NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Occupational Exposures in Petroleum Refining; Crude Oil and Major Petroleum Fuels. Lyon (FR): International Agency for Research on Cancer; 1989. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 45.)

Occupational Exposures in Petroleum Refining; Crude Oil and Major Petroleum Fuels.
Show details1. Chemical and Physical Data
1.1. Synonyms and trade names
Chem. Abstr. Services Reg. No.: 8002-05-9
Chem. Abstr. Name: Petroleum
IUPAC Systematic Name: —
Synonyms: Naphtha; petrol; rock oil; Seneca oil
1.2. Description
Crude oil is a product of the remains of prehistoric plants and animals, buried in the primaeval mud of swamps, lakes and oceans. Over the centuries, layers of mud and organic debris were subjected to enormous pressures and high temperatures, and a petroleum-saturated rock was formed.
Four elements must be present for oil to accumulate in commercially useful quantities: source rock, reservoir rock, trap and seal. These elements allow the crude oil to remain underground and available in large quantities. A source rock is usually sedimentary rock rich in organic matter. The crude oil created by the decayed matter migrates from the source rock to a reservoir rock. The reservoir rock contains many tiny pores that store the oil. A trap, either stratigraphic layers of impermeable rock or structural traps, prevents the oil from migrating from the reservoir rock. An impermeable layer, or seal, prevents the oil from rising through or around the trap to the surface (American Petroleum Institute, 1984).
Crude oil has been defined as a ‘highly complex mixture of paraffinnic, cycloparaffinic (naphthenic) and aromatic hydrocarbons, containing low percentages of sulfur and trace amounts of nitrogen and oxygen compounds’(Hawley, 1981). Crude oils are often classified on the basis of chemical composition, according to the proportion of hydrocarbon constituents. Paraffinic crude oils are rich in straight-chain and branched paraffin hydrocarbons, whereas naphthenic crude oils contain mainly naphthenic and aromatic hydrocarbons. The composition and classification of many crude oils are obtained by ring analysis and by determination of the other constituents (Sachanen, 1950). Crude oil constituents are further described in section 1.3.
Crude oils may also be classified by geological source, as arising from productive sands, sandstones and limestones. The fractional and chemical compositions of crude oil from the same producing sand are usually very similar, even if they are drawn from fairly distant pools. However, some oilfields that are close together may produce quite different crude oils from the same stratum or from different oil-bearing sands. For instance, in East Texas, USA, Woodbine sand produces almost identical crude oils in different fields (specific gravity, 0.825–0.835; sulfur content, 0.25–0.40%); and crude oils from other Woodbine oilfields close to the East Texas field differ only slightly from the East Texas crude oil. In contrast, crude oils produced from the New and Old Grozny fields in the USSR are quite different, despite being only ten miles (16 km) from each other; New Grozny crude oil is highly paraffinic, whereas Old Grozny crude oil is highly naphthenic or asphaltic (Sachanen, 1950).
A similar phenomenon is found among different oil-bearing sands of the same pool. The Old Grozny field yields at least three different types of crude oil from its 16 producing sands, while Pennsylvania fields commonly produce similar types of crude oil in a range of different producing sands and the New Grozny field produces almost identical crude oils from 24 producing sands (Sachanen, 1950).
There is no clear-cut relationship between the chemical composition of crude oils and their geological age or origin. A commonly accepted generalization for US crude oils is that those that are geologically old are paraffin- and mixed-based, while those that are geologically new are naphthenic or asphaltic. Oilfields in other countries, however, are different: in Poland, crude oils that are geologically new are asphaltic, naphthenic and paraffinic. In practice, crude oils are often identified by the oilfield alone (Sachanen, 1950).
Crude oils are also referred to as light, medium (intermediate) or heavy, depending on their density. A light crude oil generally has an API (American Petroleum Institute) gravity (see section 1.3) greater than 40 (specific gravity, <0.82), a medium crude oil between 15 and 40 (specific gravity, 0.82–0.97) and a heavy crude oil less than 15 (specific gravity, >0.97).
Crude oils are designated in industry according to their suitability for use in various products. Thus, a crude oil may be referred to as a ‘gasoline crude’, a ‘wax crude’, a ‘lube crude’, an ‘asphalt crude’, and so forth.
1.3. Chemical composition and physical properties
Crude oils are complex mixtures of a vast number of individual chemical compounds. Each crude oil is a unique mixture, not matched exactly in composition or properties by any other sample of crude oil. Two typical crude oils, for example, have been characterized by the American Petroleum Institute as shown in Figure 1. Although the mid-points of their respective boiling ranges are similar, they differ considerably in other physical properties, hydrocarbon composition and distribution and sulfur content.

Fig. 1.
Characteristics of two samples of crude oil
The bulk of the compounds present in crude oils are hydrocarbons (Speight, 1980). Crude oils generally contain the classes of hydrocarbons and other compounds described below (Cuddington & Lowther, 1977).
(a) Hydrocarbon compounds
(i) Alkanes (paraffins)
Alkanes are straight-chain normal alkanes and branched iso-alkanes with the general formula CnH2n+2. The major paraffinic components of most crude oils are in the range C1 to C35 (Speight, 1980), although smaller quantities of alkanes up to C60 or higher may be present. Crude oils vary widely in alkane content (Dickey, 1981). The ratio of n-alkanes to isoalkanes is shown in Table 1 for one crude oil sample (Ponca). The ratio ranges from a minimum of 1.7 for heptanes to a maximum of 6.9 for octanes (Speight, 1980). A Pennsylvania crude oil sample had n-alkane:isoalkane ratios of 1.3, 1.7 and 1.5 for pentanes, hexanes and heptanes, respectively (Tiratsoo, 1951). Alkenes are not generally found in crude oils (Speight, 1980).
Table 1
Alkanes isolated from a crude oil sample.
(ii) Cycloalkanes (naphthenes)
Cycloalkanes (or cycloparaffins), also called naphthenes in the petroleum industry, are saturated hydrocarbons containing structures with carbon atoms linked in a ring. The cycloalkane composition in crude oil worldwide typically varies from 30% to 60% (see also Table 3). The predominant monocycloalkanes in crude oil are in the cyclopentane series, having five carbon atoms in the ring, and in the cyclohexanes, having a six-membered ring. The most predominant monocycloalkanes and their composition ranges in crude oil are shown in Table 2 (Bestougeff, 1967). In the higher boiling fractions, such as lubricating oils, cycloalkanes with two or more rings are common, and structures containing up to ten rings have been reported. These polycyclic structures are usually composed of fused five- and six-membered rings (Table 2; Mair, 1964).
Table 3
Composition and physical characteristics of three crude oils.
Table 2
Predominant cycloalkanes isolated from crude oil.
(iii) Aromatic hydrocarbons
The most common aromatic compounds in crude oils are benzene (see IARC, 1982, 1987a), benzene derivatives (e.g., alkylbenzenes) and fused benzene ring compounds. The concentration of benzene in crude oil has been reported to range between 0.01% and 1% (Bestougeff, 1967). Table 3 shows the overall composition of three crude oil samples, including the major classes of aromatic hydrocarbons, and Table 4 gives the levels of seven specific polycyclic aromatics in two of these samples (National Research Council, 1985).
Table 4
Concentrations of individual polynuclear aromatic hydrocarbons in crude oil (10-6 g/g oil).
(b) Nonhydrocarbon compounds
(i) Sulfur compounds
Crude oils vary widely in sulfur content, which can range from <0.1% to 10% by weight. The following types of sulfur compounds have been identified in crude oils: thiols (mercaptans), sulfides, disulfides and thiophenes (Costantinides & Arich, 1967).
In the lower distillation range up to about 150°C, the most abundant sulfur compounds are thiols. In the 150–250°C distillation range, the most abundant compounds are thiocyclo-, thiobicyclo- and thiotricycloalkanes and thiophenes. These sulfur compounds are replaced, in turn, by benzothiophenes and more complex ring structures in the higher distillation ranges (Costantinides & Arich, 1967).
(ii) Nitrogen compounds
The nitrogen content of crude oils ranges from trace amounts to 0.9% by weight. The bulk of the nitrogen in fractions that boil below about 200°C is basic nitrogen. The basic nitrogen compounds often found in crude oils include pyridines and quinolines, e.g., 3-methylpyridine and quinoline, while nonbasic nitrogen compounds include pyrroles, indoles and carbazoles, e.g., carbazole, and amides (Costantinides & Arich, 1967).
(iii) Oxygen compounds
The oxygen content of crude oils ranges from 0.06% to 0.4% by weight, the majority of components being alkane and cycloalkane (naphthenic) acids. Other minor components include ketones and phenols (Costantinides & Arich, 1967). The oxygen content of crude oils increases with boiling range, so that more oxygen-containing compounds are found in distillates that boil above 400°C.
(iv) Metal-containing compounds
Traces of many metallic compounds can be found in crude oils. Nickel (see IARC, 1976, 1987b) and vanadium compounds have been identified in crude oils at levels ranging from a few parts per million to 200 ppm (mg/kg) nickel and up to 1200 ppm (mg/kg) vanadium. These metals occur primarily as complexes (porphyrins; Costantinides & Arich, 1967) which are stable and can be distilled at temperatures above 500°C.
Table 5 is a compilation of some other trace elements reported in crude oil and their typical concentrations either in crude oil or in crude oil ash (Magee et al., 1973; Valković, 1978). Most of these elements occur naturally in crude oil as a result of their presence in the rock formation or in salt-water deposits from which the crude oil was drawn, although some
Table 5
Elements in crude oil.
may also be introduced during the process of drilling, pumping, preparing and transporting crude oil to a refinery.
(v) Miscellaneous contaminants
Crude oil, as it emerges from the well-head, is typically a heterogeneous mixture of solids, liquids and gases, including, in addition to the constituents described above, sand and other sediments, water and water vapour, salts and acid gases such as hydrogen sulfide and carbon dioxide. These contaminants are at least partially separated from the crude oil in surface treatment at the well-head (see p. 132) to prepare it for transportation to the refinery (Baker et al., 1986a).
