NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Guideline Centre (UK). Chronic Heart Failure in Adults: Diagnosis and Management. London: National Institute for Health and Care Excellence (NICE); 2018 Sep. (NICE Guideline, No. 106.)
1.1. Full list of recommendations
- Take a careful and detailed history, and perform a clinical examination and tests to confirm the presence of heart failure. [2010]
- Measure N-terminal pro-B-type natriuretic peptide [NT-proBNP] in people with suspected heart failure. [2018]
- Because very high levels of NT-proBNP carry a poor prognosis, refer people with suspected heart failure and an NT-proBNP level above 2,000 ng/litre (236 pmol/litre) urgently, to have specialist assessment and transthoracic echocardiography within 2 weeks. [2018]
- Refer people with suspected heart failure and an NT-proBNP level between 400 and 2,000 ng/litre (47 to 236 pmol/litre) to have specialist assessment and transthoracic echocardiography within 6 weeks. [2018]
- Be aware that:
- an NT-proBNP level less than 400 ng/litre (47 pmol/litre) in an untreated person makes a diagnosis of heart failure less likely
- the level of serum natriuretic peptide does not differentiate between heart failure with reduced ejection fraction and heart failure with preserved ejection fraction. [2018]
- Review alternative causes for symptoms of heart failure in people with NT-proBNP levels below 400 ng/litre. If there is still concern that the symptoms might be related to heart failure, discuss with a physician with subspeciality training in heart failure. [2018]
- Be aware that:
- obesity, African or African-Caribbean family origin, or treatment with diuretics, angiotensin-converting enzyme(ACE) inhibitors , beta-blockers, angiotensin II receptor blockers (ARBs) or mineralcorticoid receptor antagonist (MRAs) can reduce levels of serum natriuretic peptides.
- high levels of serum natriuretic peptides can have causes other than heart failure (for example, age over 70 years, left ventricular hypertrophy, ischaemia, tachycardia, right ventricular overload, hypoxaemia [including pulmonary embolism], renal dysfunction [eGFR less than 60 ml/minute/1.73 m2], sepsis, chronic obstructive pulmonary disease, diabetes, or cirrhosis of the liver). [2010, amended 2018]
- Perform transthoracic echocardiography to exclude important valve disease, assess the systolic (and diastolic) function of the (left) ventricle, and detect intracardiac shunts. [2003, amended 2018]
- Transthoracic echocardiography should be performed on high-resolution equipment, by experienced operators trained to the relevant professional standards. Need and demand for these studies should not compromise quality. [2003, amended 2018]
- Ensure that those reporting echocardiography are experienced in doing so. [2003]
- Consider alternative methods of imaging the heart (for example, radionuclide angiography [multigated acquisition scanning], cardiac MRI or transoesophageal echocardiography) if a poor image is produced by transthoracic echocardiography. [2003, amended 2018]
- Perform an ECG and consider the following tests to evaluate possible aggravating factors and/or alternative diagnoses:
- chest X-ray
- blood tests:
- renal function profile
- thyroid function profile
- liver function profile
- lipid profile
- glycosylated haemoglobin (HbA1c)
- full blood count
- urinalysis
- peak flow or spirometry. [2010, amended 2018]
- Try to exclude other disorders that may present in a similar manner.[2003]
- When a diagnosis of heart failure has been made, assess severity, aetiology, precipitating factors, type of cardiac dysfunction and correctable causes. [2010]
- Refer people with heart failure caused by valve disease for specialist assessment and advice regarding follow-up. [2003]
- Review the basis for a historical diagnosis of heart failure, and manage care in accordance with this guideline only if the diagnosis is confirmed. [2003]
- If the diagnosis of heart failure is still suspected, but confirmation of the underlying cardiac abnormality has not occurred, then the person should have appropriate further investigation. [2003]
- Do not routinely advise people with heart failure to restrict their sodium or fluid consumption. Ask about salt and fluid consumption and, if needed, advise as follows:
- restricting fluids for people with dilutional hyponatraemia
- reducing intake for people with high levels of salt and/or fluid consumption. Continue to review the need to restrict salt or fluid. [2018]
- Advise people with heart failure to avoid salt substitutes that contain potassium. [2018]
- Offer people with heart failure an annual vaccination against influenza. [2003]
