normal bacterial flora on hands - WHO Guidelines on Hand Hygiene in Health CareNormal bacterial flora on hands - WHO Guidelines on Hand Hygiene in Health Care
Your browsing activity is empty.
Activity recording is turned off.
See more...