Crude oils are not analysed routinely for their content of various classes of hydrocarbons and nonhydrocarbons; rather, they are usually characterized by their physical properties (specific gravity or density, viscosity) and their sulfur content. Crude oils are also characterized in pilot-scale distillations by the volume or weight percentage in various boiling-point ranges (‘straight-run fractions’).
One of the most important physical properties of crude oil is its specific gravity —the ratio of the density of oil to the density of water, both taken at the same temperature and pressure. From the specific gravity, the ratio of aromatic (high density) to saturated (low density) hydrocarbons in crude oil samples may be estimated. An alternative expression for specific gravity, developed for petroleum applications, is:
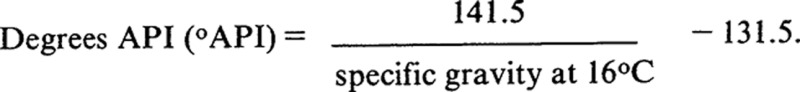
The specific gravities of petroleum usually range from about 0.8 (45.3°API) for the lighter crude oils to over 1.0 (10°API) for the heavier asphaltic crude oil (Dickey, 1981).
Crude oil is also characterized by its viscosity. Viscosity is expressed in Saybolt universal seconds (SUS) at 38°C. This value is determined by the time it takes for 60 cm3 of crude oil to flow by gravity through an orifice in a calibrated viscometer (Dickey, 1981). Viscosity may also be expressed in centipoises.
Sulfur content is the third important property of crude oil because of its effect on the refining process (in poisoning catalysts) and the malodorous and toxic properties of hydrogen sulfide and other sulfur compounds. Table 6 gives the API gravity, sulfur content and viscosity of several crude oils.
Table 6
Characteristics of some typical crude oils.
Table 7 summarizes the composition of crude oils throughout the world, based on analysis by the US Department of Energy of 800 crude oil samples from 691 major oilfields in the USA (Coleman et al., 1978) and on analysis by the US Bureau of Mines of the Department of the Interior of 169 samples of crude oil from 122 fields in 27 countries outside the USA (Ferrero & Nichols, 1972).
Table 7
Summary of worldwide crude oil compositions and characteristics.
2. Production, Use, Occurrence and Analysis
2.1. Production and use
(a) Production
Crude oil production is the process of raising well fluids to the surface and preparing them for further processing at the refinery. Since 1972, about 60 million barrels of crude oil have been produced each day worldwide, mostly in areas of sparse population or of limited industrial development (Anderson, 1984; American Petroleum Institute, 1987a; British Petroleum Company, 1988). Crude oil production begins with preparation of a well, followed by the application of a variety of natural and artificial lift mechanisms to bring the oil to the surface. There it is treated superficially to prepare it for transport to the refinery by tanker or barge, by pipeline, or by truck or rail (Baker et al., 1986a).
Worldwide, about 500 000 workers are employed in oil exploration and production (International Labour Office, 1986).
(i) Preparing the well
The production operation begins after the well has been drilled and has been evaluated as being economically favourable for production. Pipe or casing is inserted into the well bore in a concentric series to prevent contamination by fresh water, loss of circulation, sloughing or charging of shallow sands with abnormal pressures (American Petroleum Institute, 1983). The first such casing placed into a well is the conductor pipe, which may be pile driven or cemented into place and may extend to a depth of 150–1500 m.
The conductor pipe and all other casings are attached at the surface to the casing head (American Petroleum Institute, 1983). Surface casing is inserted through the conductor pipe and deeper into the well to prevent underground formations of fresh water from becoming contaminated with well fluids and to provide a mechanism for controlling the flow of fluid from the well.
(ii) Pumping the crude
Once the well has been completed, oil begins to flow up the well as a result of the inherent reservoir energy, which is manifested by an oil displacement process involving water, gas or a combination of both. Reservoir drive mechanisms — the processes by which the reservoir energy displaces the crude oil — include dissolved-gas drive, gas-cap drive, water drive and combination drive (Baker et al., 1986a; Gray, 1986). Natural drive mechanisms may, at some point in the economic life of the well, lose their inherent energy and the well will require a mechanical force to draw the oil from the reservoir. The common methods of artificial lift are surface pumping, submersible pumping and gas lift (Baker et al., 1986a; Gray, 1986).
The natural and artificial lift mechanisms provide a means of raising reservoir fluids capable of flowing into the well bore. However, fractures, channels and perforations through which the fluids flow often become blocked and diminish the production capacity of a well. These passages may be cleared and new ones created by using reservoir stimulation techniques such as acidizing and hydraulic fracturing (Baker et al., 1986a).
Acidizing is the process of treating the formation — limestone or dolomite — with hydrochloric, acetic or hydrofluoric acid. Additives such as corrosion inhibitors, surface active agents, sequestering agents and antisludge agents are mixed with the acids to prevent acid attack on tubing and casing, to help disperse the acid in the formation, to prevent precipitation of ferric iron during acidizing and to prevent formation of insoluble sludge (Giuliano, 1981; Baker et al., 1986a).
Hydraulic fracturing is used extensively and successfully on formations composed of sandstone. A fluid, such as water charged with nitrogen, is pumped under high pressure at high rates into the well to create deep penetrating fractures in the reservoir. Charging the water with nitrogen facilitates the flow of water back out of the well (Giuliano, 1981; Baker et al., 1986a).
(iii) Surface treatment
When the crude oil has been brought to the surface, the final production step is to reduce it to the form in which it will be sent to the refinery for processing. Contaminants such as sediment and water are removed, and volatile components are separated and treated by the use of separators (Giuliano, 1981; Baker et al., 1986a; Gray, 1986).
Natural gases must be treated to remove water vapour and acid gases such as carbon dioxide and hydrogen sulfide. Water vapour may be removed by bubbling the gas through a solid or liquid desiccant; the acid gases may also be removed from a natural gas stream by adsorption or absorption with an appropriate liquid or solid desiccant. This process of removing acid gases from a natural gas stream is commonly referred to as sweetening (Baker et al., 1986a).
(iv) Transportation and storage
The primary means of transporting crude oil are tankers and pipelines; trucks and railways fulfil much smaller yet significant roles. Barges are used to transport oil on inland waterways and to off-load large tankers.
Modern tankers carry over two-thirds of all crude oil produced to modern industrial societies (Baker et al., 1986b). Oil can be loaded onto tankers either from onshore facilities after transport from inland fields or from offshore platforms. The single-point (or single-buoy) mooring system is a common method for loading tankers. Oil is pumped from an offshore or onshore facility through a pipeline on the ocean bed to a marine riser which is suspended at the surface by a large mooring buoy. The oil is passed from the pipeline into a flexible hose connected to the riser, through the riser to a floating base and from there to the ship (Giuliano, 1981).
An individual oil field may contain several hundred wells. Flow lines connect individual wells in an oil field to field storage tanks and transport oil to a central location for treatment, testing and measurement. Following treatment, oil is transported from a central tank battery by intermediate ‘gathering’ lines which, like the flow lines, generally range from 5 to 30 cm in diameter (Giuliano, 1981; Baker et al., 1986b).
Pumps at a pump station move the oil into and through a pipeline. A gathering station in or close to an oil field receives oil from producers' tanks via a pipeline gathering system and moves it on to a trunk-line station located on the main ‘trunk’ line. Trunk lines are large-diameter (up to 120 cm) pipelines that carry oil over long distances to refineries, central storage or ports. Booster pump stations are placed along the trunk line as necessary to compensate for loss of pressure as the oil is moved through the line (Giuliano, 1981; Baker et al., 1986b).
Tank farms may be located along pipelines, where oil can be temporarily side-tracked from transit for holding, sorting, measuring or rerouting. A tank farm may function as a receiving station for oil that is to be moved into the pipeline transportation system. Pipelines from a tank farm converge at a station manifold which can split, merge or reroute the flow of oil as needed (Baker et al., 1986b). Highly viscous crude oil can be heated and transported via an insulated pipeline, along which reheating stations may be employed (Watkins, 1977).
Deposits accumulate on the inside wall of a pipe during the course of operations. Some crude oils deposit substantial coatings of wax on cooling; salts and other foreign materials may also build up. To clean the pipeline and remove deposits, ‘pigs’ equipped with scrapers and brushes are run through it periodically, entering and leaving via locks or pig traps, so that the line can continue to operate under pressure (Anderson, 1984).
Crude oil is also transported by truck, especially from new fields where pipeline gathering lines have not been built. However, motor carrier transport represents only a small fraction of US domestic transportation of crude oil, accounting for less than 0.3% of that total in 1982. An even smaller percentage (0.05% in 1982) of domestic crude oil transportation is by rail. Rail tank cars are used to move crude oil from ocean tankers or waterways to small inland refineries (Baker et al., 1986b).
(v) Production volumes
World crude oil production from 1947 to 1986 is shown in Table 8 by geographical region. Over the past 40 years, production has increased more than seven fold, from 3000 million barrels to 22 000 million barrels per year. Table 9 gives production data for the 20 countries that produced the most crude oil in 1976 and 1986.
Table 8
World crude oil production, 1947–86 (millions of barrels per year)a.
Table 9
World crude oil production (thousands of barrels per year): 20 leading regions.
In 1986, proven worldwide reserves of crude oil were estimated to be 700 000 million barrels (Table 10).
Table 10
Proven reserves at end 1987.
(b) Use>
The direct use of raw crude oil was reported as far back as 3000 BC. Crude oil seeping to the earth's surface was collected and used in ancient times by the Chinese, Babylonians, Assyrians and other early civilizations. With only rudimentary methods of discovery and extraction, these early peoples often located crude oil by observing natural gas escaping from the earth's crust with the petroleum liquid. They used this natural resource for its four principal components — oil, grease, asphalt and wax. The source of the crude oil and its composition determined the petroleum products for which it was useful. Among the early uses of the unrefined natural product were fuel for oil lamps, heating fuel, bitumens mixed with fibre, sand, etc. for buildings, roads and dams, medicinal oils (e.g., Seneca oil), paints, waterproofing wicker and mats, adhesives for inlay work, insecticides and rodenticides, and tool manufacture. Historical uses in Europe include lubricants for axles, lamp oil, preservatives for wood used in shipbuilding, and other applications in navigation (Cross, 1983).
