NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Eravacycline is a parenterally administered tetracycline-like antibiotic used to treat moderate-to-severe intraabdominal infections due to susceptible organisms. Eravacycline use has been associated with a low rate of serum enzyme elevations during therapy but has not been convincingly linked to instances of clinically apparent acute liver injury.
Background
Eravacycline (er" a va sye' kleen) is a parenterally administered, broad spectrum synthetic tetracycline-like antibiotic with potent activity against many gram-positive and gram-negative aerobic and anaerobic organisms including many multidrug resistant organisms. Eravacycline is similar to tigecycline both in structure and in spectrum of activity and demonstrates somewhat greater potency in vitro. Tetracycline antibiotics act by binding to bacterial ribosomes and inhibiting protein synthesis. In multiple clinical trials, intravenous eravacycline has been found to be effective in treating intraabdominal and urinary tract infections. Eravacycline was approved for use in the United States for complicated intraabdominal infections in adults in 2018 and is available as 50 mg of lyophilized powder in single dose vials for reconstitution under the brand name Xerava. The recommended dose is 1 mg per kilogram intravenously every 12 hours for total of 4 to 14 days. Oral formulations for continuation as outpatient therapy are under evaluation. Common side effects of eravacycline include gastrointestinal upset, nausea and vomiting, and infusion site reactions. Tetracyclines can cause vertigo and tinnitus, skin photosensitivity reactions, and staining of developing teeth and inhibition of bone growth in children or when taken by a pregnant mother, for which reasons eravacycline should not be used in pregnant women or children below the age of nine.
Hepatotoxicity
In preclinical trials of intravenous eravacycline, serum aminotransferase elevations were mild and no more frequent than with placebo or comparator treatment arms. There were no instances of clinically apparent liver injury that could be attributed to eravacycline. Nevertheless, other tetracycline antibiotics are well known causes of drug induced liver injury. In particular, high doses of intravenous tetracycline are known to be able to cause severe hepatic microvesicular steatosis with lactic acidosis and severe hepatic dysfunction (LASH syndrome). For this reason, intravenous tetracycline was withdrawn from use. This complication has not been reported with intravenous eravacycline, omadacycline or tigecycline. The tetracyclines have also been linked to idiosyncratic liver injury with autoimmune features that generally arise with long term use and has most commonly been associated with minocycline. This complication also has not been reported with eravacycline, omadacycline or tigecycline.
Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which eravacycline might cause liver injury is unknown. Eravacycline is metabolized in the liver by the microsomal cytochrome P450 system, largely via CYP 3A4. Eravacycline is susceptible to drug-drug interactions with modulators of CYP 3A4, inducers causing a decrease in eravacycline plasma levels.
Outcome and Management
Patients on intravenous eravacycline who develop serum aminotransferase elevations that rise above 5 times the upper limit of normal or are accompanied by jaundice or symptoms should have eravacycline discontinued. Whether there is cross sensitivity to hepatic injury among the various tetracyclines is not known, but switching to another class of antibiotics would be more appropriate than changing to another tetracycline-like agent in patients who develop significant hepatic injury while receiving eravacycline.
Drug Class: Antiinfective Agents, Tetracyclines
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Eravacycline – Xerava®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
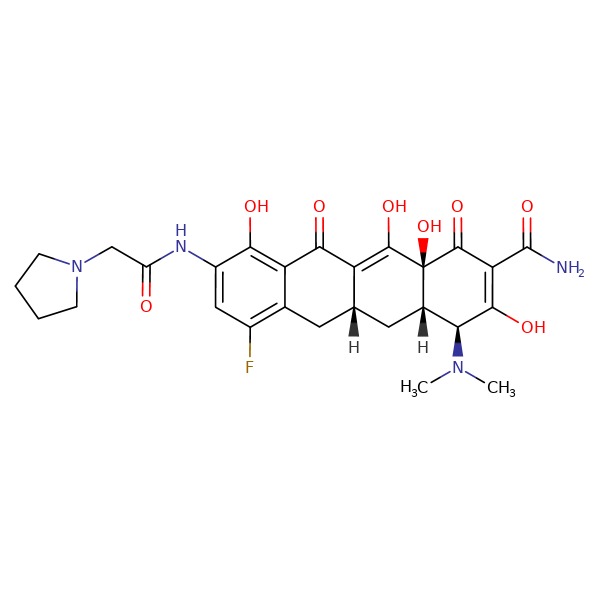
| Eravacycline | 1207283-85-9 | C29-H39-N5-O8 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 10 April 2019
- Zimmerman HJ. Tetracyclines. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999. p. 599-602.(Expert review of tetracycline and liver injury published in 1999, long before the availability of eravacycline; the tetracyclines cause two forms of drug induced liver injury, microvesicular fat and liver failure occurring after 4-10 days with high doses of parenteral tetracyclines and an idiosyncratic liver injury that occurs with the oral agents, doxycycline causing a cholestatic and minocycline a hepatocellular injury which may be associated with autoimmune features).