- Offer people with heart failure vaccination against pneumococcal disease (only required once). [2003]
- In women of childbearing potential who have heart failure, contraception and pregnancy should be discussed. If pregnancy is being considered or occurs, specialist advice should be sought. Subsequently, specialist care should be shared between the cardiologist and obstetrician. ]2003]
- Air travel will be possible for the majority of people with heart failure, depending on their clinical condition at the time of travel. [2003]
- Large Goods Vehicle and Passenger Carrying Vehicle licence: physicians should be up to date with the latest Driver and Vehicle Licensing Agency guidelines. Check the website for regular updates [2003]
- Diuretics should be routinely used for the relief of congestive symptoms and fluid retention in people with heart failure, and titrated (up and down) according to need following the initiation of subsequent heart failure therapies. [2003]
- People who have heart failure with preserved ejection fraction should usually be offered a low to medium dose of loop diuretics (for example, less than 80 mg furosemide per day). People whose heart failure does not respond to this treatment will need further specialist advice. [2003, amended 2018]
- Avoid verapamil, diltiazem and short-acting dihydropyridine agents in people who have heart failure with reduced ejection fraction. [2003, amended 2018]
- Make the decision to prescribe amiodarone in consultation with a specialist. [2003]
- Review the need to continue the amiodarone prescription at the 6-monthly clinical review. [2003, amended 2018]
- Offer people taking amiodarone liver and thyroid function tests, and a review of side effects, as part of their routine 6-monthly clinical review. [2003, amended 2018]
- For people who have heart failure and atrial fibrillation, follow the recommendations on anticoagulation in the NICE guideline on atrial fibrillation. Be aware of the effects of impaired renal and liver function on anticoagulant therapies. [2018]
- In people with heart failure in sinus rhythm, anticoagulation should be considered for those with a history of thromboembolism, left ventricular aneurysm or intracardiac thrombus. [2003]
- Offer an angiotensin-converting enzyme (ACE) inhibitor and a beta-blocker licensed for heart failure to people who have heart failure with reduced ejection fraction. Use clinical judgement when deciding which drug to start first. [2010]
- Do not offer ACE inhibitor therapy if there is a clinical suspicion of haemodynamically significant valve disease until the valve disease has been assessed by a specialist. [2003]
- Do not withhold treatment with a beta-blocker solely because of age or the presence of peripheral vascular disease, erectile dysfunction, diabetes, interstitial pulmonary disease or chronic obstructive pulmonary disease. [2010]
- Start ACE inhibitor therapy at a low dose and titrate upwards at short intervals (for example, every 2 weeks) until the target or maximum tolerated dose is reached. [2010]
- Measure serum sodium and potassium and assess renal function before and 1 to 2 weeks after starting an ACE inhibitor, and after each dose increment.[2010,amended 2018]
- Measure blood pressure before and after each dose increment of an ACE inhibitor. Follow the recommendations on measuring blood pressure, including measurement in people with symptoms of postural hypotension, in the NICE guideline on hypertension in adults. [2018]
- Once the target or maximum tolerated dose of an ACE inhibitor is reached, monitor treatment monthly for 3 months and then at least every 6 months, and at any time the person becomes acutely unwell. [2010, amended 2018]
- Introduce beta-blockers in a ‘start low, go slow’ manner. Assess heart rate, and clinical status after each titration. Measure blood pressure before and after each dose increment of a beta-blocker. [2010, amended 2018]
- Switch people whose condition is stable and who are already taking a beta-blocker for a comorbidity (for example, angina or hypertension), and who develop heart failure with reduced ejection fraction, to a beta-blocker licensed for heart failure. [2010]
- Consider an angiotensin II receptor blocker (ARB) licensed for heart failure as an alternative to an ACE inhibitor for people who have heart failure with reduced ejection fraction and intolerable side effects with ACE inhibitors. [2010]
- Measure serum sodium and potassium and assess renal function before and after starting an ARB and after each dose increment.[2010, amended 2018]
- Measure blood pressure after each dose increment of an ARB. Follow the recommendations on measuring blood pressure, including measurement in people with symptoms of postural hypotension, in the NICE guideline on hypertension in adults. [2018]