During the twentieth century, crude oil has become one of the world's most important natural raw materials. Commercial quantities are extracted from all large land masses, except Antarctica and Greenland, as well as from the earth beneath major bodies of water. The petroleum or crude oil thus obtained is a major source of the world's energy and the main feedstock for the petrochemical industry (Considine, 1974).
According to the American Petroleum Institute (1984), the use of oil refinery products as feed stocks for the petrochemical industry has resulted in more than 3000 petrochemical intermediates and products. Hoffman (1982) has published a useful table of ‘Petroleum Products, Their Uses and Compositions’.
Because crude oil varies markedly in composition and properties and, therefore, lacks consistency and reproducibility, it is no longer used directly in consumer applications, even as fuel. Today, virtually all recovered crude oil is sent to a refinery for processing into products or intermediates.
A significant and growing amount of the world's elemental sulfur is also recovered as a by-product of sour crude oil. Refineries process more sour crude oils under stricter pollution controls, with the result that the production of recovered sulfur has increased in recent years (West, 1983). The Oil and Gas Journal Data Book (Anon., 1987) lists three countries as producers of sulfur derived from crude oil, reporting production levels in tonnes per day at 1 January 1986 of 120 in Brazil, 51 in Hungary and 121.4 in the USA.
Demand for refined petroleum products by geographical region during the past two decades is shown in Table 11. Consumption of petroleum products by group (gasoline, middle distillates, fuel oil, others) is given in Table 4 of the monograph on occupational exposures in petroleum refining.
Table 11
Estimated world demand for refined petroleum products by region (millions of barrels per year).
(c) Regulatory status and guidelines
Occupational exposure limits have been established or recommended for various petroleum fractions, as well as for many of the individual substances found in crude oil. However, for crude oil itself, no exposure limit has been set.
Several national laws and multinational agreements have been established to prevent pollution of the seas and other environments by oil (Reitze, 1972; Myhre, 1980; Duck, 1983).
2.2. Occurrence
(a) Natural occurrence
Crude oil is a naturally occurring complex mixture which is found in subsurface deposits in most regions of the world.
(b) Occupational exposure
Since crude oil is a complex liquid, there is potential occupational exposure to a variety of substances: various hydrocarbons and other organic compounds, dissolved gases and metal compounds. Exposure is possible in all operations involving the product, including drilling, pumping and treating steps; transport by pipeline, ships or rail cars; storage and refinery processing (Suess et al., 1985).
The primary route of exposure is through skin contact. However, some sour crude oils contain high concentrations of hydrogen sulfide, and control of exposures, particularly during sampling and maintenance operations, is critical. Some known carcinogens, such as benzene, certain polycyclic aromatic compounds and nickel and arsenic compounds, are commonly found in crude oils. Certain crude oil condensates can contain up to 15 vol % benzene.
Other airborne contaminants identified in operations involving crude oil are mercaptans and gaseous and volatile hydrocarbons. Explosive concentrations of airborne hydrocarbons and lethal levels of hydrogen sulfide can be found at the well head and in compartments and confined spaces (Duck, 1983). No data were available to quantify occupational exposure levels to crude oil components.
(c) Environmental exposure
A recent estimate of the total input of petroleum into the marine environment from all sources is 1.7–8.8 million tonnes per year, with a best estimate of 3.2 million tonnes per year. Table 12 presents the approximate annual input of petroleum hydrocarbons into the oceans from various man-made and natural sources (Koons, 1984).
Table 12
Petroleum hydrocarbons in the marine environment.
The total amount of oil produced in Nigeria between 1980 and 1983 was approximately 350 million m3 (370 million tonnes), averaging 88 million m3 (93 million tonnes) per year and generating an average of 13 million m3 of waste water per year. The average concentration of oil dissolved in the water ranged from 11.2 to 53.9 mg/l (total range, 0.9–96.7 mg/l; Ibiebele, 1986).
In a study of estuarine and seawater samples from three Australian bodies of water, it was found that a probable source of aromatic hydrocarbons in the dissolved and particulate phases from the estuarine samples was crude oil. Other probable sources included refined petroleum products, including lubricating oil and residual fuel oil, and distillates, including gasoline and diesel fuel (Smith & Maher, 1984).
In a study of petroleum residues in the waters of the Shatt al-Arab River in the northwest region of the Arabian Gulf, DouAbul (1984) found that average total hydrocarbon concentrations ranged from 2.7 to 86.7 µg/l Kuwaiti crude oil equivalents. The highest concentrations were found at sites that were near port areas. These results were within the range of values reported for comparable areas in other parts of the world (UK marine waters, 24.0–74.0 µg/l; Canadian marine waters, 1.0–90.0 µg/l; Corella river, 2.2–200 µg/l; Halifax harbour, 1.2–71.7 µg/l).
In a similar study of seasonal variations in oil residues in the waters of the Shatt al-Arab River in Iraq, DouAbul and Al-Saad (1985) found that concentrations varied between 1.7 to 35.4 µg/l Kuwaiti crude oil equivalents. The results suggested that petroleum hydrocarbons found in the river originated from diverse sources. Hydrocarbon concentrations were highest in winter (averaging 17.4 µg/l) and lowest in summer (averaging 3.1 µg/l).
Table 13 lists some accidental releases of crude oil that have been reported in the recent past.
Table 13
Major accidental releases of crude oil in the recent past.
2.3. Analysis
Because of the extreme complexity of the composition of petroleum and petroleum products, no single analytical method can be used to measure all the components in an environmental sample. For example, methods suitable for sampling and analysis of the volatile paraffinic (alkanes) hydrocarbon components are not directly applicable to the high molecular weight aromatic and polar fractions or to metals. Moreover, because petroleum is a complex and labile mixture, the composition of a sample released into the environment begins to change almost immediately. Fractionation and separation of components begins to take place by evaporation (or condensation), dissolution (e.g., of more polar components into water) and adsorption/absorption (e.g., into soils, sediments or biological tissues). Chemical, photochemical and biochemical reactions occur, leading to further selective changes and the appearance of degradation products and metabolites.
The problem of identification and quantification of petroleum released into the environment is further complicated by the fact that many petroleum components are ubiquitous and may arise from other sources such as the incomplete combustion of fossil fuels or biogenesis.
For these reasons, a number of analytical techniques have been applied in environmental analyses of petroleum, ranging from low-resolution, relatively nonspecific techniques, such as extraction/ gravimetry and infrared spectrometry, to high-resolution, specific techniques involving capillary gas chromatography, high-pressure liquid chromatography and mass spectrometry (National Research Council, 1985). The choice of a method in any particular case depends on several factors, including the objective of the study, the medium (air, water, soil, sediment), what is known about the sample(s) and practical considerations such as cost, time restrictions and availability of equipment.
A number of reviews have been published on the environmental analysis of crude oil (e.g., Egan et al., 1979; National Research Council, 1985; US Environmental Protection Agency, 1986; American Petroleum Institute, 1987b).
3. Biological Data Relevant to the Evaluation of Carcinogenic Risk to Humans
3.1. Carcinogenicity studies in animals
Skin application1
Mouse: Groups of 25 male and 25 female outbred albino mice [stock unspecified], 10–12 weeks of age, received twice weekly skin applications of 0.2 ml of one of three crude oils: from Kuwait (paraffinic-asphaltic base), Lagunillas/Venezuela (naphthenic) and Oklahoma [unspecified] or laboratory distilled fractions of the oils (obtained by fractionation using vacuum and steam in an apparatus selected to preclude cracking) or residues for 52 weeks. A similar experiment, using the same samples and numbers of mice of different strains was carried out in another laboratory. Skin from the treated area of all mice that survived 12 weeks of treatment was prepared for histology. Surviving animals were killed at week 52 [survival rate and effective number of animals unspecified]. In 18 groups each of 50 mice in laboratory 1, the skin tumour yield per group varied between 0 and 5; that in laboratory 2 varied between 0 and 2 [tumour type unspecified]. With the crude oils and residues, only two tumours developed among mice treated with Kuwaiti crude oil and one among mice treated with its residue (Hieger & Woodhouse, 1952). [The Working Group noted the lack of information on untreated controls, lack of histological classification and the short duration of the study.]
A group of 30 mice [age, sex and strain unspecified] received thrice-weekly skin applications of crude oil (natural Saratov; 28% methane, 68% naphthenes (cycloalkanes), 4% aromatic hydrocarbons, 2.86% paraffins (alkanes), 0.34% sulfur [quantity unspecified]) for six months, followed by twice weekly applications for life. All mice died within 13 months; the first death occurred after 40 treatments (94 days) and the last after 142 treatments (393 days). Hyperkeratosis was observed at the site of treatment in 13 of 23 animals of which the skin was examined histologically, and three mice developed papillomas within 147, 149 and 154 days, respectively (Antonov & Lints, 1960). [The Working Group noted the small number of animals, the lack of controls and absence of experimental detail, and the short duration of the experiment.]
Three groups of 30 mice [sex, age and strain unspecified] received twice weekly skin applications [not otherwise specified] of crude oils [quantities unspecified] of different origins (Bitkovsk, Gozhansk and Kokhanovsk) containing different amounts of paraffins, sulfur and tar, for ten months. No squamous-cell tumour was observed, but an angiosarcoma of the skin developed in two mice treated with the Bitkovsk and Gozhansk crude oils (Shapiro & Getmanets, 1962). [The Working Group noted the absence of experimental detail and the short duration of treatment.]
Groups of ten male and ten female C3H/Bdf mice [age unspecified] received twice weekly applications on shaved skin of 3, 6, 12 or 25 mg crude oil (Wilmington, CA; benzo[a]pyrene content, 1 µg/g) in 70% cyclohexane: acetone (final volume, 50 µl) for 30 weeks and were observed for a further 20 weeks. A group of 50 mice received applications of vehicle only. No skin tumour was observed in either treated or control animals (Holland et al., 1979). [The Working Group noted the small number of animals and the short duration of treatment.]
