NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Industrial Chemicals. Lyon (FR): International Agency for Research on Cancer; 2000. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 77.)
1. Exposure Data
1.1. Chemical and physical data
1.1.1. Nomenclature
- Chem. Abstr. Serv. Reg. No.: 102-71-6
- Deleted CAS Reg. Nos: 36549-53-8; 36549-54-9; 36549-55-0; 36659-79-7; 10565527-4; 126068-67-5
- Chem. Abstr. Name: 2,2′,2″-Nitrilotris[ethanol]
- IUPAC Systematic Name: 2,2′,2″-Nitrilotriethanol
- Synonyms: Alkanolamine 244; nitrilotriethanol; TEA; TEA (amino alcohol); TEOA; triethanolamin; tris(β-hydroxyethyl)amine; tris(2-hydroxyethyl)amine
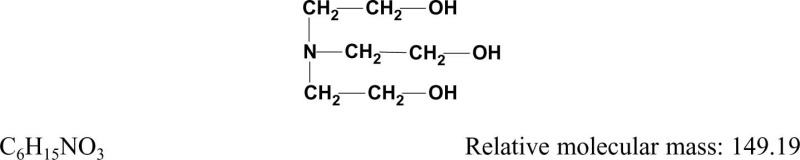
1.1.2. Structural and molecular formulae and relative molecular mass
1.1.3. Chemical and physical properties of the pure substance
- (a)
Description: Hygroscopic crystals, or colourless, viscous liquid with a mild ammoniacal odour (Lide & Milne, 1996; Budavari, 1998)
- (b)
Boiling-point: 335.4 °C (Lide & Milne, 1996)
- (c)
Melting-point: 20.5 °C (Lide & Milne, 1996)
- (d)
Density: 1.1242 g/cm3 at 20 °C (Lide & Milne, 1996)
- (e)
Spectroscopy data: Infrared (proton [10636]; grating [18547]), nuclear magnetic resonance (proton [7209]; C-13 [1871]) and mass spectral data have been reported (Sadtler Research Laboratories, 1980; Lide & Milne, 1996)
- (f)
Solubility: Miscible with water, acetone, ethanol and methanol; soluble in chloroform; slightly soluble in benzene, diethyl ether and lignans (Lide & Milne, 1996; Budavari, 1998)
- (g)
Volatility: Vapour pressure, < 1.3 Pa at 20 °C; relative vapour density (air = 1), 5.14 (Verschueren, 1996); flash-point, 185 °C (Budavari, 1998)
- (h)
Stability: Incompatible with metals such as aluminium and copper, halogenated organics, strong acids, oxidizing materials and absorbent materials (cellulose, sawdust) (Dow Chemical Company, 1999a)
- (i)
Octanol/water partition coefficient (P): log P, –2.3 (Verschueren, 1996)
- (j)
Conversion factor1: mg/m3 = 6.10 × ppm
1.1.4. Technical products and impurities
Triethanolamine is commercially available with the following specifications: purity, 99.0% min.; monoethanolamine, 0.05% max.; diethanolamine, 0.40% max. (see monograph in this volume); and water content, 0.20% max. (Dow Chemical Company, 1999b). Triethanolamine is also available in several other grades, including a blend of 85% triethanolamine and 15% diethanolamine [TEA 85]; a low freeze-grade blend (85% TEA 85 and 15% deionized water) for use in colder temperatures; and a blend of 85% triethanolamine and 15% deionized water [TEA 99 Low Freeze Grade] (Dow Chemical Company, 1998).
Trade names for triethanolamine include Daltogen, Sterolamide, Sting-Kill, Thiofaco T-35, and Trolamine.
1.1.5. Analysis
Triethanolamine can be determined in workplace air by drawing the air sample through aqueous hexanesulfonic acid and analysing by ion chromatography. The limit of detection for this method is 20 µg per sample (Eller, 1994).
Triethanolamine can be determined in metalworking and cutting fluids by gas chromatography–mass selective detection of silylated derivatives, by isotachophoresis, by capillary zone electrophoresis with indirect ultraviolet detection, and by spectrophotometry (Kenyon et al., 1993; Fernando, 1995; Schubert et al., 1996; Sollenberg, 1997); and in cosmetics and pharmaceuticals by ion-exclusion chromatography and by reversed-phase high performance liquid chromatography (Fukui et al., 1992; Maurer et al., 1996).
1.2. Production
Ethanolamines became available commercially in the early 1930s; they assumed steadily growing commercial importance as intermediates after 1945, because of the large-scale production of ethylene oxide. Since the mid-1970s, economical production of very pure, colourless ethanolamines has been possible. Ethanolamines are produced on an industrial scale exclusively by reaction of ethylene oxide (see IARC, 1994) and excess ammonia. This reaction takes place slowly, but is accelerated by water. An anhydrous procedure uses a fixed-bed ion-exchange resin catalyst (Hammer et al., 1987).
