Introduction
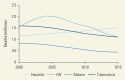
Viral hepatitis is caused by five distinct viruses (hepatitis A, B, C, D, and E), which have different routes of transmission and varying courses of disease (). According to the Global Health Estimates, deaths from acute and chronic hepatitis in 2012 were the tenth leading cause of death and the sixteenth leading cause of disability. In 2013, an estimated 1.45 million persons (95 percent uncertainty interval 1.38 million to 1.54 million) died from viral hepatitis; this estimate includes deaths due to acute hepatitis, as well as hepatitis-related liver cancer and cirrhosis (Stanaway and others 2016). Furthermore, while deaths from infectious diseases such as HIV/AIDS, malaria, and tuberculosis are decreasing, deaths from hepatitis increased by 63 percent between 1990 and 2013. Most (96 percent) hepatitis deaths are caused by hepatitis B virus (HBV) and hepatitis C virus (HCV)—these two viruses cause chronic, lifelong infection resulting in progressive liver damage leading to cirrhosis and hepatocellular carcinoma ().
Characteristics of Main Types of Viral Hepatitis Infections.
Number of Deaths per Year Due to Hepatitis, HIV/AIDS, Tuberculosis, and Malaria, 2000–15.
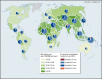
The burden of hepatitis infection is not equally distributed globally. Mortality rates from hepatitis are highest in West Africa and parts of Asia; in absolute numbers, East Asia and South Asia account for the greatest number of people dying from hepatitis—51 percent of the total number of deaths.
Effective interventions exist to prevent transmission of viral hepatitis (). Safe and effective vaccines have been developed to prevent hepatitis A, B, and E, and protection from hepatitis B infection by immunization also prevents hepatitis D.
Elements of a Comprehensive Hepatitis Prevention Program.
Hepatitis B and C chronic infections can be treated effectively. The new direct acting antiviral (DAA) medicines for hepatitis C can cure more than 90 percent of those with chronic infection with a two to three month course of treatment. Hepatitis C treatment could also reduce hepatitis C transmission because people who have been cured do not transmit the infection. There is no cure for chronic hepatitis B, but effective antiviral treatments can suppress viral replication and prevent disease progression.
Incidence, Prevalence, and Distribution
Because infection with the hepatitis viruses is often asymptomatic, there are no reliable estimates of the incidence of acute and chronic viral hepatitis. An estimated 250 million people live with chronic hepatitis B infection; 80 million have chronic hepatitis C infection (Gower and others 2014; Schweitzer and others 2015).
The prevalence of hepatitis B and C varies considerably in different regions. The areas of highest prevalence for hepatitis B are West Africa, where in some countries more than 8 percent of the population is infected, and East and Central Asia (). For hepatitis C infection, the regions with the highest prevalence are West and Central Africa, Eastern Europe, and Central Asia. The prevalence of hepatitis C infection is extremely high in a few countries, most notably, the Arab Republic of Egypt and Pakistan, where high incidence continues, largely the result of weak prevention measures, such as reuse of syringes and needles in health care settings (Ahmed and others 2013; Mostafa and others 2010).
Hepatitis Mortality Rates and Virus Distribution, by Global Burden of Disease Region, 2013.
Natural History and Mortality from Viral Hepatitis
The natural history of the hepatitis viruses can be categorized based on whether they cause chronic infection. All hepatitis viruses cause acute hepatitis, which can result in fulminant hepatitis in rare cases and may be fatal. Hepatitis B and hepatitis C also can cause chronic infection. Hepatitis D is an incomplete virus that can only replicate and cause infection in the presence of hepatitis B.
