NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Sotalol is a nonselective beta-adrenergic blocker used largely in the therapy cardiac arrhythmias. Sotalol has been linked to at least one instance of clinically apparent liver injury.
Background
Sotalol (soe' ta lol) is a nonselective beta-blocker, acting on both beta-1 and beta-2 adrenergic receptors. Beta-1 adrenergic blockade reduces the heart rate and myocardial contractility by slowing the AV conduction and suppressing automaticity. Beta-2 blockade affects peripheral vascular resistance and can also cause bronchospasm and hypoglycemia. In addition, sotalol has unique antiarrhythmic activity not shared by all beta-blockers. Sotalol is indicated for the management of atrial and ventricular arrhythmias. While it has antihypertensive effects, sotalol is not generally used for treatment of hypertension alone. Sotalol was approved for use in the United States in 1992 and its current indications include maintenance of normal sinus rhythm in patients with symptomatic atrial fibrillation and treatment of severe or life-threatening ventricular arrhythmias. Sotalol is available in tablets of 80, 120, 160 and 240 mg in generic forms and under the trade names Betapace, Betapace AF and Sorine. The typical initial dose of sotalol in adults is 80 mg twice daily, with subsequent dose adjustment based upon clinical response and tolerance, the typical maintenance dose being 160 to 320 mg daily. Common side effects of sotalol include bradycardia, hypotension, fatigue, dizziness, depression, memory loss, impotence, cold limbs and, less commonly, severe hypotension, heart failure and bronchospasm. Sudden withdrawal can trigger rebound hypertension. Beta-blockers are contraindicated in patients with asthma, bradycardia and heart failure and should be used cautiously in the elderly and in patients with diabetes.
Hepatotoxicity
Mild-to-moderate elevations in serum aminotransferase levels occur in less than 2% of patients on sotalol and are usually transient and asymptomatic, resolving even with continuation of therapy. Sotalol has been linked to a single case of clinically apparent liver injury, with onset of an acute hepatitis-like syndrome with prolonged jaundice 12 weeks after sotalol was initiated. The injury improved but did not resolve with discontinuation of sotalol. In large case series of drug induced liver disease and acute liver failure, sotalol has not been listed as a cause. Other beta-blockers have been linked to rare instances of acute clinically apparent liver injury with a latency to onset ranging from 4 to 24 weeks, a hepatocellular pattern of serum enzyme elevations and a mild, self-limiting course without evidence of hypersensitivity or autoimmune reactions.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Sotalol undergoes minimal metabolism by the liver and is excreted largely unchanged in the urine. The reason why sotalol might cause liver injury is unknown. The acute liver injury attributed to other beta-blockers is likely to be idiosyncratic.
References to the safety and potential hepatotoxicity of sotalol are provided in the overview on Beta-Adrenergic Receptor Antagonists, last updated in June 2019.
Drug Class: Beta-Adrenergic Receptor Antagonists
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Sotalol – Generic, Betapace®
DRUG CLASS
Beta-Adrenergic Receptor Antagonists
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
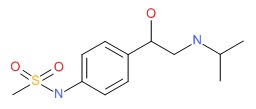
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Sotalol | 3930-20-9 | C12-H20-N2-O3-S |
 |
- PubChem SubstanceRelated PubChem Substances
- Review Sotalol: a new beta-adrenergic blocker for ventricular arrhythmias.[Prog Cardiovasc Dis. 1995]Review Sotalol: a new beta-adrenergic blocker for ventricular arrhythmias.Cavusoglu E, Frishman WH. Prog Cardiovasc Dis. 1995 May-Jun; 37(6):423-40.
- COMBINED PHARMACOLOGICAL THERAPY INCLUDING SEVERAL ANTIARRHYTHMIC AGENTS FOR TREATMENT OF DIFFERENT DISORDERS OF CARDIAC RHYTHM.[Georgian Med News. 2021]COMBINED PHARMACOLOGICAL THERAPY INCLUDING SEVERAL ANTIARRHYTHMIC AGENTS FOR TREATMENT OF DIFFERENT DISORDERS OF CARDIAC RHYTHM.Kapustnick Y, Lutsenko R, Sуdorenko A. Georgian Med News. 2021 Jun; (315):85-93.
- Review The Use of Intravenous Sotalol in Cardiac Arrhythmias.[Heart Lung Circ. 2018]Review The Use of Intravenous Sotalol in Cardiac Arrhythmias.Samanta R, Thiagalingam A, Turner C, Lakkireddy DJ, Kovoor P. Heart Lung Circ. 2018 Nov; 27(11):1318-1326. Epub 2018 Mar 29.
- Review Sotalol: a new agent for the treatment of ventricular arrhythmias.[Am J Med Sci. 1994]Review Sotalol: a new agent for the treatment of ventricular arrhythmias.Samoil D, Grubb BP, Temesy-Armos PN. Am J Med Sci. 1994 Jan; 307(1):49-53.
- Review Suppressant effects of conventional beta blockers and sotalol on complex and repetitive ventricular premature complexes.[Am J Cardiol. 1990]Review Suppressant effects of conventional beta blockers and sotalol on complex and repetitive ventricular premature complexes.Deedwania PC. Am J Cardiol. 1990 Jan 2; 65(2):43A-50A; discussion 51A-52A.
- Sotalol - LiverToxSotalol - LiverTox
- Prominent superficial veinsProminent superficial veinsMedGen
- C1837785[conceptid] (1)MedGen
- KANSL1L-AS1 KANSL1L antisense RNA 1 [Homo sapiens]KANSL1L-AS1 KANSL1L antisense RNA 1 [Homo sapiens]Gene ID:101928020Gene
- Gm25541 predicted gene, 25541 [Mus musculus]Gm25541 predicted gene, 25541 [Mus musculus]Gene ID:115489638Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...