Estimated annual production of triethanolamine in the United States is presented in Table 1. Worldwide production has been estimated at 100 000–500 000 tonnes per year and European production at 50 000–100 000 tonnes per year (United Nations Environment Program Chemicals, 2000).
Table 1
Estimated annual production of triethanolamine in the United States (thousand tonnes).
Information available in 1999 indicated that triethanolamine was manufactured by six companies in India, five companies in the United States, three companies each in China, France, Germany and Mexico, two companies each in Italy and the Russian Federation and one company each in Australia, Belgium, Brazil, Czech Republic, Iran, Japan, Spain and the United Kingdom (Chemical Information Services, 1999).
1.3. Use
Triethanolamine is used as a corrosion inhibitor in metal-cutting fluids (see General Remarks), a curing agent for epoxy and rubber polymers, as a copper–triethanolamine complex to control freshwater algae on lakes and ponds and as a neutralizer–dispersing agent in agricultural herbicide formulations. It is also extensively used in emulsifiers, thickeners and wetting agents in the formulation of consumer products such as cosmetics, detergents, shampoos and other personal products (Beyer et al., 1983; Santa María et al., 1996; West & Gonsior, 1996).
Other applications of triethanolamine include: adhesives, antistatic agents, cement and concrete work, coatings, in electroless and electroplating, in fuels, printing inks, lithography, metal-cleaning and lubricating, mining, paint and pigments, petroleum and coal production, as a pharmaceutical intermediate and an ointment-emulsifier, in polymers and polymer production, rubber processing, soldering flux, textile finishing, polyurethane production and use and wood pulping (Dow Chemical Company, 1998). Table 2 presents estimates of the percentages used in major applications (Knaak et al., 1997) in the United States.
Table 2
Major uses of triethanolamine in the United States.
1.4. Occurrence
1.4.1. Natural occurrence
Triethanolamine is not known to occur as a natural product.
1.4.2. Occupational exposure
No data on the number of workers exposed to triethanolamine were available from the 1981–83 National Occupational Exposure Survey (NOES, 1999) conducted by the National Institute for Occupational Safety and Health (NIOSH).
Triethanolamine is present in machining and grinding fluids and has been measured in the metal manufacturing industry. It was present in bulk cutting fluids at levels ranging from 0.3 to 40%. Personal air exposures ranged from 0.02 to 244 µg/m3 (n = 110) (Kenyon et al., 1993). Concentrations were generally higher for workers engaged in transfer operations and lowest for assembly workers (who did not use machining fluids themselves).
In a German study (1992–94), triethanolamine was measured in metalworking fluid samples (n = 69). The proportion of samples containing triethanolamine varied over time between 50 and 85% (Pfeiffer et al., 1996).
1.4.3. Environmental occurrence
The broad utility of triethanolamine in a large number of industrial applications and consumer products may result in its release to the environment through various waste streams (Beyer et al., 1983; Santa María et al., 1996; West & Gonsior, 1996).
Dermal exposure to triethanolamine-containing products (principally personal care products) is the primary route of general population exposure to triethanolamine (Jones & Kennedy, 1988; Batten et al., 1994).
In 1981, triethanolamine was reported to be an ingredient (generally at a concentration of less than or equal to 5%) in 2720 out of 22 572 cosmetic products which may be applied to or come into contact with skin, eyes, hair, nails, mucous membrane and respiratory epithelium. Small amounts may be ingested from lipsticks. Product formulations containing also monoethanolamine (triethanolamine–ethanolamine) may be in contact with the skin for variable periods of time following each application. Daily or occasional use may extend over many years (Beyer et al., 1983).
1.5. Regulations and guidelines
Occupational exposure limits and guidelines for triethanolamine are presented in Table 3.
Table 3
Occupational exposure limits and guidelines for triethanolamine.
The Food and Drug Administration (1999) permits the use of triethanolamine as a component of adhesives in food packaging as an indirect food additive, as a component of the uncoated or coated food contact surface of paper and paper board for use with dry solid foods with no free fat or oil on the surface, and to adjust pH during the manufacture of amino resins permitted for use as components of paper and paper board in the United States.