- Once the target or maximum tolerated dose of an ARB is reached, monitor treatment monthly for 3 months and then at least every 6 months, and at any time the person becomes acutely unwell. [2010 amended 2018]
- If neither ACE inhibitors nor ARBs are tolerated, seek specialist advice and consider hydralazine in combination with nitrate for people who have heart failure with reduced ejection fraction. [2010]
- Offer a mineralcorticoid receptor antagonist (MRA) in addition to an angiotensin-converting enzyme inhibitor (ACE) or ARB and beta-blocker, to people who have heart failure with reduced ejection fraction if they continue to have symptoms of heart failure. [2018]
- Measure serum sodium and potassium and assess renal function before and after starting an MRA and after each dose increment. [2018]
- Measure blood pressure before and after each dose increment of MRA. Follow the recommendations on measuring blood pressure, including measurement in people with symptoms of postural hypotension, in the NICE guideline on hypertension in adults. [2018]
- Once the target, or maximum tolerated, dose of an MRA is reached, monitor treatment monthly for 3 months and then at least every 6 months, and at any time the person becomes acutely unwell. [2018]
- Ivabradine is recommended as an option for treating chronic heart failure for people:
- with New York Heart Association (NYHA) class II to IV stable chronic heart failure with systolic dysfunction and
- who are in sinus rhythm with a heart rate of 75 beats per minute (bpm) or more and
- who are given ivabradine in combination with standard therapy including beta-blocker therapy, angiotensin-converting enzyme (ACE) inhibitor and aldosterone antagonists, or when beta-blocker therapy is contraindicated or not tolerated and
- Ivabradine should only be initiated after a stabilisation period of 4 weeks on optimised standard therapy with ACE inhibitors, beta-blockers and aldosterone antagonists. [2012]
- Ivabradine should be initiated by a heart failure specialist with access to a multidisciplinary heart failure team. Dose titration and monitoring should be carried out by a heart failure specialist, or in primary care by either a GP with a special interest in heart failure or a heart failure specialist nurse. [2012]
- Sacubitril valsartan is recommended as an option for treating symptomatic chronic heart failure with reduced ejection fraction, only in people
- With New York Heart Association (NYHA) class II to IV symptoms and
- With a left ventricular ejection fraction of 35% or less and
- Who are already taking a stable dose of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor-blockers (ARBS) [2016]
- Treatment with sacubitril valsartan should be started by a heart failure specialist with access to a multidisciplinary heart failure team. Dose titration and monitoring should be performed by the most appropriate team members as defined in NICE’s guideline on chronic heart failure in adults: diagnosis and management. [2016]
- This guidance is not intended to affect the position of patients whose treatment with sacubitril valsartan was started within the NHS before this guidance was published. Treatment of those patients may continue without change to whatever funding arrangements were in place for them before this guidance was published until they and their NHS clinician consider it appropriate to stop. [2016]
- Hydralazine in combination with nitrate
- Seek specialist advice and consider offering hydralazine in combination with nitrate (especially if the person is of African or Caribbean family origin and has moderate to severe heart failure [NYHA class III/IV] with reduced ejection fraction).[2010]
- Digoxin
- For recommendations on digoxin for people with atrial fibrillation see the section on rate and rhythm control in the NICE guideline on atrial fibrillation
- Digoxin is recommended for worsening or severe heart failure with reduced ejection fraction despite first line treatment for heart failure. Seek specialist advice before initiating.[2010, amended 2018]
- Routine monitoring of serum digoxin concentrations is not recommended. A digoxin concentration measured within 8–12 hours of the last dose may be useful to confirm a clinical impression of toxicity or non-adherence[2003]
- the serum digoxin concentration should be interpreted in the clinical context as toxicity may occur even when the concentration is within the ‘therapeutic range’. [2003]
- For people who have heart failure with reduced ejection fraction and chronic kidney disease with an eGFR of 30 ml/min/1,73 m2 or above: (estimated glomerular filtration rate) as follows.