Groups of 15 male and 15 female C3H/Bdf mice [age unspecified] received thrice weekly applications on shaved skin of 25 mg of a composite petroleum sample (Wilmington, CA, USA (20%); South Swan Hills, Alberta, Canada (20%); Prudhoe Bay, AK, USA (20%); Gach Sach, Iran (20%); Louisiana–Mississippi, USA, Sweet (10%); Arabian Light (10%); polycyclic aromatic hydrocarbon content, 2.6%; benzo[a]pyrene content, 1 µg/g) in 70% cyclohexane:30% acetone (final volume, 50 µ/l) for 22 weeks, followed by a 22-week observation period. A group of 25 males and 25 females received the vehicle only. None of the animals developed skin tumours (Holland et al., 1979). [The Working Group noted the small number of animals and short duration of treatment.]
Groups of 25 male and 25 female C3H/Bdf mice [age unspecified] received thrice weekly applications on shaved skin of 0, 0.08, 0.3, 0.4 or 2.0 mg of the same composite petroleum samples as described above for 24 months. A group of 25 males and 25 females served as vehicle controls. Among mice treated with the highest dose, four skin carcinomas developed (8%), with an average latency of 658 (± 22) days. No tumour was observed among mice treated with lower doses or with the solvent only (Holland et al., 1979). [The Working Group noted the low doses tested.]
Groups of 20 male C3H mice [age unspecified] were treated on the clipped dorsal skin with 50 µl of a crude oil sample of Texan origin (benzo[a]pyrene content, 0.002%) or 50 µl of an asphaltic type (benzo[a]pyrene content, 0.0005%) two to three times per week [duration not specified]. No skin tumour developed in the animals. Benzo[a]pyrene (0.005% and 0.2% in toluene) produced high numbers of skin papillomas (6/50 and 3/30) and carcinomas (1/50 and 27/30; Bingham & Barkley, 1979). [The Working Group noted the small number of animals and the lack of experimental details.]
Groups of 25 male and 25 female C3H/Bdf mice, ten weeks of age, received thrice weekly applications on shaved skin of 0.08, 0.3, 0.4 or 2.0 mg of a natural composite petroleum sample (Wilmington, CA, USA (10%); South Swan Hills, Alberta, Canada (20%); Prudhoe Bay, AK, USA (20%); Gach Sach, Iran (20%); Louisiana–Mississippi, USA, Sweet (10%); Arabian Light (20%)) in 70% acetone:30% cyclohexane (final volume, 50 µl) for 24 months. The numbers of animals that died in the respective groups were 15, 11, 14 and 10. No skin tumour developed in the mice. Further groups of 25 males and 25 females treated with 0.006, 0.03 or 0.15 mg benzo[a]pyrene per week developed skin tumours at the application site: low-dose, 43/50; mid-dose, 49/50; high-dose, 48/50. No skin tumour was observed among solvent-treated mice (Holland et al., 1981). [The Working Group noted the low doses of the petroleum mixture tested.]
Groups of 50 C3H mice [sex and age unspecified] received twice weekly skin applications of 50 mg crude oil from either Kuwait (paraffinic with high sulfur content) or southern Louisiana, USA (naphthenic with low sulfur content), for 80 weeks and were observed for a further 40 weeks. Of the Kuwaiti oil-treated animals, 38% developed squamous-cell tumours [histological type not specified] with an average tumour latency of 64 weeks; of the Louisiana oil-treated mice, 20% had skin tumours with an average tumour latency of 69 weeks. In a similar experiment conducted separately, a group of 20 mice received skin applications of southern Louisiana crude oil; tumour incidence was also 20%, but average tumour latency was 86 weeks. In an experiment conducted in another laboratory, 40 C3H mice [sex and age unspecified] received thrice weekly applications of 5 mg southern Louisiana crude oil (as described above) in a 30:70% cyclohexane:acetone mixture on the skin for 78 weeks and were observed for an additional 22 weeks. Skin tumours [histologically unspecified] developed in 92% of animals with an average tumour latency of 67 weeks (Coomes & Hazer, 1984). [The Working Group noted the lack of appropriate controls and of histological characterization of the tumours.]
Groups of 50 male C3H/HeJ mice, eight weeks of age, received twice weekly applications of 50 mg of one of two undiluted samples of crude oils (‘C’, predominantly naphthenic; ‘D’, predominantly paraffinic with a high sulfur content) or distilled fractions of the oils with boiling ranges corresponding to various refinery streams (petroleum ether, D-1; naphthas or gasoline components, C-2 and D-2; kerosene, C-3 and D-3; gas oil, C-4 and D-4; heavy oils, C-5 and D-5; and residual, C-6 and D-6) on clipped interscapular skin for 18 months. One group of mice received no treatment on the clipped skin and another treated with toluene only on the clipped skin served as negative controls; a further group treated with 0.05 or 0.15% benzo[a]pyrene in toluene on clipped skin served as positive controls. Total polycyclic aromatic hydrocarbon and benzo[a]pyrene contents, when determined, and details of the experiments are summarized in Table 14 [effective number of animals unspecified]. Fractions D-l and C-6 produced no tumour and fractions D-4 and D-6 produced one carcinoma and one papilloma, respectively. All other samples produced numerous tumours, the most potent being the C-5 and D-5 fractions (boiling range, 371–577°C). Both crude oils induced tumours; however, the paraffinic sample (D) produced more tumours with slightly shorter arithmetic average time to appearance of the first tumour in weeks than the naphthenic (C) sample (56% and 64 weeks versus 30% and 69 weeks; Lewis, 1983; Lewis et al., 1984; Cragg et al., 1985). [The Working Group noted that the authors were not the original investigators of the study.]
Table 14
Carcinogenic activity of crude oil samples and their fractions.
Rabbit: A group of 30 male rabbits (from different stocks) [age unspecified] received twice weekly applications of 0.3 ml of crude oils from Kuwait (paraffinic-asphaltic), Lagunillas/Venezuela (naphthenic) or Oklahoma, USA [unspecified], on six different areas (∼3 cm2) of shaved skin for 52 weeks. Another group of 75 male rabbits received twice weekly applications of 0.3 ml of laboratory distilled fractions (obtained by fractionation using vacuum and steam in an apparatus selected to preclude cracking) or residues of the same crude oils on seven different areas of shaved skin for 52 weeks. A similar experiment using the same samples and equal numbers of animals of different stocks was carried out in another laboratory (2). Surviving animals were killed at 52 weeks. Treatment with Oklahoma crude oil resulted in the development of two skin tumours in laboratory 2. Twenty-one, 34 and six skin tumours were induced by the fractions in laboratory 1 and 13, 12 and 12 by the fractions and residues in laboratory 2 by the Kuwaiti, Lagunillas and Oklahoma oils, respectively. The heavy fraction of each crude oil was the most active (Hieger & Woodhouse, 1952). [The Working Group noted the lack of information on controls and the lack of histological classification.]
A group of eight rabbits [sex, strain and age unspecified] received thrice weekly applications of crude oil (natural Saratov; 28% methane, 68% naphthenes, 4% aromatic hydrocarbons, 2.86% paraffins, 0.34% sulfur) [quantity unspecified] on the entire internal surface of one ear for six months followed by twice weekly applications for life. The first death occurred at 25 months and the last at 31 months from the start of the experiment. Six rabbits that were studied microscopically had all developed papillomas at the application site; the first tumour appeared 14 months after the start of the experiment (Antonov & Lints, 1960). [The Working Group noted the small number of animals and the lack of controls and the uncertainty about the cause of death.]
Five groups of six rabbits [sex, strain and age unspecified] received thrice weekly skin applications [not otherwise specified] of crude oils [quantity unspecified] of different origin (Bitkovsk, Gozhansk, Kokhanovsk, Romashkinsk and Radchenkovsk) with different paraffin, sulfur and tar contents for 10–17 months. Papillomas developed in all groups [survival, effective number of animals and number of tumours unspecified] (Shapiro & Getmanets, 1962). [The Working Group noted the lack of experimental details.]
3.2. Other relevant data
(a) Experimental systems
Absorption, distribution, excretion and metabolism
No data were available to the Working Group on the absorption, distribution, excretion and metabolism of crude oil in laboratory animals.
Toxicokinetic studies have been reported in non-laboratory mammals, birds and aquatic organisms (Engelhardt et al., 1977; Lee, 1977; Lawler et al., 1978a,b; Gay et al., 1980; Engelhardt, 1981; Neff & Anderson, 1981; Oritsland et al., 1981).
Toxic effects
Oral administration of Prudhoe Bay crude oil (5.0 ml/kg bw daily for two days) to male Charles River CD-1 mice resulted in increases in liver weight, hepatic proteins, RNA, glycogen, total lipids, cholesterol, triglycerides and phospholipids (Khan et al., 1987a).
Epidermal ornithine decarboxylase was induced following application of Prudhoe Bay crude oil to the backs of female Charles River CD-1 mice; a maximal induction of over 60 fold was seen 6 h after application of 50 µl. Concurrently, epidermal putrescine levels were elevated 4.7 fold over those in controls. Intraperitoneal administration of the crude oil led to an increase (15–20 fold, maximal activity 12 h following administration of 4 ml/kg bw) in hepatic ornithine decarboxylase activity but to a 45% decrease in the renal enzyme activity. Hepatic putrescine levels were elevated 34 fold over those in controls (Rahimtula et al., 1987).
Application of Kuwaiti crude oil (0–200 µg) to the skin of male Sprague-Dawley rats increased dermal benzo[a]pyrene 3-hydroxylase by 15 fold and diphenyloxazole hydroxylase by six fold (Rahimtula et al., 1984).
Platelets isolated from male Sprague-Dawley rats 24 h after oral treatment with a Prudhoe Bay crude oil showed a substantial inhibition of aggregation induced by adenosine diphosphate, arachidonic acid or epinephrine (Chaudhury et al., 1987a). Inhibition of aggregation was effected with as little as 0.1 ml crude oil/kg bw (Chaudhury et al., 1987b). Aggregation was also inhibited by aliphatic, heterocyclic and aromatic fractions of the crude oil (Chaudhury et al., 1987a).