2. Studies of Cancer in Humans
The Working Group was not aware of any studies that specifically examined the risk of cancer among persons exposed to triethanolamine. However, ethanolamines have been used as additives for metalworking fluids since the 1950s (see General Remarks). There are three major types of metalworking fluid; straight (generally mineral oils), soluble (straight oils diluted with water and additives) and synthetic (water and additives). Ethanolamines, either triethanolamine or diethanolamine, are very common additives to both soluble and synthetic metalworking fluids (see Sections 1.3 and 1.4.2). A number of studies have examined the risk of cancer among workers exposed to metalworking fluids. Only studies which stated that ethanolamines (no study indicated triethanolamine alone) were used as additives or that presented results for workers primarily exposed to soluble or synthetic fluids were considered by the Working Group. The characteristics of these studies are presented in Table 4 of the monograph on diethanolamine in this volume and a summary of the results of these studies for specific cancer sites is presented in Table 5 of the same monograph. The use of ethanolamines and nitrites together as additives to metalworking fluids can lead to the formation of N-nitrosodiethanolamine. Studies stating that ethanolamines and nitrites were used together as additives or which presented results for exposure to nitrosamines are described in detail in the monograph in this volume on N-nitrosodiethanolamine. The other studies are described in detail in the monograph on diethanolamine.
Table 4
Genetic and related effects of triethanolamine.
3. Studies of Cancer in Experimental Animals
3.1. Oral administration
3.1.1. Mouse
Groups of 40 male and 40 female ICR-JCL mice, six weeks of age, were fed diets prepared by adding 0.03 (low dose) or 0.3% w/w (high dose) triethanolamine (analytical grade) to a powdered diet (heated for 40 min at 100 °C and formed into pellets) throughout their lifespan. Control animals received untreated diet [heating not specified]. The survival rate was 50% at 85 weeks in females and at 65 weeks in males for both treated and control animals. There was a statistically significant (p < 0.05, test unspecified) increase in the incidence of lymphomas in female mice (controls, 1/36; low dose, 7/37; high dose, 9/36), but no increase in the incidence of tumours at any site in male mice (Hoshino & Tanooka, 1978). [The Working Group noted the lack of historical control data on the incidence of lymphomas in female mice, as well as the possibility that heating of the triethanolamine in the diet may have produced degradation products.]
Groups of 50 male and 50 female B6C3F1 mice, six weeks of age, were given drinking-water containing triethanolamine (reagent grade, containing 1.9% diethanolamine as a contaminant) at concentrations of 0% (control), 1% (low dose) or 2% (high dose) for 82 weeks, at which time the study was terminated. The high dose was estimated to be the maximum tolerated dose. The percentage of mice surviving at week 82 was: females—all groups, 100%; males—control, 86%; low-dose, 92%; high-dose, 96%. There was no significant difference between the body weights of treated and untreated mice. There was no treatment-related increase in tumour incidence in either sex (Konishi et al., 1992).
3.1.2. Rat
Groups of 50 male and 50 female Fischer (F344/DuCrj) rats, six weeks of age, were given drinking-water containing triethanolamine (reagent grade, containing 1.9% diethanolamine as a contaminant) at concentrations of 0% (control), 1% (low dose) or 2% (high dose) for two years. At approximately experimental week 60, loss of body weight gain and mortality increased in the female 2% group. Administration of triethanolamine was therefore ceased in both female treatment groups at week 68 for one week and thereafter, from week 69, dietary concentrations in both female treatment groups were reduced by half. After week 104, untreated drinking-water was given to all rats and observation was continued until week 113, when all surviving animals were killed. Treatment with triethanolamine produced a reduction in weight in both males and females and, in females, a dose-dependent increase in mortality due to nephrotoxicity was seen. The percentage mortality in the control, low- and high-dose groups was 32, 32 and 34 in males, and 16, 32 and 42 in females, respectively. No treatment-related increase in the incidence of tumours was observed (Maekawa et al., 1986).
3.2. Skin application
3.2.1. Mouse
Groups of 60 male and 60 female B6C3F1 mice, six weeks of age, were administered triethanolamine (purity, 99%) topically in acetone on five days per week for 103 weeks. Male mice received 0, 200, 630 or 2000 mg/kg bw and female mice received 0, 100, 300 or 1000 mg/kg bw triethanolamine. Although there was an apparent association with hepatocellular tumours in both sexes and hepatoblastomas in males, the authors noted that the mice were chronically infected with Helicobacter hepaticus, an organism that is known to induce hepatitis, so that interpretation of any relationship between triethanolamine and liver neoplasms was inconclusive (Fox et al., 1998; National Toxicology Program, 1999). [The Working Group did not consider this study in its evaluation of carcinogenicity since it was considered inadequate by the National Toxicology Program.]
3.2.2. Genetically modified mouse
Groups of 15–20 female Tg.Ac mice, which carry a zeta-globin v-Ha-ras gene on an FVB background, 14 weeks of age, were administered triethanolamine in acetone topically (the triethanolamine used was from the same chemical batch as that used in the National Toxicology Program mouse study (National Toxicology Program, 1999)). Triethanolamine was administered in 200-µL volumes, five times per week for 20 weeks. The concurrent negative control groups were treated with 200 µL acetone. The positive control group was treated with 1.25 µg 12-O-tetradecanoylphorbol 13-acetate (TPA; approximately 99% pure) three times per week or with 1.5 µg twice per week for 20 weeks. The doses of triethanolamine selected were based on the maximum tolerated dose (MTD) used earlier (National Toxicology Program, 1999) and were 3, 10 or 30 mg triethanolamine per mouse per application. Lesions were diagnosed as papillomas when they reached at least 1 mm in size and persisted for at least three weeks. Animals that did not survive until the end of week 10 were not included in the data summaries or calculations. Six weeks after the last application, all surviving mice were killed. There was no evidence of chronic irritation or ulceration at the site of application during the exposure period. In contrast to the positive controls, which developed multiple papillomas in 19/20 animals, there was no increase in the incidence of skin tumours in triethanolamine-treated animals (Spalding et al., 2000).