The risk of developing chronic hepatitis B infection depends on the age at infection; it declines from more than 90 percent among children infected during the first year to 20 percent to 30 percent for children infected between the ages of 1 and 5 years, and 6 percent for children ages 5 to 15 years (Edmunds and others 1993). For hepatitis C, approximately one-third of individuals will spontaneously clear infection, while the remaining two-thirds will develop chronic infection. For both viruses, chronic infection is marked by continued replication of the virus in the liver, which can lead to cirrhosis, hepatocellular carcinoma, or both (Chen, Iloeje, and Yang 2007; Thein and others 2008). These two conditions account for more than 90 percent of all deaths due to viral hepatitis () (Stanaway and others 2016). Chronic hepatitis B or hepatitis C infections cause an estimated 78 percent of all liver cancer and 57 percent of all liver cirrhosis (Perz and others 2006). Because of the higher prevalence of hepatitis B and hepatitis C in Asia and Sub-Saharan Africa, countries in these regions, which are least able to deal with these diseases, experience the highest rates of death due to viral hepatitis.
Number of Hepatitis-Related Deaths, by Virus Type, 2013.
Transmission of Hepatitis Viruses
Hepatitis A and hepatitis E are transmitted through the fecal-oral route by contact with contaminated food or water. Hepatitis E is also a zoonotic infection that can be spread by eating undercooked or uncooked pork or deer meat (Kamar and others 2012). Sexual transmission of hepatitis A by frequent oral-anal contact is common among men who have sex with men (Jin and others 2007). Hepatitis B, C, and D are transmitted through blood and bodily fluids. Globally, most hepatitis B infections occur through mother-to-child and early-life horizontal transmission between family members. Among adults, transmission occurs through sexual intercourse, as well as through unsafe injection practices and transfusion of unscreened blood and sexual transmission (Goldstein and others 2005). Most infections with HCV occur through unsafe injections, either in medical settings from reuse of medical equipment and substandard application of infection control measures, or through unsafe practices among people who inject drugs. Sexual transmission of hepatitis C is rare in heterosexual couples but more common among HIV/AIDS-infected men who have sex with men (Tohme and Holmberg 2010).
Interventions
summarizes the effective interventions for preventing the transmission of viral hepatitis.
Sanitation
Hepatitis A and E infections can be prevented through improved sanitation. Although no reliable estimates are available, it is likely that the incidence of hepatitis A and E has declined as part of the overall reduction in the number of deaths due to diarrhea. Between 1990 and 2012, all regions experienced major declines in the annual number of diarrheal diseases attributable to inadequate water, sanitation, and hygiene, from 1.8 million to 842,000 (WHO 2014).
Vaccination
Hepatitis A
An effective hepatitis A vaccine exists, and 18 countries have introduced universal childhood hepatitis A vaccination. Whether universal vaccination is appropriate depends on the socioeconomic status of a country. In countries where sanitation practices are improving as a result of improved socioeconomic conditions, many children escape hepatitis A infection, thus leaving them susceptible when they become adults. In these countries, wide-scale hepatitis A vaccination is likely to be cost-effective and is encouraged (Jacobsen and Wiersma 2010).
Hepatitis B
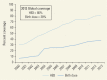
The most notable achievement in hepatitis prevention is the reduction in incidence of acute and chronic hepatitis B infection as a result of universal childhood hepatitis B vaccination. At the end of 2013, 183 out of 194 countries had introduced universal childhood vaccination; global coverage with three doses of hepatitis B vaccine is estimated to be 81 percent () (WHO 2015b). Universal infant vaccination with high coverage levels has led to major reductions in the prevalence of chronic hepatitis B infection among children. In China, the prevalence of chronic hepatitis B infection declined from approximately 8 percent in 1992 to 1 percent in 2006 among children ages one to four years (Liang and others 2009). The World Health Organization (WHO) Western Pacific Region, which includes China and several other high-prevalence countries, is on track to reach its goal of reducing the prevalence of chronic hepatitis B infection to less than 1 percent by 2017 (Wiesen, Diorditsa, and Li 2016). Countries where hepatitis B vaccine coverage has been high for several decades have noted reductions in death rates from hepatocellular carcinoma (Hung and others 2015).
Global Coverage of Childhood Hepatitis Immunization and Birth Dose, 2000–13.