Alteration in cellular calcium sequestration has been postulated to be a primary mechanism in initiating irreversible cell damage. Administration of 5 ml/kg bw Prudhoe Bay crude oil intraperitoneally or orally daily for two days to male Sprague-Dawley rats that were sacrificed 24 h later resulted in an abrupt drop in liver mitochondrial and microsomal adenosine triphosphate-dependent calcium uptake. In-vitro incubation of either mitochondria or microsomes with dimethyl sulfoxide (DMSO) extracts of the crude oil resulted in a concentration-dependent inhibition of calcium influx. The release of calcium from calcium-loaded mitochondria and microsomes was also observed in the presence of the crude oil extract. At concentrations which affect calcium sequestration, the crude oil extract produced swelling of mitochondria. Microsomal adenosine triphosphatase activity in the presence or absence of calcium was unaffected by the crude oil. The results indicate that increased permeability of the mitochondrial and microsomal membranes to calcium is a contributing factor in the inhibition of calcium uptake by Prudhoe Bay crude oil (Khan et al., 1986).
Administration of a single oral dose (5-10 ml/kg bw) of Prudhoe Bay crude oil to pregnant Sprague-Dawley rats resulted in induction in maternal hepatic microsomal cytochrome P450 levels and various monooxygenases in a dose-dependent manner after 24 h. Maximal induction of glutathione S-transferase, uridine 5'-diphospho (UDP) glucuronyl-transferase and DT-diaphorase (NADH, NADPH quinone oxido reductase) activities were observed 72 h after administration of the crude oil (Khan et al., 1987b).
Many studies on the toxic effects of crude oil in non-laboratory mammals, birds, and aquatic organisms have been reported and reviewed (Rice et al., 1977; Engelhardt, 1984; Holmes, 1984; Engelhardt, 1985; Leighton et al., 1985; Payne et al., 1987).
Effects on reproduction and prenatal toxicity
The effects of petroleum and petroleum products on reproduction have been reviewed (Schreiner, 1984).
Prudhoe Bay crude oil was administered orally to pregnant Sprague-Dawley rats as a single dose (5 ml/kg bw) on various gestation days (3, 6, 11, 15 or 17), as a single variable dose (2–10 ml/kg bw) on gestation day 6, or as daily doses (1 or 2 ml/kg bw) on days 6–17 of pregnancy. Administration during the earlier stages of pregnancy (day 3, 6 or 11) significantly increased the number of resorptions and decreased fetal weight and length. No adverse effect was observed following administration on gestation day 15 or 17. Multiple exposure to crude oil also caused a significant reduction in maternal body weight (Khan et al., 1987c). [The Working Group noted that no information on gross external abnormalities was reported and that the embryotoxic effects might have been a consequence of maternal toxicity.]
Both placental and fetal hepatic enzyme systems were induced on gestation day 18 following treatment of pregnant Sprague-Dawley rats with a single 5 ml/kg bw dose of Prudhoe Bay crude oil on gestation days 11, 15 or 17. Liver microsomal P450 levels, benzo[a]pyrene hydroxylase and ethoxyresorufin O-deethylase activities were increased two, two to three and 10–12 fold, respectively in 18-day-old fetuses. Similar trends were noticed in the placenta. Activities of phase II enzymes such as glutathione S-transferase, UDP glucuronyltransferase and DT-diaphorase were also significantly elevated (Khan et al., 1987b).
Several studies have demonstrated pronounced effects of crude oil on the reproductive capacity of birds (decreased hatchability, deformed bills, incomplete ossification, incomplete feather formation, gross structural abnormalities, dead embryos) after application on the shell surface or after oral administration (Grau et al., 1977; Albers, 1978; Holmes et al., 1978; Hoffman, 1978, 1979a,b; Lee et al., 1986; Walters et al., 1987). [The Working Group noted that the avian system is a sensitive model for embryotoxic effects; results should be interpreted with caution with respect to possible effects in mammalian systems.]
Genetic and related effects
A large number of studies have been reported on the mutagenicity of crude oil and its fractions to Salmonella typhimurium (Table 15). Crude oil did not induce mutagenicity in any of the studies reported, either in the presence or absence of an exogenous metabolic system. Some neutral/aromatic (including polycyclic aromatic) fractions of crude oil were mutagenic in the presence of an exogenous metabolic system.
Table 15
Mutagenicity of crude oils and their fractions in Salmonella typhimurium.
Aromatic fractions (one to three rings and four rings and more) of Prudhoe Bay crude oil caused a significant increase in the frequency of sister chromatid exchange in cultured Chinese hamster ovary cells only in the presence of an exogenous metabolic system; no increase in the frequency of chromosomal aberrations was observed (Ellenton & Hallett, 1981). Wilmington crude oil did not increase the number of sister chromatid exchanges in human lymphocytes in vitro in the presence of an exogenous metabolic system (Lockard et al., 1982).
Intraperitoneal administration of Wilmington crude oil (five doses of 1 or 2.1 g/kg bw) did not induce sperm abnormalities in B6C3F1/Hap mice, and micronuclei were not induced in bone marrow of outbred Swiss male mice given 6.1 g/ kg bw intraperitoneally; an increase in the number of sister chromatid exchanges in bone-marrow cells of male outbred Swiss mice was observed at 7.2 g/kg bw intraperitoneally, but not at 1.8 or 3.6 g/kg (Lockard et al., 1982).
(b) Humans
Absorption, distribution, excretion and metabolism
No data were available to the Working Group.
Toxic effects
A labourer who had aspirated crude oil developed aspiration pneumonia and hepatic and renal toxicity, from which he recovered completely (Wojdat & Winnicki, 1964).
Adverse skin effects including dryness, pigmentation, hyperkeratosis, pigmented plane warts and eczematous reactions have been observed among petroleum field workers in contact with crude oil (Mierzecki, 1965; Dzhafarov, 1970; Gusein-Zade, 1982). In one study in the USSR, a higher prevalence of skin effects was noted among transport workers in crude oil production than among petroleum field workers (Gusein-Zade, 1982). Skin diseases (hyperkeratosis and follicular lesions) were 1.5–2.5 times more frequent in petroleum field workers than in control groups (Chernov et al., 1970).
Effects on reproduction and prenatal toxicity
No data were available to the Working Group.
Genetic and related effects
No data were available to the Working Group.
3.3. Epidemiological studies and case reports of carcinogenicity to humans
(a) Cohort study
A large retrospective cohort mortality study of US petroleum producing and pipeline workers was reported by Divine and Barron (1987). To be included in the study, men had to have been employed for at least six months at a producing or pipeline location and to have worked at some time during the period 1946–80. Vital status was ascertained for 97.8% of the cohort, which comprised 11 098 white men; death certificates were obtained for all but 3.4% of the deceased. Complete occupational histories were available from company records. Standardized mortality ratios (SMRs) were calculated in comparison with rates for US white males, and mortality was studied by length of employment, latency, whether producing or pipeline workers, and selected job categories. The SMR for all causes of death was significantly low (1886 observed; SMR, 0.63; 95% confidence interval [CI], 0.61–0.66), as was that for all cancers (393 observed; SMR, 0.68; 95% CI, 0.61–0.75). There was a significant excess of thyroid cancer among men employed as pumper-gaugers in petroleum production, but this was based on four cases only. A significant deficit of lung cancer (109 observed; SMR, 0.61; 95% CI, 0.50–0.73) was found among producing and pipeline workers, and no death from testicular cancer was observed although 3.2 were expected.
(b) Case-control studies
(i) Lung cancer
In an attempt to explain an excess of lung cancer cases observed in a cluster of parishes in Louisiana, USA, Gottlieb et al. (1979) conducted a case-control study, the design of which is described in the monograph on occupational exposures in petroleum refining (p. 102). An elevated risk for lung cancer was observed among black men aged over 53 years who had been employed in petroleum exploration and production (odds ratio, 1.6; 95% CI, 1.0–2.6). By logistic analysis, the ratio associated with crude oil exploration and drilling was three fold among persons over the age of 62 in parishes with petroleum or paper industries. [The Working Group noted that, since information used in this study was extracted directly from death certificates and since no account was taken of cigarette smoking, caution should be applied in interpreting the results.]
Gottlieb (1980) reanalysed the risk of lung cancer in relation to work in the petroleum mining and refining industry in the men included in the previous study. A group of 200 men with lung cancer and 170 control men who had worked in petroleum mining (125 cases, 112 controls) and refining (75 cases, 58 controls) were identified. The odds ratio for lung cancer associated with employment in the petroleum industry (mining and refining combined) was estimated at 1.2 (95% CI, 1.1–1.4). For welders, operators, boiler makers and painters, and oil-field workers taken as a group (mining and refining combined), the odds ratio was 2.3 (95% CI, 1.4–3.9). [The Working Group noted that information on exposure was extracted directly from death certificates; that no information on cigarette smoking was available; that cases were older than controls, which, in itself, may explain the difference observed; and that mining and refining occupations were combined.]
(ii) Testicular cancer
Mills et al. (1984) studied 347 hospital patients with histologically confirmed germ-cell tumour of the testis in the USA and matched them by age, sex, race and residence with 347 hospital controls, most of whom had tumours other than cancer of the testis. The ascertainment period was from 1 January 1977 to 31 August 1980. Occupational histories were extracted from medical records; when the type of industry was not apparent in the record, this was ascertained from the employer. An excess risk for testicular cancer was observed among petroleum and natural gas extraction workers (odds ratio, 2.3; 95% CI, 1.0–5.1). [The Working Group noted that information was obtained only on current occupation.]
Sewell et al. (1986) conducted a population-based study in New Mexico, USA, in which cases were identified at the New Mexico Tumor Registry. In order to be included in the study, the cases had to have had histologically confirmed testicular cancer registered in 1966–84, to have been 15 years old or more at the time of diagnosis and to have died of the disease. Controls consisted of persons who had died from other cancers, matched by age, year of diagnosis, race and sex. A total of 81 cases and 311 controls was identified. The source of occupational data was either death certificates (99%) or information on file at the tumour registry (1%). No excess risk for testicular cancer was observed among petroleum and gas workers (odds ratio, 0.57; 95% CI, 0.16–2.0). The authors noted the limited power of the study, that an association might have been obscured by the restriction to fatal cases and that information on exposure was limited.