3.2.3. Rat
Groups of 60 male and 60 female Fischer 344/N rats, six weeks of age, were administered triethanolamine (purity, 99%) topically in acetone on five days per week for 103 weeks. Male rats received 0, 32, 63 or 125 mg/kg bw and females received 0, 63, 125 or 250 mg/kg bw triethanolamine. The survival rates of males were 21/50, 11/50, 18/49 and 19/50 and of females were 25/50, 29/50 and 18/50 in the control, low-, mid- and high-dose rats respectively. The mean body weight of females receiving 250 mg/kg bw ranged from 9% to 12% lower than that of the vehicle controls from weeks 73 to 93, and by the end of the study, was 7% lower than that of the vehicle control group. There was no significant increase in the incidence of tumours at any site (National Toxicology Program, 1999).
4. Other Data Relevant to an Evaluation of Carcinogenicity and its Mechanisms
4.1. Absorption, distribution, metabolism and excretion
4.1.1. Humans
No toxicokinetic data related directly to triethanolamine were available.
4.1.2. Experimental systems
(a) Absorption, distribution, metabolism and excretion
Toxicokinetic data for triethanolamine have been reviewed (Beyer et al., 1983; Melnick & Tomaszewski, 1990; Gillner & Loeper, 1993; Knaak et al., 1997; National Toxicology Program, 1999).
Absorption in the gastrointestinal tract of triethanolamine administered orally to Wistar rats is rapid; 63% of the dose disappeared from intestines within 65 min (Kohri et al., 1982). In dermal toxicity studies, the peak blood levels of [14C]triethanolamine were observed 2 h after its application in C3H/HeJ mice (2000 mg/kg bw), whereas in Fischer 344 rats (1000 mg/kg bw), the blood levels (expressed as radioactivity) indicated that triethanolamine was absorbed less rapidly than by mice. Data from various studies in mice and rats (1000–2000 mg/kg bw) suggest that absorption of dermally administered triethanolamine is almost complete in 24 h (Waechter & Rick, 1988, cited in Knaak et al., 1997).
The elimination of [14C]triethanolamine from the blood of mice administered 1.0 mg/kg bw intravenously showed first-order biphasic kinetics with a rapid (0.58-h half-life) and a slow phase (10.2-h half-life). The slow phase half-lives for elimination of triethanolamine in mice after dermal exposure to 1000 and 2000 mg/kg bw in acetone were 9.7 h and 18.6 h. Skin absorption rates (as blood concentration–time curves) after dermal application of aqueous and neat [14C]triethanolamine to mouse skin (2000 mg/kg bw, enclosed by a glass ring) showed no significant change with the use of water as the vehicle (Waechter & Rick, 1988, cited in Knaak et al., 1997).
After topical application of 2000 mg/kg bw [14C]triethanolamine in acetone to 2cm2 of mouse back skin, blood levels, expressed as radioactivity/mL, were about fivefold higher than those after application of 1000 mg/kg [14C]triethanolamine to 1cm2 of skin, and about 5000-fold higher than that after a 1.0 mg/kg bw intravenous dose (Waechter & Rick, 1988, cited in Knaak et al. 1997).
After a single application of [14C]triethanolamine for 48 h to the skin of rats (1000 mg/kg bw; 1.75 cm2 area) and mice (2000 mg/kg bw; 1.75 cm2 area), the absorption rate through rat skin (2.4 mg/cm2/h) was estimated to be greater than that through mouse skin (0.4 mg/cm2/h). This was evaluated by analysis of [14C]triethanolamine-equivalents in the blood. The sixfold difference in the absorption rates was explained by the sixfold higher concentration of triethanolamine applied to rat skin rather than by a greater permeability (kp) of rat skin, since the kp values for rats (1.85 × 10−2 cm/h) and mice (1.8–2.0 × 10–2 cm/h) were similar (Waechter & Rick, 1988, cited in Knaak et al., 1997). Triethanolamine thus enhances its own penetration.