Challenges remain to further reductions in incidence. Full protection for children requires that they receive the first vaccine dose within 24 hours of birth; this dose is termed a birth dose. Of 194 countries, 94 have introduced the birth dose in the vaccine schedule, and an estimated 38 percent of children globally receive this early dose (WHO 2015c). There are many logistical challenges to the delivery of the birth dose, including that most births occur at home in some countries. Treating pregnant women who have a high viral load of hepatitis B with antiviral medications during pregnancy can prevent mother-to-child transmission of hepatitis B (Pan and others 2016).
Hepatitis E
Outbreaks of hepatitis E occur primarily in Africa and South-East Asia through contaminated drinking water. An effective vaccine has been developed against one of the genotypes of hepatitis E. However, its effectiveness in outbreak settings and among children has not been assessed. For these reasons, the WHO has not made a recommendation for its use in national vaccination programs (WHO 2015c).
Safety of Health Care Injections
In 2000, unsafe health care injections accounted for an estimated 32 percent of hepatitis B infections, 40 percent of hepatitis C infections, and 5 percent of HIV infections acquired in low- and middle-income countries (LMICs) (Hauri, Armstrong, and Hutin 2004). An updated analysis estimated that between 2000 and 2010, the number of infections due to unsafe injections declined by 91 percent for hepatitis B and 83 percent for hepatitis C, primarily attributable to a reduction in the reuse of injection equipment (Pepin and others 2014).
Addressing health care–associated infections, particularly unsafe injections, is difficult because in many countries the health system is fragmented and poorly regulated. In some areas, the health care system overuses injections because of the perception that injected medicines are more effective than oral medicines. The approach to reducing this risk needs to combine raising awareness about the effectiveness of oral medicines with the introduction of needles and syringes with reuse-prevention features (WHO 2015d).
Safety of Blood Supply
The risk of transmission of hepatitis B and C viruses through the transfusion of unsafe blood can be dramatically lowered by appropriate selection of donors and universal, quality-assured testing of blood donations. Many countries, however, still collect a significant proportion of the blood for transfusion from donors who have a high prevalence of hepatitis infection, for example, paid donors, and not all countries screen 100 percent of their blood collections for hepatitis B or C. Accordingly, the risk of transfusion-related transmission of these viruses remains unacceptably high in these settings (WHO 2011).
Harm Reduction Programs
People who inject drugs are the group most highly affected by viral hepatitis. Globally, an estimated 67 percent of people who inject drugs have evidence of hepatitis C infection and 8 percent have hepatitis B (Nelson and others 2011). An effective package of intervention, termed harm reduction, has been developed and includes the provision of sterile injecting equipment and opioid substitution therapy (WHO, UNODC, and UNAIDS 2009). Countries that have provided these services as part of a public health response to injecting drug use have been most successful in addressing the epidemics of injecting drug use, hepatitis, and HIV/AIDS (Palmateer and others 2014). Despite solid public health evidence demonstrating the effectiveness of harm reduction interventions, many decision makers remain reluctant to implement or scale up such programs because of their controversial nature. Ongoing dedicated advocacy efforts are still needed to initiate and sustain harm reduction programs across the globe.
Access to Treatment
Even a comprehensive hepatitis prevention program cannot prevent deaths occurring among persons already infected. Fortunately, effective treatment exists to treat persons with chronic hepatitis B and C infections. HBV replication can be suppressed, and therapy is lifelong in most cases (Yapali, Talaat, and Lok 2014). By effectively suppressing the replication of HBV, hepatitis B treatment can result in a reversal of cirrhosis (Marcellin and others 2013). Hepatitis C can be cured in as little as eight weeks by providing treatment with DAA medications. Persons cured of hepatitis C have improved quality of life and lower rates of liver-related and overall mortality (Smith-Palmer, Cerri, and Valentine 2015).