(iii) Multiple sites
In a large case-control study of cancer at many sites conducted in Montreal, Canada, which is described in detail in the monograph on gasoline, p. 185, an association was seen between exposure to crude oil and rectal cancer (five cases; adjusted odds ratio 3.7; 90% CI, 1.3–10.6) and squamous-cell lung cancer (seven cases; adjusted odds ratio, 3.5; 90% CI, 1.5–8.2) (Siemiatycki et al., 1987). It was indicated, however, that these associations might only be apparent since they are based on very small numbers. The authors suggested that one of the main groups exposed to crude oil, namely seamen, would probably have had life styles very different from those of the rest of the study population.
4. Summary of Data Reported and Evaluation
4.1. Exposure data
Crude oil, which may be broadly characterized as paraffinic or naphthenic, is a complex mixture of alkanes, cycloalkanes and aromatic hydrocarbons containing low percentages of sulfur, nitrogen and oxygen compounds and trace quantities of many other elements. Worldwide, about 500 000 workers are employed in crude oil exploration and production. Occupational exposures during drilling, pumping and transportation of crude oil, including maintenance of equipment used for these processes, may involve inhalation of volatile compounds, including hydrocarbons and hydrogen sulfide. Skin contact with crude oils, which contain polycyclic aromatic compounds, may also occur during these operations. Accidental releases of crude oil into the aquatic environment are also potential sources of human exposure.
4.2. Experimental data1
Samples of crude oil from single sources and composite blends were tested for carcinogenicity by skin application in ten experiments in mice. Four samples of crude oil from single sources produced benign and malignant or unspecified skin tumours in two experiments. In one experiment, a composite sample produced a low incidence of skin carcinomas; in a similar experiment using the same treatment regimen but a blend of slightly different composition, no skin tumour was observed. The conduct and/or reporting of the results of six other experiments in mice were inadequate for evaluation.
Skin application to mice of fractions of two crude oil samples distilled under laboratory conditions and corresponding to various refinery streams produced skin tumours.
One sample of crude oil produced skin papillomas in rabbits in one experiment. Two other experiments were inadequate for evaluation.
4.3. Human data
In a retrospective cohort mortality study of a large group of male employees in petroleum producing and pipeline operations, mortality from all types of cancer was low, except from thyroid cancer. There was a significant deficit of lung cancer and no death from testicular cancer.
In a population-based case-control study, an elevated risk for lung cancer was observed among older men who had been employed in petroleum exploration and production. Reanalysis of the risk for lung cancer among men who had worked in the petroleum mining and refining industry showed an elevated risk for lung cancer among welders, operators, boiler makers, painters and oil-field workers taken as a group; no data were available on smoking habits.
In one of two case-control studies, an excess risk for testicular cancer was observed among petroleum and natural gas extraction workers. No such excess was found in the other study.
In a case-control study of cancer at many sites, an association was observed between exposure to crude oil and rectal and squamous-cell lung cancer. However, the association was based on small numbers and may have been confounded by life style factors.
4.4. Other relevant data
Crude oil induces dermal xenobiotic metabolizing enzymes and ornithine decarboxylase after skin application in mice.
In single studies of mice treated in vivo, crude oil induced an increase in the number of sister chromatid exchanges at the highest dose tested but did not induce micronuclei in bone-marrow cells or sperm abnormalities. Crude oil did not increase the number of sister chromatid exchanges in cultured human lymphocytes. Aromatic fractions of crude oil induced sister chromatid exchange, but not chromosomal aberrations, in cultured mammalian cells. Crude oil extracts did not induce mutation in bacteria; when fractionated, neutral fractions of crude oil, which contain aromatic or polycyclic aromatic compounds, generally had mutagenic activity in bacteria. (See Appendix 1.)
4.5. Evaluation1
There is inadequate evidence for the carcinogenicity in humans of crude oil.
There is limited evidence for the carcinogenicity in experimental animals of crude oil.
Overall evaluation
Crude oil is not classifiable as to its carcinogenicity to humans (Group 3).
5. References
- Albers P.H. The effects of petroleum on different stages of incubation in bird eggs. Bull. environ. Contam. Toxicol. 1978;19:624–630. [PubMed: 667391]
- American Petroleum Institute (1983) Introduction to Oil and Gas Production, Book 1, 4th ed., Dallas, TX.
- American Petroleum Institute (1984) Facts About Oil, Washington DC, pp. 8–9.
- American Petroleum Institute (1987a) Basic Petroleum Data Book: Petroleum Industry Statistics, Vol. VII, No. 3, Washington DC.
- American Petroleum Institute (1987b) Manual of Sampling and Analytical Methods for Petroleum Hydrocarbons in Groundwater and Soil (API Publ. No. 4449), Washington DC.
- Anderson, R.O. (1984) Fundamentals of the Petroleum Industry, Norman, OK, University of Oklahoma Press.
- Anon. Oil spils: how serious a problem? J. Water Pollut. Control. 1973;45:583–585.
- Anon. (1987) Oil and Gas Journal Data Book, Tulsa, OK, PennWell Publishing.
- Antonov A.M., Lints A.M. The blastomogenic action of natural Saratov oil. Probl. Oncol. 1960;6:1629–1634.
- Baker, A.M., Baker, R., Cyrus, C., Gerding, M., House, R., Morris, J., Pietrobono, J.T., Stelzner, I. & Stemerick, M. (1986a) Production. In: Gerding, M., ed., Fundamentals of Petroleum, 3rd ed., Austin, TX, Petroleum Extension Service, pp. 176–245.
- Baker, A.M., Baker, R., Cyrus, C., Gerding, M., House, R., Morris, J., Pietrobono, J.T., Stelzner, I. & Stemerick, M. (1986b) Transportation. In: Gerding, M., ed., Fundamentals of Petroleum, 3rd ed., Austin, TX, Petroleum Extension Service, pp. 247–320.
- Berne S., Bodennec G. Evaluation of hydrocarbons after the Tanio oil spill – a comparison with the Amoco Cadiz accident. Ambio. 1984;13:109–114.
- Bestougeff, M.A. (1967) Petroleum hydrocarbons. In: Nagy, B. & Colombo, U., eds, Fundamental Aspects of Petroleum Geochemistry, Amsterdam, Elsevier, pp. 77–108.
- Bingham, E. & Barkley, W. (1979) Bioassay of complex mixtures derived from fossil fuels. Environ. Health Perspect., 30, 157–163. [PMC free article: PMC1637712] [PubMed: 446446]
- British Petroleum Company (1986) BP Statistical Review of World Energy, June 1986, London.
- British Petroleum Company (1988) BP Statistical Review of World Energy, June 1988, London.
- Carver J.H., MacGregor J.A., King R.W. Mutagenicity and chemical characterization of two petroleum distillates. J. appl. Toxicol. 1984;4:163–169. [PubMed: 6491148]
- Chaudhury S., Macko S., Rahimtula A.D. Inhibition of rat platelet aggregation by a Prudhoe Bay crude oil and its aliphatic, aromatic, and heterocyclic fractions. Toxicol. appl. Pharmacol. 1987a;90:347–356. [PubMed: 3114914]
- Chaudhury S., Martin M., Payne J.F., Rahimtula A. Alterations in platelet aggregation and microsomal benzo-α-pyrene hydroxylase activities after exposure of rats to a Prudhoe Bay crude oil. J. Biochem. Toxicol. 1987b;2:93–104. [PubMed: 3508482]
- Chernov B.S., Karimov M.A., Rakhimova G.K. Dermatoses in workers in oil-fields (Russ.). Vestn. Dermatol. Venereol. 1970;44:65–68. [PubMed: 5507870]
- Clark C.R., Walter M.K., Ferguson P.W., Katchen M. Comparative dermal carcinogenesis of shale and petroleum-derived distillates. Toxicol. ind. Health. 1988;4:11–22. [PubMed: 3388444]
- Coleman, H.J., Shelton, E.M., Nichols, D.T. & Thompson, C.J. (1978) Analyses of 800 Crude Oils from United States Oilfields (BETC/RI-78/14), Bartlesville, OK, Bartlesville Energy Technology Center.
- Considine, D.M., ed. (1974) Chemical and Process Technology Encyclopedia, New York, McGraw-Hill, pp. 848–861.
- Coomes R.M. & Hazer, K.A. (1984) Statistical analyses of crude oil and shale oil carcinogenic test data. In: MacFarland, H.N., Holdsworth, C.E., MacGregor, J.A., Call, R.W. & Lane, M.L., eds, Advances in Modern Environmental Toxicology, Vol. VI, Applied Toxicology of Petroleum Hydrocarbons, Princeton, NJ, Princeton Scientific Publishers, pp. 167–186.
- Costantinides, G. & Arich, G. (1967) Non-hydrocarbon compounds in petroleum. In: Nagy, B. & Colombo, U., eds, Fundamental Aspects of Petroleum Geochemistry, Amsterdam, Elsevier, pp. 109–175.
- Cragg S.T., Conaway C.C., MacGregor J.A. Lack of concordance of the Salmonella/microsome assay with the mouse dermal carcinogenesis bioassay for complex petroleum hydrocarbon mixtures. Fundam. appl. Toxicol. 1985;5:382–390. [PubMed: 3886469]
- Cross, W. (1983) Petroleum, Chicago, IL, Regensteiner.
- Cuddington, K.S. & Lowther, N.F. (1977) The character of crude oil. In: Our Industry Petroleum, London, British Petroleum Company, pp. 208–221.
- Dickey, P.A. (1981) Petroleum Development Geology, 2nd ed., Tulsa, OK, PennWell Publishing, pp. 194–226.
- Divine B.J., Barron V. Texaco mortality study: III. A cohort study of producing and pipeline workers. Am. J. ind. Med. 1987;11:189–202. [PubMed: 3826079]
- DouAbul A.A.Z. Petroleum residues in the waters of the Shatt al-Arab River and the northwest region of the Arabian Gulf. Environ. int. 1984;10:265–267.