About 60% of the radioactivity in [14C]triethanolamine applied to mouse skin (1000 mg/kg bw) was excreted in 48 h in urine and 20% in faeces, with less than 10% found in the skin at the site of application. The biotransformation of [14C]triethanolamine to monoethanolamine and diethanolamine was specifically investigated in mice after both intravenous and dermal treatments. Neither of the hypothetical metabolites was detected in urine (by mass spectral analysis), whereas more than 95% of the radioactivity detected in urine was identified as unchanged triethanolamine (Waechter & Rick, 1988, cited in Melnick and Thomaszewski, 1990). In vitro, triethanolamine had an inhibitory effect on the incorporation of [32P]phosphate into phospholipids from rabbit and human tissues (Morin & Lim, 1970). Cytochrome P450 monooxygenasedependent oxidative N-dealkylation of triethanolamine does occur in microorganisms, with formation of diethanolamine, ethanolamine and glyoxylate as reaction products (Fattakhova et al., 1991).
The disposition of triethanolamine in male Wistar (180–250 g) rats after administration of a single oral dose (350 mg/rat) was measured in a four-day follow-up study. The cumulative percentage recoveries reported for unchanged triethanolamine were 53 and 57% in urine and 20 and 23% in faeces at day 1 and day 3, respectively. Also, after multiple oral administration to male and female rats, triethanolamine was mainly excreted unchanged. The urinary and faecal excretion ratio of unchanged triethanolamine remained constant throughout the treatment period (for five to six days) in both males and females. A small amount of triethanolamine (1.4–2.7%) was excreted as glucuronide conjugates (Kohri et al., 1982).
4.2. Toxic effects
4.2.1. Humans
Triethanolamine appeared to be without irritating effects below a concentration of 5% in most people; above this concentration (mild) skin irritation is observed. In some volunteers, however, skin irritation was observed at lower concentrations of triethanolamine, particularly in persons who had a history of contact dermatitis and scarified skin (Beyer et al., 1983). Among patients with contact dermatitis, triethanolamine was found to be the most frequent sensitizer (Tosti et al., 1990). When a total of 1357 patients suspected of having allergic eczematous contact dermatitis were patch-tested with triethanolamine, 41 had positive reactions. Of these, 29 had applied lotion-based medicaments locally for some time (Scheuer, 1983). Many case reports on people exposed to cosmetics and investigations in groups of workers exposed to cutting fluids (containing triethanolamine among many other components) indicate that contact dermatitis and allergic reactions to triethanolamine occur (e.g., Calas et al., 1978; Herman, 1983; Shrank, 1985; Jones & Kennedy, 1988; Batten et al., 1994; Hüner et al., 1994; Hamilton & Zug, 1996; Blum & Lischka, 1997).
Triethanolamine has been clinically tested with other model irritant compounds for potency to stimulate signal release of proinflammatory mediators in human skin in order to find biomarkers of irritancy. Neat or aqueous triethanolamine was applied to the lower arm of 12 male volunteers; after 24 h, suction blister fluid specimens were taken from the site of treated skin. Triethanolamine caused no significant increase in arachidonic acid and prostaglandin concentrations in suction blister fluid samples, in contrast to the irritants sodium lauryl sulfate, benzalkonium chloride and Tween 80 that gave positive test results (Müller-Decker et al., 1998).
4.2.2. Experimental studies
The toxicology of triethanolamine has been reviewed (Knaak et al., 1997). In general, rather high doses of triethanolamine are well tolerated by rats and mice. Major sites of toxicity in rats and mice are liver and kidney, while skin toxicity occurs after dermal application, especially when undiluted triethanolamine is applied.
Irritation to the eye and skin was minimal to slight 72 h after application of pure (98%) triethanolamine to New Zealand White rabbits (Dutertre-Catella et al., 1982).
Subchronic inhalation exposure to triethanolamine aerosols has been studied in both male and female Fischer 344 rats and B6C3F1 mice at concentrations of 125– 2000 mg/m3 for 6 h per day on five days a week over a 16-day period (an unpublished study reported by Mosberg et al., 1985, Battelle Columbus Division Laboratories, and reviewed in Knaak et al., 1997). In rats, several minor changes were observed such as a decrease in body weight and an increase in kidney weight, but no histopathological changes were apparent, indicating little toxicity under these conditions. In mice, various haematological changes were considered not to be dose-related.
Signs of kidney and liver toxicity were observed by Kindsvatter (1940) in guinea-pigs and albino rats during oral administration of triethanolamine (200–1600 mg/kg bw per day). Maekawa et al. (1986) reported that exposure to triethanolamine in the drinking-water led to kidney toxicity at a concentration of 1–2% (approximately 1000 mg/kg bw per day intake) in both male and female Fischer 344 rats. Body weight gain was depressed by 10–14% after two years on this regimen, compared with controls. Kidney weights were greatly increased, and major histopathological changes were observed in the kidneys, suggesting a dose-related acceleration of chronic nephropathy, which is common in ageing Fischer 344 rats. Mineralization and necrosis of the renal papilla were found. In a 14-day study, no histopathological changes were observed in Fischer 344 rats exposed to 2% triethanolamine in the drinking-water, equivalent to an intake of approximately 2500–2800 mg/kg bw per day (an unpublished study reported by Hejtmancik et al., 1985, cited by Knaak et al., 1997).