Despite the availability of these medicines, very few people, particularly in LMICs, are treated. The high price of the medicines is an important factor. Prices for DAAs in high-income countries (HICs) generally exceed US$50,000 per person. Through price negotiations, middle-income countries such as Brazil are able to obtain the same medicines for approximately US$7,000 (Andrieux-Meyer and others 2015). In low-income countries, through the introduction of generic medications, the price to treat one person is less than US$500. Hepatitis B medicines are available in generic formulations and prices can be as low as US$4/month. Other important challenges to scaling up are the delay in drug registration of the DAAs and the lack of a workforce trained in hepatitis treatment. An additional important barrier is that most persons with chronic hepatitis infection are still undiagnosed and lack the opportunities to be tested because of the low availability, high complexity, and high cost of diagnostics.
Costs, Cost-Effectiveness, and Extended Cost-Effectiveness Analyses Results
The quality and availability of data on the cost-effectiveness of the interventions to prevent and treat hepatitis infection vary by intervention and are primarily limited to HICs ().
Cost-Effectiveness of Interventions for Hepatitis.
Immunization
Hepatitis B childhood immunization is the most cost-effective of the interventions to prevent infection, given the high morbidity associated with chronic hepatitis B infection and the low cost of vaccination. The cost-effectiveness of vaccination is directly linked to the population prevalence of infection; according to one analysis, the cost per year of life saved ranged from US$6 to US$51 in LMICs and upper-middle-income countries but was much higher (US$8,712–US$12,197) in HICs because of higher administration costs and lower prevalence of hepatitis B infection. In certain country-specific analyses, for example, in China, universal hepatitis B vaccination is cost saving as is catch-up vaccination of children and adolescents (Hutton, So, and Brandeau 2010; Lu and others 2013). An estimated 5.3 million to 6.0 million future hepatitis B–related deaths could be averted if 90 percent of children in 94 LMICs were vaccinated, compared with no vaccination. The only vaccine with greater estimated impact is that for measles (WHO 2013).
Assessing the cost-effectiveness of hepatitis A vaccination is complicated because the risk of symptomatic disease is linked with increasing age at time of infection. In countries where nearly the entire population is infected in childhood, when most infections are asymptomatic, there is very little morbidity, and thereby cost, associated with hepatitis A infection. In countries where the incidence of infection is lower, analyses show that universal childhood vaccination is more cost-effective than targeted approaches. Hepatitis A vaccination of adults is less cost-effective than for children. In a systematic review, 64 percent of studies assessing universal vaccination in children had cost-effectiveness ratios of less than US$28,606 per year of life saved or quality-adjusted life year (QALY) gained, compared with only 29 percent of studies in adults (Anonychuk and others 2008).
Injection-Associated Infections
Only limited data are available on the economic aspects of the prevention of health care–associated hepatitis infection. An analysis of the cost-effectiveness of measures to reduce unnecessary and unsafe injections resulted in a cost-effectiveness ratio between US$20.02 and US$3,280 per disability-adjusted life year (DALY) averted, depending on the region (Dziekan and others 2003).
Needle- and syringe-exchange programs for people who inject drugs are shown to be effective in preventing HIV/AIDS, but with regard to the effectiveness of these programs in the prevention of hepatitis C infection, the data are more limited and only from HICs. One analysis concluded that the cost to prevent one hepatitis C infection was between US$357,586 and US$1.4 million; a study from Australia assessing the cost-effectiveness of harm reduction to prevent hepatitis C and HIV/AIDS infections found that a needle- and syringe-exchange program resulted in US$801–US$16,840 per QALY gained (Kwon and others 2012; Pollack 2001).
Treatment
The high cost of the new hepatitis C medicines has engendered considerable controversy. Economic analyses concerning treatment have yielded widely varying results, partly because of differences in the assessed treatment regimens; virus genotypes; and types of patient populations, for example, those undergoing treatment for the first time versus those who failed previous treatment. Most of the analyses show that treatment with DAAs for hepatitis C genotype 1 regardless of the stage of liver disease is highly cost-effective in the United States, with an incremental cost-effectiveness ratio of less than US$71,517 (Tice and others 2015). Few studies have assessed the cost-effectiveness of the treatments in LMICs. These analyses are complicated by the varying and rapidly changing prices of DAAs in these countries (Andrieux-Meyer and others 2015).