- DouAbul A.A.Z., Al-Saad H.T. Seasonal variations of oil residues in water of Shattal-Arab River, Iraq. Water Air Soil Pollut. 1985;24:237–246.
- Duck, B.W. (1983) Petroleum, extraction and transport by sea of. In: Parmeggiani, L., ed., Encyclopaedia of Occupational Health and Safety, 3rd (rev.) ed., Vol. 2, Geneva, International Labour Office, pp. 1652–1656.
- Dzhafarov F.A. Results of dermatological examination of oilmen occupationally exposed to the effect of crude oil(Russ.). Gig. Tr. prof. Zabol. 1970;14:37–41.
- Egan H., Castegnaro M., Bogovski P., Kunte H., Walker E.A., editors. Environmental Carcinogens — Selected Methods of Analysis. Lyon: International Agency for Research on Cancer; Analysis of Polycyclic Aromatic Hydrocarbons in Environmental Samples (IARC Scientific Publications No. 29). 1979;3
- Ellenton J.A., Hallett D.J. Mutagenicity and chemical analysis of aliphatic and aromatic fractions of Prudhoe Bay crude oil and fuel oil No. 2. J. Toxicol. environ. Health. 1981;8:959–972. [PubMed: 7200152]
- Engelhardt, F.R. (1981) Oil pollution in polar bears: exposure and clinical effects. In: Proceedings of the Fourth Arctic Marine Oilspill Program Technical Seminar, Edmonton, Alberta, Ottawa, Environmental Protection Service, pp. 139–179.
- Engelhardt, F.R. (1984) Environmental effects of petroleum on mammals. In: Hodgson, E., ed., Reviews in Environmental Toxicology, Vol. I, Amsterdam, Elsevier, pp. 319–337.
- Engelhardt, F.R. (1985) Effects of petroleum on marine mammals. In: Engelhardt, F.R., ed., Petroleum Effects in the Arctic Environment, London, Elsevier, pp. 217–243.
- Engelhardt F.R., Geraci J.R., Smith T.G. Uptake and clearance of petroleum hydrocarbons in the ringed seal, Phoca hispida. J. Fish. Res. Board Can. 1977;34:1143–1147.
- Epler J.L., Young J.A., Hardigree A.A., Rao T.K., Guerin M.R., Rubin I.B., Ho C.-H., Clark B.R. Analytical and biological analyses of test materials from the synthetic fuel technologies. I. Mutagenicity of crude oils determined by the Salmonella typhimurium/microsomal activation system. Mutat. Res. 1978;57:265–276. [PubMed: 353548]
- Ferrero, E.P. & Nichols, D.T. (1972) Analyses of 169 Crude Oils from 122 Foreign Oilfields (Information Circular 8542), Washington DC, US Department of the Interior.
- Foster M., Charters A.C., Neushul M. The Santa Barbara oil spill. Part 1: Initial quantities and distribution of pollutant crude oil. Environ. Pollut. 1971;2:97–113 .
- Gay M.L., Belisle A.A., Patton J.F. Quantification of petroleum-type hydrocarbons in avian tissue. J. Chromatogr. 1980;187:153–160 . [PubMed: 7358812]
- Giuliano, F.A., ed. (1981) Introduction to Oil and Gas Technology, 2nd ed., Boston, MA, International Human Resources Development Corp.
- Gottlieb M.S. Lung cancer and the petroleum industry in Louisiana. J. occup. Med. 1980;22:384–388. [PubMed: 7373452]
- Gottlieb M.S., Pickle L.W., Blot W.J., Fraumeni J.F. Jr. Lung cancer in Louisiana: death certificate analysis. J. natl Cancer Inst. 1979;63:1131–1137 . [PubMed: 291745]
- Grau C.R., Roudybush T., Dobbs J., Wathen J. Altered yolk structure and reduced hatchability of eggs from birds fed single doses of petroleum oils. Science. 1977;195:779–781. [PubMed: 836586]
- Gray, F. (1986) Petroleum Production for the Nontechnical Person, Tulsa, OK, PennWell Publishing.
- Guerin M.R., Rubin I.B., Rao T.K., Clark B.R., Epler J.L. Distribution of mutagenic activity in petroleum and petroleum substitutes. Fuel. 1981;60:282–288.
- Gundlach E.R., Hayes M.O. The Urquiola oil spill La Coruna, Spain: case history and discussion of methods of control and clean-up. Mar. Pollut. Bull. 1977;8:132–136.
- Gusein-Zade K.M. Characteristics of dermatoses morbidity in workers of Apsheron oil fields in relation to physico-chemical properties of the oil produced (Russ). Vestn. Dermatol. Venereol. 1982;9:63–66.
- Hawley, G.G. (1981) The Condensed Chemical Dictionary, 10th ed., New York, Van Nostrand Reinhold, p. 792.
- Hieger I., Woodhouse D.L. The value of the rabbit for carcinogenicity tests on petroleum fractions. Br. J. Cancer. 1952;6:293–299. [PMC free article: PMC2007827] [PubMed: 12987555]
- Hoffman D.J. Embryotoxic effects of crude oil in mallard ducks and chicks. Toxicol. appl. Pharmacol. 1978;46:183–190. [PubMed: 725942]
- Hoffman D.J. Embryotoxic and teratogenic effects of petroleum hydrocarbons in mallards, (Anas platyrhynchos). J. Toxicol. environ. Health. 1979a;5:835–844. [PubMed: 513150]
- Hoffman D.J. Embryotoxic and teratogenic effects of crude oil on mallard embryos on day one of development. Bull. environ. Contam. Toxicol. 1979b;22:632–637 . [PubMed: 486764]
- Hoffman, H.L. (1982) Petroleum (products). In: Grayson, M., ed., Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed., Vol. 17, New York, John Wiley & Sons, pp. 257–271.
- Holland J.M., Rahn R.O., Smith L.H., Clark B.R., Chang S.S., Stephens T.J. Skin carcinogenicity of synthetic and natural petroleums. J. occup. Med. 1979;21:614–618. [PubMed: 490222]
- Holland J.M., Wolf D. A., Clark B.R. Relative potency estimation for synthetic petroleum skin carcinogens. Environ. Health Perspect. 1981;38:149–155. [PMC free article: PMC1568427] [PubMed: 7238444]
- Holmes, W.N. (1984) Petroleum pollutants in the marine environment and their possible effects on seabirds. In: Hodgson, E., ed., Reviews in Environmental Toxicology, Vol. I, Amsterdam, Elsevier, pp. 251–317.
- Holmes W.N., Cavanaugh K.P., Cronshaw J. The effects of ingested petroleum on oviposition and some aspects of reproduction in experimental colonies of mallard ducks (Anas platyrhynchos). J. Reprod. Fertil. 1978;54:335–347 . [PubMed: 722682]
- IARC (1976) IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man, Vol. 11, Cadmium, Nickel, Some Epoxides, Miscellaneous Industrial Chemicals, and General Considerations on Volatile Anaesthetics, Lyon, pp. 75–112. [PubMed: 992654]
- IARC (1982) IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 29, Some Industrial Chemicals and Dyestuffs, Lyon, pp. 93–148, 391–398. [PubMed: 6957379]
- IARC (1986) Information Bulletin on the Survey of Chemicals Being Tested for Carcinogenicity, No. 12, Lyon, p. 287.
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl. 7. Lyon: Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs. 1987a;1 to 42:120–122. [PubMed: 3482203]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl. 7. Lyon: Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs. 1987b;1 to 42:264–269. [PubMed: 3482203]
- Ibiebele, D.D. (1986) Point-source inputs of petroleum wastewater into the Niger Delta, Nigeria. Sci. total Environ., 52, 2330238.
- International Labour Office (1986) Petroleum Committee, 10th Session, Report I, General Report, Geneva, p. 146.
- Khan, S., Payne, J.F. & Rahimtula, A.D. (1986) Mechanisms of petroleum hydrocarbon toxicity: destruction of liver microsomal and mitochondrial calcium pump activities by Prudhoe Bay crude oil. J. Biochem. Toxicol., 1, 31–43. [PubMed: 3271883]
- Khan S., Irfan M., Rahimtula A.D. The hepatotoxic potential of a Prudhoe Bay crude oil: effect on mouse liver weight and composition. Toxicology. 1987a;46:95–105. [PubMed: 2444021]
- Khan S., Martin M., Rahimtula A.D., Payne J.F. Effect of a Prudhoe Bay crude oil on hepatic and placental drug metabolism in rats. Can. J. Physiol. Pharmacol. 1987b;65:2400–2408. [PubMed: 3449197]
- Khan S., Martin M., Payne J.F., Rahimtula A.D. Embryotoxic evaluation of a Prudhoe Bay crude oil in rats. Toxicol. Lett. 1987c;38:109–114. [PubMed: 3629623]
- Koons C.B. Input of petroleum to the marine environment. Mar. Technol. Soc. J. 1984;18:4–10.
- Lawler G.C., Loong W.-A., Laseter J.L. Accumulation of saturated hydrocarbons in tissues of petroleum-exposed mallard ducks (Anas platyrhynchos). Environ. Sci. Technol. 1978a;12:47–51.
- Lawler G.C., Loong W.-A., Laseter J.L. Accumulation of aromatic hydrocarbons in tissues of petroleum-exposed mallard ducks (Anas platyrhynchos). Environ. Sci. Technol. 1978b;12:51–54.
- Lee, R.F. (1977) Accumulation and turnover of petroleum hydrocarbons in marine organisms. In: Wolfe, D.A., ed., Fate and Effect of Petroleum in Marine Organisms and Ecosystems, Oxford, Pergamon Press, pp. 60–70.