In a chronic study in B6C3F1 mice given 1 or 2% triethanolamine (containing 2% diethanolamine) in the drinking-water (see Section 3.1.1), little toxicity was observed in either sex (Konishi et al., 1992). [The mice consumed up to 3000 mg/kg bw per day at the high dose.]
Dermal application of high concentrations and, in particular, of undiluted triethanolamine to rats and mice in (sub-)chronic studies led to a necrotizing, chronic– active inflammatory process on the skin as observed in several studies and reported extensively in a National Toxicology Program (1999) study. In a 13-week study in Fischer 344 rats given 125–2000 mg/kg bw dermally per day on five days per week, animals receiving 2000 mg/kg bw per day showed body weight gain reductions and kidney weight increases. Hypertrophy of the pars intermedia of the pituitary gland also occurred. The effects were dose-related and were similar in both males in females. In a subsequent 103-week study, doses of 32–125 mg/kg bw per day were applied to the skin of males and of 63–250 mg/kg per day to the skin of females. At the 15-month interim evaluation, inflammation and ulceration were present at the site of application in both males and females. In females, kidney weights increased after application of 250 mg/kg bw per day. At the end of the study, hyperplasia in the kidney was observed, which was more severe in males than in controls, although the incidence of hyperplasia was the same.
The National Toxicology Program (1999) study also included B6C3F1 mice. In the 13-week study (250–4000 mg/kg bw per day on five days per week), acanthosis and inflammation at the site of application were observed in both males and females at 4000 mg/kg bw per day, and liver and kidney weights were increased at that dose. In the subsequent 103-week study, females received doses of 100–1000 mg/kg bw per day and males 200–2000 mg/kg bw per day. Skin inflammation was found in both males and females at the site of application. Infection with Helicobacter hepaticus in these mice (Fox et al., 1998) complicates the interpretation of the results. Another subchronic study of triethanolamine administered dermally to C3H/HeJ mice (140–2000 mg/kg bw in males and 160–2300 mg/kg bw in female mice, three times per week for 95 days) found no signs of toxicity except for slight epidermal hyperplasia at the application site; the internal organs showed no change in weight and no histopathological signs were observed (DePass et al., 1995).
4.3. Reproductive and developmental effects
4.3.1. Humans
No data were available to the Working Group.
4.3.2. Experimental systems
Triethanolamine was administered to groups of 10 male and 10 female Fischer 344/N rats and B6C3F1 mice for 13 weeks by topical application at dose levels of 0, 500, 1000 or 2000 mg/kg bw per day and 0, 1000, 2000 or 4000 mg/kg bw per day, respectively. Body weight gains were significantly lower in the highest-dose group of male and female rats, but there was no change in mice at any dose level. In neither rats nor mice was there any significant change in sperm motility, morphology or number and there was no change in the mean duration of the estrous cycle (National Toxicology Program, 1999).
No embryotoxic or teratogenic effects were produced when pregnant rats were exposed by topical administration to their shaved skin of semipermanent hair-dye preparations containing 0.1–1.5% triethanolamine on gestational days 1, 4, 7, 10, 13, 16 and 19 (Burnett et al., 1976). [This study was not reviewed in detail by the Working Group because of the low proportion of triethanolamine in the complex mixtures tested.]
The reproductive and developmental toxicity of triethanolamine tested without the complication of many other accompanying substances has been reviewed (Knaak et al., 1997). This review is used as the reference source to the following studies because they have not been reported in the open literature (Battelle reports).
Triethanolamine was administered as a solution in acetone to the skin of male and female Fischer 344 rats at dose levels of 0 or 500 mg/kg bw per day for 10 weeks before mating and then to the females during gestation and lactation. No effect on mating, fertility or offspring growth and survival was observed. In a similar study with CD-1 mice administered triethanolamine doses of 0 or 2000 mg/kg bw per day, no effect of treatment was observed.
4.4. Genetic and related effects
Triethanolamine has been reviewed by an expert panel for cosmetic ingredient review (Beyer et al., 1983), by Knaak et al. (1997) and by the National Toxicology Program (1999).
4.4.1. Humans
No data were available to the Working Group.
4.4.2. Experimental systems (see Table 4 for references)
Triethanolamine was not mutagenic to Salmonella typhimurium strains TA98, TA100, TA1535, TA1537 or TA1538 in the presence or absence of exogenous metabolic activation in a number of studies. Triethanolamine did not induce mutations in Escherichia coli WP2 uvrA and WP2 try– in the presence or absence of exogenous metabolic activation in two studies. In a single study, triethanolamine was not mutagenic to Bacillus subtilis strains carrying uvrA or uvrA and polA mutations in the presence or absence of exogenous metabolic activation. However, when triethanolamine was mixed with sodium nitrite, mutations were induced in this system without exogenous metabolic activation; this activity was lost in the presence of exogenous metabolic activity.