The economics of treatment for hepatitis B differ from those for hepatitis C. Although the price of medicines is much lower on a per-day basis, treatment is lifelong for most patients with HBV infection; however, analyses show hepatitis B treatment to be cost-effective. In a systematic review, most of the studies conducted in HICs showed that treating compared to not treating resulted in an incremental cost-effectiveness ratio of less than US$70,000 per QALY gained (Buti and others 2013). Several studies have assessed the economics of treatment in China, which has the largest number of persons with chronic hepatitis B infection. In one study, entecavir or tenofovir monotherapy treatment in non-cirrhotic patients would prevent 49 percent to 69 percent of liver-related deaths and 26 percent to 31 percent of hepatocellular carcinoma deaths; treatment would be cost saving at a drug price of less than US$32–US$75 a month (Toy, Hutton, and So 2015).
Conclusions
The arrival of highly effective treatments for hepatitis B and C and an improved understanding of the attendant burden of disease have resulted in a call for increased action to eliminate hepatitis. In view of the differences in the distribution of hepatitis viruses and the related burden of disease, policy makers will need to develop national plans that are tailored to their epidemiologic patterns and response capacity. The goals of the United Nations 2030 Agenda for Sustainable Development include a goal to combat the epidemic of hepatitis (UN 2015). The WHO has developed a global hepatitis strategy to eliminate hepatitis B and C as major public health threats by 2030 (WHO 2016). The means to achieve these goals exist. What is needed is increased advocacy and country-level investment and action.
Note
World Bank Income Classifications as of July 2014 are as follows, based on estimates of gross national income (GNI) per capita for 2013:
Low-income countries (LICs) = US$1,045 or less
Middle-income countries (MICs) are subdivided:
| alpha a. | lower-middle-income = US$1,046 to US$4,125 |
| alpha b. | upper-middle-income (UMICs) = US$4,126 to US$12,745 |
High-income countries (HICs) = US$12,746 or more.
Corresponding author: Stefan Z. Wiktor, School of Public Health, University of Washington, Seattle, Washington, United States; ude.wu@srotkiw.
References
Ahmed B, Ali T, Qureshi H, Hamid S.
2013. “Population-Attributable Estimates for Risk Factors Associated with Hepatitis B and C: Policy Implications for Pakistan and Other South Asian Countries.”
Hepatology Internatonal
7 (2): 500–507. [
PubMed: 26201782]
Andrieux-Meyer I, Cohn J, Araujo E S de, Hamid S S.
2015. “Disparity in Market Prices for Hepatitis C Virus Direct-Acting Drugs.”
The Lancet Global Health
3 (11): e676–77. doi:10.1016/S2214-109X(15)00156-4. [
PubMed: 26475012]
Anonychuk A M, Tricco A C, Bauch C T, Pham B, Gilca V., others. 2008. “Cost-Effectiveness Analyses of Hepatitis A Vaccine: A Systematic Review to Explore the Effect of Methodological Quality on the Economic Attractiveness of Vaccination Strategies.”
Pharmacoeconomics
26 (1): 17–32. [
PubMed: 18088156]
Buti M, Oyaguez I, Lozano V, Casado M A.
2013. “Cost Effectiveness of First-Line Oral Antiviral Therapies for Chronic Hepatitis B: A Systematic Review.”
Pharmacoeconomics
31 (1): 63–75. doi:10.1007/s40273-012-0009-2. [
PubMed: 23329593]
Chen C J, Iloeje U H, Yang H I.
2007. “Long-Term Outcomes in Hepatitis B: The REVEAL-HBV Study.”
Clinics in Liver Disease
11 (4): 797–816, viii. [
PubMed: 17981229]
Creese A, Floyd A, Alban A, Guinness L.
2002. “Cost-Effectiveness of HIV/AIDS Interventions in Africa: A Systematic Review of the Evidence.”