- Moseley RH. Tetracyclines. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 468.(Expert review of tetracycline induced liver injury; mentions that the hepatotoxicity of intravenous tetracycline is of historic interest only as it is no longer given parenterally; both doxycycline and minocycline have been associated with idiosyncratic liver injury; tigecycline and eravacycline are not discussed).
- MacDougall C. Protein synthesis inhibitors and miscellaneous antibacterial agents. In, Brunton LL, Hilal-Dandan R, Knollman KC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1049-65.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/scripts/cder/daf/ (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; mentions that the toxicity profile of eravacycline is consistent with other tetracycline-like antibiotics, and while ALT elevations above twice ULN arose in 9-20% of eravacycline treated subjects, these rates were no different than with comparator antibiotics–such as meropenem and ertapenem [10-19%] and no patient developed clinically apparent liver injury attributable to eravacycline). - Solomkin JS, Ramesh MK, Cesnauskas G, Novikovs N, Stefanova P, Sutcliffe JA, Walpole SM, et al. Phase 2, randomized, double-blind study of the efficacy and safety of two dose regimens of eravacycline versus ertapenem for adult community-acquired complicated intra-abdominal infections. Antimicrob Agents Chemother 2014; 58: 1847-54. [PMC free article: PMC4023720] [PubMed: 24342651](Among 139 patients with intraabdominal infections treated with eravacycline [1.0 mg/kg every 12 hours or 1.5 mg/kg every 24 hours] vs ertapenem [1 g every 24 hours], clinical success rates were similar [100% and 93% vs 92%] and no “safety signals as evaluated by laboratory tests… were identified”).
- Zhanel GG, Cheung D, Adam H, Zelenitsky S, Golden A, Schweizer F, Gorityala B, et al. Review of eravacycline, a novel fluorocycline antibacterial agent. Drugs 2016; 76: 567-88. [PubMed: 26863149](Review of the structure, antibacterial spectrum of activity, pharmacology, clinical efficacy and safety of eravacycline; mentions that the most common adverse event is nausea and vomiting, but that other event rates are similar to those in comparator arm subjects; no discussion of ALT elevations or hepatotoxicity).
- Solomkin J, Evans D, Slepavicius A, Lee P, Marsh A, Tsai L, Sutcliffe JA, et al. Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the investigating gram-negative infections treated with eravacycline (IGNITE 1) trial: a randomized clinical trial. JAMA Surg 2017; 152: 224-32. [PubMed: 27851857](Among 541 patients with complicated intraabdominal infections treated with eravacycline [1 mg/kg every 12 hours] or ertapenem [1 gm every 24 hours] for 4-14 days, rates of clinical cure were similar [87% vs 88%] while adverse events were more frequent with eravacycline [42% vs 28%], although severe adverse event rates were similar [6% vs 5.6%]; no mention of ALT elevations or hepatotoxicity).
- Newman JV, Zhou J, Izmailyan S, Tsai L. Randomized, double-blind, placebo-controlled studies of the safety and pharmacokinetics of single and multiple ascending doses of eravacycline. Antimicrob Agents Chemother 2018; 62. pii: e01174-18. [PMC free article: PMC6201080] [PubMed: 30150464](Pharmacokinetic studies in healthy subjects indicated that a 1 mg/kg dose of eravacycline was most appropriate and that it was generally well tolerated with dose related nausea, infusion site reactions and superficial phlebitis being the most common adverse events).
- Solomkin JS, Gardovskis J, Lawrence K, Montravers P, Sway A, Evans D, Tsai L. IGNITE4: Results of a phase 3, randomized, multicenter, prospective trial of eravacycline vs. meropenem in the treatment of complicated intra-abdominal infections. Clin Infect Dis 2018 Dec 18. [Epub ahead of print] [PMC free article: PMC6735687] [PubMed: 30561562](Among 400 hospitalized patients with intraabdominal infections treated with eravacycline or meropenem intravenously for 4-14 days, clinical cure rates were similar [91% vs 91%], and adverse events included nausea [5%], vomiting [4%] and diarrhea [3%]; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Goserelin.[LiverTox: Clinical and Researc...]Review Goserelin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Leuprolide.[LiverTox: Clinical and Researc...]Review Leuprolide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Pemetrexed.[LiverTox: Clinical and Researc...]Review Pemetrexed.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Trimetrexate.[LiverTox: Clinical and Researc...]Review Trimetrexate.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Pralatrexate.[LiverTox: Clinical and Researc...]Review Pralatrexate.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Eravacycline - LiverToxEravacycline - LiverTox
- Physiology, Ovulation - StatPearlsPhysiology, Ovulation - StatPearls
- History of the Controversy - HIV And The Blood SupplyHistory of the Controversy - HIV And The Blood Supply
- major allergen Bet v 1 [Betula pendula]major allergen Bet v 1 [Betula pendula]gi|1321724|emb|CAA96543.1|Protein
- 9536[uid] AND (alive[prop]) (1)Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...