Triethanolamine did not induce gene conversion in Saccharomyces cerevisiae in the presence or absence of exogenous metabolic activation in one study. In a single study, sex-linked recessive lethal mutations were not induced in Drosophila melanogaster by treatment with triethanolamine either by diet or injection.
Unscheduled DNA synthesis was not induced in rat primary hepatocytes exposed to triethanolamine in two studies.
Triethanolamine did not induce sister chromatid exchanges in Chinese hamster ovary cells in either the presence or absence of exogenous metabolic activation. Chromosomal aberrations were not induced in rat liver cells, Chinese hamster lung cells or Chinese hamster ovary cells by in-vitro exposure to triethanolamine. It did not induce cell transformation in Syrian hamster embryo cells.
Treatment of male and female mice with triethanolamine for 13 weeks by dermal application did not result in any change in the frequency of micronuclei in their blood cells.
4.5. Mechanistic considerations
Triethanolamine is rapidly absorbed and excreted in urine (about 60%) and faeces (about 20%) mainly in the unchanged form. Biodegradation of triethanolamine to monoethanolamine or diethanolamine or to any other putative metabolite has not been shown in rodents, nor its incorporation into natural products. In spite of the search for a possible mode of bioactivation of triethanolamine, no mechanism has been reported in mammals to date.
It has been hypothesized that endogenous nitrosation of triethanolamine may produce a potent liver carcinogen, N-nitrosodiethanolamine (Lijinsky et al., 1980; Lijinsky & Kovatch, 1985), or that some other endogenous reactions convert triethanolamine to a putative carcinogen (Hoshino & Tanooka, 1978). The formation of N-nitrosodiethanolamine in amounts that would cause liver cancer in vivo appears, however, unlikely since no treatment-related liver cancers have been observed in oral or dermal triethanolamine carcinogenicity studies in mice or rats (Hoshino & Tanooka, 1978; Maekawa et al., 1986; Konishi et al., 1992). The potential reported for triethanolamine to undergo nitrosative dealkylation and form N-nitrosodiethanolamine under physiological conditions (including gastric pH) is, in general, considered negligible in comparison with the nitrosation of secondary amines (Knaak et al., 1997).
In human keratinocyte cultures, triethanolamine was categorized as a weak inducer of a delayed (≥ 4 h) stimulation of the release of key mediators (arachidonic acid, eicosanoids, interleukin-1α) that are known to be indicative of hyperproliferative and inflammatory events in human skin (Müller-Decker et al., 1994). In line with the in-vitro irritancy tests, triethanolamine was found to be a non-irritant in a clinical patch testing study of human skin in 20 male volunteers (Müller-Decker et al., 1998).
5. Summary of Data Reported and Evaluation
5.1. Exposure data
Triethanolamine is a viscous liquid widely used as a corrosion inhibitor, a surface-active agent and an intermediate in various products including metalworking fluids, oils, fuels, paints, inks, cement, cosmetic and personal products, as well as herbicide and algicide formulations. Occupational exposure may occur by inhalation and dermal contact, particularly in metal-machining occupations. No data were available on environmental exposure to this substance. The general population may be exposed through contact with a variety of personal care products.
5.2. Human carcinogenicity data
Two cohort studies, two proportionate mortality studies and two nested case–control studies looked at cancer mortality or incidence among workers using metalworking fluids with ethanolamines as additives, with or without sodium nitrite. Small excesses were observed for cancers at various sites, in particular, stomach, oesophagus and larynx. In most of these studies, only associations with use of soluble oils or synthetic fluids were presented and no results were given specifically in relation to triethanolamine exposure. It is difficult to draw conclusions regarding triethanolamine using data from studies of exposures to these complex mixtures.
5.3. Animal carcinogenicity data
Triethanolamine was adequately tested for carcinogenicity in one study in mice and in one study in rats by oral administration in the drinking-water. No increase in the incidence of tumours was observed. It was also tested by dermal application in one study in rats and no increase in the incidence of tumours was found.
In a Tg.AC transgenic mouse model, dermal application of triethanolamine produced no increase in tumours.
5.4. Other relevant data
Triethanolamine is rapidly absorbed and excreted in rodent urine (about 60%) and faeces (about 20%), mainly in the unchanged form. Biodegradation of triethanolamine to monoethanolamine or diethanolamine or to any other putative metabolite has not been shown in rodents, nor has its incorporation into endogenous macromolecules. There is no evidence for formation of N-nitrosodiethanolamine from triethanolamine under physiological conditions.