- Offer the treatment outlined in section 6.2.7 and
- If the person’s eGFR is 45 ml/min/1.73 m2 or below, consider lower doses and/or slower titration of dose of ACE inhibitors or ARBs, mineralcorticoid receptor antagonists and digoxin. [2018]
- For people who have heart failure with reduced ejection fraction and chronic kidney disease with an eGFR below 30 ml/min/1.73 m2 the specialist heart failure MDT should consider liaising with a renal physician [2018]
- Monitor the response to titration of medicines closely in people who have heart failure with reduced ejection fraction and chronic kidney disease, taking into account the increased risk of hyperkalaemia. [2018]
- Do not routinely offer coronary revascularisation to people who have heart failure with reduced ejection fraction and coronary artery disease. [2018]
- Specialist referral for transplantation should be considered for people with severe refractory symptoms or refractory cardiogenic shock [2003]
- Offer people with heart failure a personalised, exercise-based cardiac rehabilitation programme, unless their condition is unstable. The programme:
- should be preceded by an assessment to ensure that it is suitable for the person
- should be provided in a format and setting (at home, in the community or in the hospital) that is easily accessible for the person
- should include a psychological and educational component
- may be incorporated within an existing cardiac rehabilitation programme
- should be accompanied by information about support available from healthcare professionals when the person is doing the programme. [2018]
- All people with chronic heart failure need monitoring. This monitoring should include:
- a clinical assessment of functional capacity, fluid status, cardiac rhythm (minimum of examining the pulse), cognitive status and nutritional status
- a review of medication, including need for changes and possible side effects
- an assessment of renal function. [2010, amended 2018]
- More detailed monitoring will be needed if the person has significant comorbidity or if their condition has deteriorated since the previous review. [2003]
- The frequency of monitoring should depend on the clinical status and stability of the person. The monitoring interval should be short (days to weeks) if the clinical condition or medication has changed, but is needed at least 6-monthly for stable people with proven heart failure. [2003]
- People with heart failure who wish to be involved in monitoring of their condition should be provided with sufficient education and support from their healthcare professional to do this, with clear guidelines as to what to do in the event of deterioration. [2003]
- Consider measuring NT-proBNP (N-terminal pro-B-type natriuretic peptide) as part of a treatment optimisation protocol only in a specialist care setting for people aged under 75 who have heart failure with reduced ejection fraction and an eGFR above 60 ml/min/1.73 m2. [2018]
- The core specialist heart failure multidisciplinary team (MDT) should work in collaboration with the primary care team, and should include:
- a lead physician with a subspecialty interest in heart failure (usually a consultant cardiologist) who is responsible for making the clinical diagnosis
- a specialist heart failure nurse
- a healthcare professional with expertise in specialist prescribing for heart failure.
[2018] - The specialist heart failure MDT should directly involve, or refer people to, other services, including rehabilitation services, and tertiary and palliative care, as needed. [2018]
- The specialist heart failure MDT should:
- diagnose heart failure
- give information to people newly diagnosed with heart failure (see section 9.4.6)
- manage newly diagnosed, recently decompensated or advanced heart failure (NYHA [New York Heart Association] class III to IV) heart failure
- optimise treatment
- start new medicines that need specialist supervision
- continue to manage care after an interventional procedure such as implantation of a cardioverter defibrillator or cardiac resynchronisation device
- manage heart failure that is not responding to treatment. [2018]
- The primary care team should carry out the following for people with heart failure at all times, including periods when the person is also receiving specialist heart failure from the MDT:
- ensure effective communication links between different care settings and clinical services involved in the person’s care
- lead a full review of the person’s heart failure care, which may form part of a long-term conditions review
- recall the person at least every 6 months and update the summary and clinical record
- ensure that changes to the clinical record are understood and agreed by the person with heart failure and shared with the specialist heart failure MDT
- arrange access to specialist heart failure services if needed. [2018]
For recommendations on the diagnosis and management of acute heart failure see NICE’s guideline on acute heart failure. - People with heart failure should generally be discharged from hospital only when their clinical condition is stable and the management plan is optimised. Timing of discharge should take into account the wishes of the person and their family or carer, and the level of care and support that can be provided in the community. [2003]
- The primary care team working within the specialist heart failure MDT should take over routine management of heart failure as soon as it has been stabilised and its management optimised. [2018]
- The specialist heart failure MDT should write a summary for each person with heart failure that includes:
- diagnosis and aetiology
- medicines prescribed, monitoring of medicines, when medicines should be reviewed and any support the person needs to take the medicines
- functional abilities and any social care needs
- social circumstances, including carers’ needs. [2018]
- The summary should form the basis of a care plan for each person, which should include.
