NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Oxycodone is a semisynthetic, moderately potent, orally available opioid that is widely used for acute or chronic management of moderate- or moderately severe pain either alone or in combination with acetaminophen. Oxycodone by itself has not been linked to serum enzyme elevations during therapy or to clinically apparent liver injury. However, the combination of oxycodone with acetaminophen has been linked to many cases of acute liver failure caused by unintentional overdose with acetaminophen.
Background
Oxycodone (ox” i koe’ done) is a semisynthetic derivative of thebaine, a natural alkaloid found in the resin of poppy seeds (Papaver somniferum). It is well absorbed orally and has moderate opiate activity, acting as an agonist of the u type opiate receptor. Oxycodone alone or in combination with acetaminophen has been shown to be effective in ameliorating moderate- to moderately severe pain and is widely used for temporary as well as chronic management of pain states. Oxycodone has been in use since its first synthesis in 1917, but was formally approved for use in the United States in 1991. Since then, it has become one of the most commonly prescribed drugs in medical practice. Oxycodone is available in multiple formulations including oral tablets of 5, 7.5, 10 and 20 mg, as well as capsules of 5 mg, suppositories of 10 and 20 mg and oral solution in various concentrations generically. Higher dose oral formulations of 10 to 80 mg are also available generically and under the brand name OxyContin for therapy of persistent, moderate- to moderately severe pain that requires 24 hour opioid therapy. Oxycodone is also available in fixed combinations with other analgesics, including aspirin, ibuprofen, but particularly acetaminophen. These combinations are commonly used and available generically and under the brand names such as Percocet, Tylox and Endocet. The dose of oxycodone is typically 5 to 10 mg and acetaminophen 300 to 600 mg per tablet. Side effects of oxycodone include sedation, respiratory depression, mental clouding, euphoria, agitation, itching, constipation, diarrhea, abdominal bloating, nausea, vomiting, headache and dizziness. Severe adverse events include life-threatening respiratory depression, addiction, abuse, opioid withdrawal, serotonin syndrome (when used with serotonergic agents) and adrenal insufficiency. Oxycodone is a controlled substance and classified as a Schedule II drug, indicating that it has medical usefulness, but also a high potential for physical and psychological dependency and abuse. Unfortunately, oxycodone has become one of the most frequently abused prescription medications and some formulations can be dissolved and injected intravenously. With oxycodone dependence, patients may turn to illegal opiate drug use. Ironically, in some communities, heroin is more available and less expensive than oxycodone by prescription. Tamper deterrent formulations have now become available.
Hepatotoxicity
Despite wide scale use for many decades, oxycodone has not been convincingly linked to instances of clinically apparent acute liver injury. However, oxycodone and other opioid-acetaminophen combinations have become a common cause of acute liver injury, which is usually the result of excessive use of the medication for the opioid effect, which leads secondarily and unintentionally to an overdose of acetaminophen. In 2014, the FDA warned against the use of opioid combinations in which the dose of acetaminophen is greater than 325 mg per tablet or unit dose. Because of their potential for hepatotoxicity, opioid combinations in which the dose of acetaminophen is greater than 325 mg per tablet or capsule were discontinued.
Oxycodone, like other opiates, is metabolized in the liver by the P450 microsomal oxidizing enzyme system, and levels can be significantly affected by either inhibitors of CYP 3A4 (which increase levels and can lead to toxicity) or inducers of the enzyme (which decrease levels and reduce efficacy).
Likelihood score: E (unlikely cause of drug-induced liver injury).
References on the safety and potential hepatotoxicity of oxycodone are given in the overview section of the Opioids.
Drug Class: Opioids
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Oxycodone – Generic, OxyContin®
DRUG CLASS
Opioids
Product labeling at DailyMed, National Library of Medicine, NIH
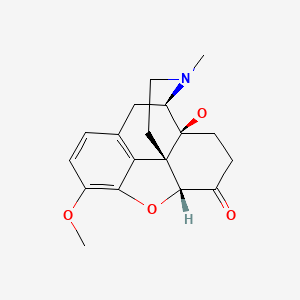
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Oxycodone | 76-42-6 | C18-H21-N-O4 |
|
- PubChem SubstanceRelated PubChem Substances
- Review Opioids.[LiverTox: Clinical and Researc...]Review Opioids.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Hydrocodone.[LiverTox: Clinical and Researc...]Review Hydrocodone.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Opioid-related overdose and chronic use following an initial prescription of hydrocodone versus oxycodone.[PLoS One. 2022]Opioid-related overdose and chronic use following an initial prescription of hydrocodone versus oxycodone.Weiner SG, Hendricks MA, El Ibrahimi S, Ritter GA, Hallvik SE, Hildebran C, Weiss RD, Boyer EW, Flores DP, Nelson LS, et al. PLoS One. 2022; 17(4):e0266561. Epub 2022 Apr 5.
- Comparison of the efficacy and safety of dual-opioid treatment with morphine plus oxycodone versus oxycodone/acetaminophen for moderate to severe acute pain after total knee arthroplasty.[Clin Ther. 2013]Comparison of the efficacy and safety of dual-opioid treatment with morphine plus oxycodone versus oxycodone/acetaminophen for moderate to severe acute pain after total knee arthroplasty.Richards P, Gimbel JS, Minkowitz HS, Kelen R, Stern W. Clin Ther. 2013 Apr; 35(4):498-511. Epub 2013 Mar 29.
- Analgesic efficacy and tolerability of oxycodone 5 mg/ibuprofen 400 mg compared with those of oxycodone 5 mg/acetaminophen 325 mg and hydrocodone 7.5 mg/acetaminophen 500 mg in patients with moderate to severe postoperative pain: a randomized, double-blind, placebo-controlled, single-dose, parallel-group study in a dental pain model.[Clin Ther. 2005]Analgesic efficacy and tolerability of oxycodone 5 mg/ibuprofen 400 mg compared with those of oxycodone 5 mg/acetaminophen 325 mg and hydrocodone 7.5 mg/acetaminophen 500 mg in patients with moderate to severe postoperative pain: a randomized, double-blind, placebo-controlled, single-dose, parallel-group study in a dental pain model.Litkowski LJ, Christensen SE, Adamson DN, Van Dyke T, Han SH, Newman KB. Clin Ther. 2005 Apr; 27(4):418-29.
- major allergen [Daucus carota]major allergen [Daucus carota]gi|2154732|emb|CAB03715.1|Protein
- Oxycodone - LiverToxOxycodone - LiverTox
- KRTAP5-9 keratin associated protein 5-9 [Homo sapiens]KRTAP5-9 keratin associated protein 5-9 [Homo sapiens]Gene ID:3846Gene
- 3846[uid] AND (alive[prop]) (1)Gene
- CCDC39-AS1 CCDC39 antisense RNA 1 [Homo sapiens]CCDC39-AS1 CCDC39 antisense RNA 1 [Homo sapiens]Gene ID:100874112Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...