- Lee Y.-Z., O'Brien P.J., Payne J.F., Rahimtula A.D. Toxicity of petroleum crude oils and their effect on xenobiotic metabolizing enzyme activities in the chicken embryo in ovo. Environ. Res. 1986;39:153–163. [PubMed: 3943505]
- Leighton F.A., Lee Y.-Z., Rahimtula A.D., O'Brien P.J., Peakall D.B. Biochemical and functional disturbances in red blood cells of herring gulls ingesting Prudhoe Bay crude oil. Toxicol. appl. Pharmacol. 1985;81:25–31. [PubMed: 4049418]
- Lewis S.C. Crude petroleum and selected fractions. Skin cancer bioassays. Progr. exp. Tumor Res. 1983;26:68–84. [PubMed: 6302736]
- Lewis, S.C., King, R.W., Cragg, S.T. & Hillman, D.W. (1984) Skin carcinogenic potential of petroleum hydrocarbons: crude oil, distillate fractions and chemical class subfractions. In: MacFarland, H.N., Holdsworth, C.E., MacGregor, J.A., Call, R.W. & Lane, M.L., eds, Advances in Modern Environmental Toxicology, Vol. VI, Applied Toxicology of Petroleum Hydrocarbons, Princeton, NJ, Princeton Scientific Publishers, pp. 139–150.
- Lockard J.M., Prater J.W., Viau C.J., Enoch H.G., Sabharwal P.S. Comparative study of the genotoxic properties of eastern and western US shale oils, crude petroleum and coal-derived oil. Mutat. Res. 1982;102:221–235. [PubMed: 6755232]
- Ma C.Y., Ho C.-H., Quincy R.B., Guerin M.R., Rao T.K., Allen B.E., Epler J.L. Preparation of oils for bacterial mutagenicity testing. Mutat. Res. 1983;118:15–24. [PubMed: 6346084]
- MacGregor, J.A., Conaway, C.C. & Cragg, S.T. (1982) Predictivity of the Salmonella/microsome assay for carcinogenic and noncarcinogenic complex petroleum hydrocarbon mixtures. In: MacFarland, H.N., Holdsworth, C.E., MacGregor, J.A., Call, R.W. & Kane, M.L., eds, Proceedings of the Symposium on the Toxicology of Petroleum Hydrocarbons, Washington DC, American Petroleum Institute.
- Magee, E.M., Hall, H.J. & Varga, G.M., Jr (1973) Potential Pollutants in Fossil Fuels (EPA-R2-72-249/ PB 225-039), Linden, NJ, Esso Research and Engineering.
- Mair B.J. Here's a complete up-to-date list of the hydrocarbons isolated from petroleum. Oil Gas J. 1964;62:130–134.
- Mierzecki H. Chemical sensitization in the petroleum industry (Ger.). Berufsdermatosen. 1965;13:350–359. [PubMed: 4223274]
- Miller A.J., Ott G.L. Major oil spill on the Delaware River, September 1985. US Geol. Surv. Water-Supply Pap. 1986;2300:47–48.
- Mills P.K., Newell G.R., Johnson D.E. Testicular cancer associated with employment in agriculture and oil and natural gas extraction. Lancet. 1984;i:207–210. [PubMed: 6141346]
- Moldan A.G.S., Jackson L.F., McGibbon S., van der Westhuizen J. Some aspects of the Castillo de Bellver oil spill. Mar. Pollut. Bull. 1985;16:97–102.
- Myhre, W. (1980) Review of Federal and State Oil Pollution Laws, Washington DC, Preston, Thorgrimson, Ellis & Holman.
- National Research Council (1985) Oil in the Sea. Inputs, Fates and Effects, Washington DC, National Academy Press.
- Neff, J.M. & Anderson, J.W. (1981) Response of Marine Animals to Petroleum and Specific Petroleum Hydrocarbons, London, Applied Science Publishers, pp. 93–142.
- Oritsland, N.A., Engelhardt, F.R., Juck, F.A., Hurst, R.J. & Watts, P.D. (1981) Effect of Crude Oil on Polar Bears (Environmental Studies No. 24), Ottawa, Northern Environmental Protection Branch, Indian and Northern Affairs.
- Payne J.F., Fancey L.L., Rahimtula A.D., Porter E.L. Review and perspective on the use of mixed-function oxygenase enzymes in biological monitoring. Comp. Pharmacol. Physiol. 1987;86C:233–245. [PubMed: 2882912]
- Pelroy R.A., Sklarew D.S., Downey S.P. Comparison of the mutagenicities of fossil fuels. Mutat. Res. 1981;90:233–245. [PubMed: 7035938]
- Petrilli F.L., De Renzi G.P., De Flora S. Interaction between polycyclic aromatic hydrocarbons, crude oil and oil dispersants in the Salmonella mutagenesis assay. Carcinogenesis. 1980;1:51–56. [PubMed: 22282980]
- Rahimtula, A.D., O'Brien, P.J. & Payne, J.F. (1984) Induction of xenobiotic metabolism in rats on exposure to hydrocarbon-based oils. In: MacFarland, H.N., Holdsworth, C.E., MacGregor, J.A., Call, R.W. & Lane, M.L., eds, Advances in Modern Environmental Toxicology, Vol. VI, Applied Toxicology of Petroleum Hydrocarbons, Princeton, NJ, Princeton Scientific Publishers, pp. 71–79.
- Rahimtula A.D., Lee Y.-Z., Silva J. Induction of epidermal and hepatic ornithine decarboxylase by a Prudhoe Bay crude oil. Fundam. appl. Toxicol. 1987;8:408–414. [PubMed: 3569711]
- Reitze, A.W., Jr (1972) Environmental Law, 2nd ed., Washington DC, North American International.
- Rice, S.D., Short, J.W. & Karinen, J.F. (1977) Comparative oil toxicity and comparative animal sensitivity. In: Wolfe, D.A., ed., Fate and Effect of Petroleum in Marine Organisms and Ecosystems, Oxford, Pergamon Press, pp. 78–94.
- Sachanen, A.N. (1950) Hydrocarbons in petroleum. In: Brooks, B.T. & Duhstan, A.E., eds, The Science of Petroleum, Vol. V, Part I, Crude Oils. Chemical and Physical Properties, London, Oxford University Press, pp. 72–77.
- Sadiq M., Zaidi T.H. Vanadium and nickel content of Nowruz spill tar flakes on the Saudi Arabian coastline and their probable environmental impact. Bull. environ. Contam. Toxicol. 1984;32:635–639. [PubMed: 6743851]
- Schreiner, C.A. (1984) Petroleum and petroleum products: a brief review of studies to evaluate reproductive effects. In: Christian, M.S., Galbraith, W.M., Voytek, P. & Mehlman, M.A., eds, Advances in Modern Environmental Toxicology, Vol. III, Assessment of Reproductive and Teratogenic Hazards, Princeton, NJ, Princeton Scientific Publishers, pp. 29–45.
- Sewell C.M., Castle S.P., Hull H.F., Wiggins C. Testicular cancer and employment in agriculture and oil and natural gas extraction. Lancet. 1986;i:553. [PubMed: 2869275]
- Shapiro D.D., Getmanets I.Y. Blastomogenic properties of petroleum of different sources (Russ.). Gig. Sanit. 1962;27:38–42. [PubMed: 13911288]
- Sheppard E.P., Wells R.A., Georghiou P.E. The mutagenicity of a Prudhoe Bay crude oil and its residues from an experimental in situ burn. Environ. Res. 1983;30:427–441. [PubMed: 6339232]
- Siemiatycki J., Dewar R., Nadon L., Gérin M., Richardson L., Wacholder S. Associations between several sites of cancer and twelve petroleum-derived liquids. Results from a case-referent study in Montreal. Scand. J. Work Environ. Health. 1987;13:493–504. [PubMed: 3433051]
- Smith J.D., Maher W.A. Aromatic hydrocarbons in waters of Port Phillip bay and the Yarra river estuary. Aust. J. mar. Freshwater Res. 1984;35:119–128.
- Speight, J.G. (1980) The Chemistry and Technology of Petroleum, New York, Marcel Dekker, pp. 49–78.
- Suess, M.J., Grefen, K, Reinisch, D.W., eds (1985) Ambient Air Pollutants from Industrial Sources, Amsterdam, Elsevier, pp. 279–282.
- Tiratsoo, E.N. (1951) Petroleum Geology, London, Methuen, pp. 1–22.
- US Environmental Protection Agency (1986) Test Methods for Evaluating Solid Waste. Physical/Chemical Methods (Publ. SW-846), 3rd ed., Washington DC.
- Valković, V. (1978) Trace Elements in Petroleum, Part 2, Trace Element Levels in Crude Oils and Products, Tulsa, OK, Petroleum Publishing, pp. 62-101.
- Vandermeulen J.H., Foda A., Stuttard C. Toxicity vs mutagenicity of some crude oils, distillates and their water soluble fractions. Water Res. 1985;19:1283–1289.
- Walters P., Khan S., O'Brien P.J., Payne J.F., Rahimtula A.D. Effectiveness of a Prudhoe Bay crude oil and its aliphatic, aromatic and heterocyclic fractions in inducing mortality and aryl hydrocarbon hydroxylase in chick embryo in ovo. Arch. Toxicol. 1987;60:454–459. [PubMed: 3662820]
- Watkins, R.E. (1977) Transport of crude oil, gas and products by pipeline. In: Our Industry Petroleum, London, British Petroleum Company, pp. 179–196.
- West, J.R. (1983) Sulphur recovery. In: Grayson, M., ed., Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed., Vol. 22, New York, John Wiley & Sons, pp. 267–297.
- Wojdat W., Winnicki S. A case of bronchial aspiration pneumonia due to crude oil (Pol.). Bull. Inst. mar. Med. Gdansk. 1964;15:83–85. [PubMed: 14152242]
Footnotes
- 1
The Working Group was aware of studies by skin painting in progress in mice using three distillate fractions of a high-nitrogen crude oil (IARC, 1986).
- 1
Subsequent to the meeting, the Secretariat became aware of a study in which skin tumours were reported in mice after application to the skin of East Wilmington crude oil (Clark et al, 1988).
- 1
For definitions of the italicized terms, see Preamble, pp. 25-28.
- CRUDE OIL - Occupational Exposures in Petroleum Refining; Crude Oil and Major Pe...CRUDE OIL - Occupational Exposures in Petroleum Refining; Crude Oil and Major Petroleum Fuels
Your browsing activity is empty.
Activity recording is turned off.
See more...