The Lancet
359 (9318): 1635–43. [
PubMed: 12020523]
Dziekan G, Chisholm D, Johns B, Rovira J, Hutin Y J.
2003. “The Cost-Effectiveness of Policies for the Safe and Appropriate Use of Injection in Healthcare Settings.”
Bulletin of the World Health Organization
81 (4): 277–85. [
PMC free article: PMC2572434] [
PubMed: 12764494]
Edmunds W J, Medley G F, Nokes D J, Hall A J, Whittle H C.
1993. “The Influence of Age on the Development of the Hepatitis B Carrier State.”
Proceedings of the Royal Society: Biological Sciences
253 (1337): 197–201. doi: 10.1098/rspb.1993.0102. [
PubMed: 8397416]
GBD 2013 Mortality and Causes of Death Collaborators. 2015. “Global, Regional, and National Age-Sex Specific All-Cause and Cause-Specific Mortality for 240 Causes of Death, 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013.”
The Lancet
385 (9963): 117–71. doi:10.1016/S0140-6736(14)61682-2. [
PMC free article: PMC4340604] [
PubMed: 25530442]
Goldstein S T, Zhou F, Hadler S C, Bell B P, Mast E E., others. 2005. “A Mathematical Model to Estimate Global Hepatitis B Disease Burden and Vaccination Impact.”
International Journal of Epidemiology
34 (6): 1329–39. doi:10.1093/ije/dyi206. [
PubMed: 16249217]
Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H.
2014. “Global Epidemiology and Genotype Distribution of the Hepatitis C Virus Infection.”
Journal of Hepatology
61 (Suppl 1): S45–57. doi:10.1016/j.jhep.2014.07.027. [
PubMed: 25086286]
Hauri A M, Armstrong G. L, Hutin Y J.
2004. “The Global Burden of Disease Attributable to Contaminated Injections Given in Health Care Settings.”
International Journal of STD and AIDS
15 (1): 7–16. doi:10.1258/095646204322637182. [
PubMed: 14769164]
Hung G Y, Horng J L, Yen H J, Lee C Y, Lin L Y.
2015. “Changing Incidence Patterns of Hepatocellular Carcinoma among Age Groups in Taiwan.”
Journal of Hepatology
63 (6): 1390–96. doi:10.1016/j.jhep.2015.07.032. [
PubMed: 26256438]
Hutton D W, So S. K, Brandeau M L.
2010. “Cost-Effectiveness of Nationwide Hepatitis B Catch-Up Vaccination among Children and Adolescents in China.”
Hepatology
51 (2): 405–14. doi:10.1002/hep.23310. [
PMC free article: PMC3245734] [
PubMed: 19839061]
Jacobsen K H, Wiersma S T.
2010. “Hepatitis A Virus Seroprevalence by Age and World Region, 1990 and 2005.”
Vaccine
28 (41): 6653–57. doi:10.1016/j.vaccine.2010.08.037. [
PubMed: 20723630]
Jin F, Prestage G P, Zablostka I, Rawstone R, Kippax S C., others. 2007. “High Rates of Sexually Transmitted Infections in HIV Positive Homosexual Men: Data from Two Community Based Cohorts.”
Sexually Transmitted Infections
83: 397–99. [
PMC free article: PMC2659038] [
PubMed: 17556503]
Kamar N, Bendall R, Legrand-Abravanel F, Xia N S, Ijaz S., others. 2012. “Hepatitis E.”
The Lancet
379 (9835): 2477–88. doi:10.1016/S0140-6736(11)61849-7. [
PubMed: 22549046]
Kwon J A, Anderson J, Kerr C C, Thein H H, Zhang L., others. 2012. “Estimating the Cost-Effectiveness of Needle-Syringe Programs in Australia.”