- plans for managing the person’s heart failure, including follow-up care, rehabilitation and access to social care
- symptoms to look out for in case of deterioration
- a process for any subsequent access to the specialist heart failure MDT if needed
- contact details for:
- a named healthcare coordinator (usually a specialist heart failure nurse)
- local heart failure specialist care providers, for urgent care or review
- additional sources of information for people with heart failure. [2018]
- Give a copy of the care plan to the person with heart failure, ther family or carer if appropriate, and all health and social care professionals involved in their care. [2018]
- When giving information to people with heart failure, follow the recommendations in the NICE guideline on patient experience in adult NHS services. [2018]
- Discuss the person’s prognosis in a sensitive, open and honest manner. Be frank about the uncertainty in predicting the course of their heart failure. Revisit this discussion as the person’s condition evolves. [2018]
- Provide information whenever needed throughout the person’s care. [2018]
- Consider training in advanced communication skills for all healthcare professionals working with people who have heart failure. [2018]
- The specialist heart failure MDT should offer people newly diagnosed with heart failure an extended first consultation, followed by a second consultation, to take place within 2 weeks if possible. At each consultation:
- discuss the person’s diagnosis and prognosis
- explain heart failure terminology
- discuss treatments
- address the risk of sudden death, including any misconceptions about that risk
- encourage the person and their family or carers to ask any questions they have. [2018]
- Do not offer long-term home oxygen therapy for advanced heart failure. Be aware that long-term home oxygen therapy may be offered for comorbidities, such as for some people with chronic obstructive pulmonary disease (see section 1.2.5 on oxygen in the NICE guidline on chronic obstructive pulmonary disease in over 16s. [2018]
- If it is thought that a person may be entering the last 2 to 3 days of life, follow the NICE guideline on care of dying adults in the last days of life. [2018]
- See NICE’s technology appraisal guidance on implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure.
- When discussing implantation of a cardioverter defibrillator:
- explain the risks, benefits and consequences of cardioverter defibrillator implantation, following the principles on shared decision making in the NICE guideline on patient experience in adult NHS services
- ensure the person knows that the defibrillator can be deactivated without affecting any cardiac resynchronisation or pacing, and reactivated later
- explain the circumstances in which deactivation might be offered
- discuss and dispel common misconceptions about the function of the device and the consequences of deactivation
- provide the person and, if they wish, their family or carers with written information covering the information discussed. [2018]
- Review the benefits and potential harms of a cardioverter defibrillator remaining active in a person with heart failure:
- at each 6-monthly review of their heart failure care
- whenever their care goals change
- as part of advance care planning if it is thought they are nearing the end of life. [2018]
- Do not use prognostic risk tools to determine whether to refer a person with heart failure to palliative care services. [2018]
- If the symptoms of a person with heart failure are worsening despite optimal specialist treatment, discuss their palliative care needs with the specialist heart failure MDT and consider a needs assessment for palliative care. [2018]
- People with heart failure and their families or carers should have access to professionals with palliative care skills within the heart failure team. [2003]
1.2. Diagnostic algorithm
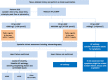
Figure 1
Diagnostic algorithm.
1.3. Therapeutic algorithm
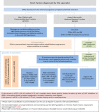
Figure 2
Therapeutic algorithm.
1.4. Key research recommendations
- What is the optimal NT-proBNP threshold for the diagnosis of heart failure in people with atrial fibrillation?
- What are the optimal NT-proBNP thresholds for diagnosing heart failure in people with IIIb, IV or V chronic kidney disease?
- What is the optimal threshold for NT-proBNP for the diagnosis of heart failure in people with suspected heart failure: 400 ng/ml or 125 ng/ml?
- What is the optimal imaging technique for the diagnosis of heart failure?
No recommendation
No recommendation
- In people with advanced heart failure and significant peripheral fluid overload, what is the clinical and cost effectiveness of oral, subcutaneous and intravenous diuretic therapy in the community?
- What is the most accurate prognostic risk toos in predicting 1 year mortality from heart failure at specific clinically relevant thresholds (for example, sensitivity, specificity, negative predictive value and positive predictive value at a threshold of 50% risk of mortality at 1 year)?
- Guideline summary - Chronic Heart Failure in AdultsGuideline summary - Chronic Heart Failure in Adults
Your browsing activity is empty.
Activity recording is turned off.
See more...