AIDS
26 (17): 2201–10. doi:10.1097/QAD.0b013e3283578b5d. [
PubMed: 22914579]
Liang X, Bi S, Yang W, Wang L, Cui G., others. 2009. “Epidemiological Serosurvey of Hepatitis B in China: Declining HBV Prevalence Due to Hepatitis B Vaccination.”
Vaccine
27 (47): 6550–57. doi:10.1016/j.vaccine.2009.08.048. [
PubMed: 19729084]
Lu S Q, McGhee S M, Xie X, Cheng J, Fielding R.
2013. “Economic Evaluation of Universal Newborn Hepatitis B Vaccination in China.”
Vaccine
31 (14): 1864–69. doi:10.1016/j.vaccine.2013.01.020. [
PubMed: 23384752]
Marcellin P, Gane E, Buti M, Afdhal N, Sievert W., others. 2013. “Regression of Cirrhosis during Treatment with Tenofovir Disoproxil Fumarate for Chronic Hepatitis B: A 5-Year Open-Label Follow-Up Study.”
The Lancet
381 (9865): 468–75. doi:10.1016/S0140-6736(12)61425-1. [
PubMed: 23234725]
Miller M A, McCann L.
2000. “Policy Analysis of the Use of Hepatitis B, Haemophilus influenzae Type b-, Streptococcus pneumoniae-Conjugate and Rotavirus Vaccines in National Immunization Schedules.”
Health Economics
9 (1): 19–35. [
PubMed: 10694757]
Mostafa A, Taylor S M, el-Daly M, el-Hoseiny M, Bakr I., others. 2010. “Is the Hepatitis C Virus Epidemic Over in Egypt? Incidence and Risk Factors of New Hepatitis C Virus Infections.”
Liver International
30 (4): 560–66. doi:10.1111/j.1478-3231.2009.02204.x. [
PubMed: 20141592]
Nelson P K, Mathers B M, Cowie B, Hagan H, Jarlais D Des. others. 2011. “Global Epidemiology of Hepatitis B and Hepatitis C in People Who Inject Drugs: Results of Systematic Reviews.”
The Lancet
378 (9791): 571–83. doi:10.1016/S0140-6736(11)61097-0. [
PMC free article: PMC3285467] [
PubMed: 21802134]
Palmateer N E, Taylor A, Goldberg D J, Munro A, Aitken C., others. 2014. “Rapid Decline in HCV Incidence among People Who Inject Drugs Associated with National Scale-Up in Coverage of a Combination of Harm Reduction Interventions.”
PLoS One
9 (8): e104515. doi:10.1371/journal.pone.0104515. [
PMC free article: PMC4128763] [
PubMed: 25110927]
Pan C Q, Duan Z, Dai E, Zhang S, Han G., others. 2016. “Tenofovir to Prevent Hepatitis B Transmission in Mothers with High Viral Load.”
New England Journal of Medicine
374 (24): 2324–34. doi:10.1056/NEJMoa1508660. [
PubMed: 27305192]
Pepin J, Abou C. N, Chakra E, Pepin V Nault, Valiquette L.
2014. “Evolution of the Global Burden of Viral Infections from Unsafe Medical Injections, 2000–2010.”
PLoS One
9 (6): e99677. doi:10.1371/journal.pone.0099677. [
PMC free article: PMC4049770] [
PubMed: 24911341]
Perz J F, Armstrong G L, Farrington L A, Hutin Y J, Bell B. P.
2006. “The Contributions of Hepatitis B Virus and Hepatitis C Virus Infections to Cirrhosis and Primary Liver Cancer Worldwide.”
Journal of Hepatology
45 (4): 529–38. doi:10.1016/j.jhep.2006.05.013. [
PubMed: 16879891]
Pollack H A.
2001. “Cost-Effectiveness of Harm Reduction in Preventing Hepatitis C among Injection Drug Users.”
Medical Decision Making
21 (5): 357–67. [
PubMed: 11575485]
Schweitzer A, Horn J, Mikolajczyk R T, Krause G, Ott J J.
2015. “Estimations of Worldwide Prevalence of Chronic Hepatitis B Virus Infection: A Systematic Review of Data Published between 1965 and 2013.”
The Lancet
386 (10003): 1546–55. doi:10.1016/S0140-6736(15)61412-X. [
PubMed: 26231459]
Smith-Palmer J, Cerri K, Valentine W.
2015. “Achieving Sustained Virologic Response in Hepatitis C: A Systematic Review of the Clinical, Economic and Quality of Life Benefits.”
BMC Infectious Diseases
15: 19. doi:10.1186/s12879-015-0748-8. [
PMC free article: PMC4299677] [
PubMed: 25596623]
Stanaway J D, Flaxman A D, Naghavi M, Fitzmaurice C, Vos T., others. 2016. “The Global Burden of Viral Hepatitis from 1990 to 2013: Findings from the Global Burden of Disease Study 2013.”
The Lancet. doi:10.1016/S0140-6736(16)30579-7. [
PMC free article: PMC5100695] [
PubMed: 27394647]
Thein H H, Yi Q, Dore G J, Krahn M D.
2008. “Estimation of Stage-Specific Fibrosis Progression Rates in Chronic Hepatitis C Virus Infection: A Meta-Analysis and Meta-Regression.”
Hepatology
48 (2): 418–31. doi:10.1002/hep.22375. [
PubMed: 18563841]
Tice J A, Ollendorf D A, Chahal H S, Kahn J G, Marseille E., others. 2015. The Comparative Clinical Effectiveness and Value of Novel Combination Therapies for the Treatment of Patients with Genotype 1 Chronic Hepatitis C Infection. A Technology Assessment Final Report. Boston. MA: Institute for Clinical and Economic Review.
Tohme R A, Holmberg S D.
2010. “Is Sexual Contact a Major Mode of Hepatitis C Virus Transmission?” Hepatology
52 (4): 1497–505. doi:10.1002/hep.23808. [
PubMed: 20635398]
Toy M, Hutton D. W, So S K.
2015. “Cost-Effectiveness and Cost Thresholds of Generic and Brand Drugs in a National Chronic Hepatitis B Treatment Program in China.”
PLoS One
10 (11): e0139876. doi:10.1371/journal.pone.0139876. [
PMC free article: PMC4633043] [
PubMed: 26536626]
WHO (World Health Organization). 2011. Global Database on Blood Safety. Blood Transfusion Safety [web page]. WHO, Geneva.
WHO (World Health Organization). 2013. Global Vaccine Action Plan 2011–2020. Geneva: WHO.
WHO (World Health Organization). 2015c. “Hepatitis E Vaccine: WHO Position Paper. May 2015.”
Weekly Epidemiological Record
90 (18): 185–200. [
PubMed: 25935931]
WHO and UNICEF (United Nations Children’s Fund). 2015. Achieving the Malaria MDG Target: Reversing the Incidence of Malaria 2000–2015. Geneva: WHO and UNICEF.
WHO, UNODC (United Nations Office on Drugs and Crime), and UNAIDS. 2009. Technical Guide for Countries to Set Targets for Universal Access to HIV Prevention, Treatment and Care for Injecting Drug Users. Geneva: WHO, Regional Office for Europe, UNAIDS, UNODC.
Wiesen E, Diorditsa S, Li X.
2016. “Progress towards Hepatitis B Prevention through Vaccination in the Western Pacific, 1990–2014.”
Vaccine
34 (25): 2855–62. doi: 10.1016/j.vaccine.2016.03.060. [
PubMed: 27020710]
Yapali S, Talaat N, Lok A S.
2014. “Management of Hepatitis B: Our Practice and How It Relates to the Guidelines.”
Clinical Gastroenterology and Hepatology
12 (1): 16–26. [
PubMed: 23660419]
Younossi Z, Henry L L.
2014. “The Impact of the New Antiviral Regimens on Patient Reported Outcomes and Health Economics of Patients with Chronic Hepatitis C.”
Digestive and Liver Disease
46 (Suppl 5): S186–96. [
PubMed: 25458773]