In humans, triethanolamine is reported to be a skin sensitizer. Repeated dermal application of high concentrations of triethanolamine to rats led to a necrotizing inflammatory process in the skin.
Data on reproductive and developmental effects in humans were not available. No reproductive or developmental effects were produced when rats and mice were exposed by topical administration. Other routes of exposure have not been studied.
No data on the genetic and related effects of triethanolamine in humans were available to the Working Group.
Triethanolamine did not induce mutations in bacteria, unless nitrite was also present. It did not influence the frequency of micronuclei in mouse peripheral blood in vivo after dermal application. Triethanolamine did not induce unscheduled DNA synthesis, sister chromatid exchange, chromosomal aberrations or cell transformation in rodent cells in vitro. Triethanolamine had no effect on sex-linked recessive lethal mutations in Drosophila melanogaster or on gene conversion in Saccharomyces cerevisiae.
5.5. Evaluation
There is inadequate evidence in humans for the carcinogenicity of triethanolamine. There is inadequate evidence in experimental animals for the carcinogenicity of triethanolamine.
Overall evaluation
Triethanolamine is not classifiable as to its carcinogenicity to humans (Group 3).
6. References
- American Conference of Governmental Industrial Hygienists (1999)TLVs and other Occupational Exposure Values—1999 CD-ROM, Cincinnati, OH.
- Batten T.L., Wakeel R.A., Douglas W.S., Evans C., White M.I., Moody R., Ormerod A.D. Contact dermatitis from the old formula E45 cream. Contact Derm. 1994;30:159–161. [PubMed: 8187515]
- Beyer K.H. Jr, Bergfeld W.F., Berndt W.O., Boutwell R.K., Carlton W.W., Hoffmann D.K., Schroeder A.L. Final report on the safety assessment of triethanolamine, diethanolamine and monoethanolamine. J. Am. Coll. Toxicol. 1983;2:183–235.
- Blum A., Lischka G. Allergic contact dermatitis from mono-, di- and triethanolamine (Short communication). Contact Derm. 1997;36:166. [PubMed: 9145273]
- Bollmeier, A.F. (1992) Alkanolamines. In: Kroschwitz, J.I. & Howe-Grant, M., eds, Kirk-Othmer Encyclopedia of Chemical Technology, 4th Ed., Vol. 2, New York, John Wiley, pp. 1–34.
- Budavari, S., ed. (1998) The Merck Index, 12th Ed., Version 12:2, Whitehouse Station, NJ, Merck & Co. [CD-ROM]
- Burnett C., Goldenthal E.I., Harris S.B., Wazeter F.X., Strausburg J., Kapp R., Voelker R. Teratology and percutaneous toxicity studies on hair dyes. J. Toxicol. environ. Health. 1976;1:1027–1040. [PubMed: 966314]
- Calas E., Castelain P.-Y., Piriou A. [Epidemiology of contact dermatitis in Marseilles]. Ann. Dermatol. Venereol. 1978;105:345–347. (in French) [PubMed: 150247]
- Chemical Information Services (1999)Directory of World Chemical Producers (Version 99.1.0), Dallas, TX [CD-ROM]
- DePass L.R., Fowler E.H., Leung H.-W. Subchronic dermal toxicity study of triethanolamine in C3H/HeJ mice. Food chem. Toxicol. 1995;33:675–680. [PubMed: 7672740]
- Dean B.J., Brooks T.M., Hodson-Walker G., Hutson D.H. Genetic toxicology testing of 41 industrial chemicals. Mutat. Res. 1985;153:55–77. [PubMed: 3883152]
- Dow Chemical Company (1998)The Specifier's Guide to Buying and Applying Ethanolamines, Midland, MI.
- Dow Chemical Company (1999a) Material Safety Data Sheet: Triethanolamine 99, Midland, MI.
- Dow Chemical Company (1999b) Sales Specification: Triethanolamine, Midland, MI.
- Dutertre-Catella H., Lich N.P., Huyen V.N., Truhaut R. [Comparative study of skin and eye irriration by ethanolamines (mono, di, tri and poly)]. Arch. mal. prof. 1982;43:455–460. (in French)
- Eller, P.M., ed. (1994) NIOSH Manual of Analytical Methods (DHHS (NIOSH) Publ. No. 94113), 4th Ed., Cincinnati, OH, United States National Institute for Occupational Safety and Health [Method 3509]
- Fattakhova A.N., Ofitserov E.N., Garusov A.V. Cytochrome P-450-dependent catabolism of triethanolamine in Rhodotorula mucilaginosa. Biodegradation. 1991;2:107–113. [PubMed: 1368151]
- Fernando L.A. Spectrophotometric determination of ethanolamines in lubricating emulsions. J. Soc. Tribol. Lubric. Engin. 1995;51:701–704.
- Food and Drug Administration (1999)Food and drugs. US Code Fed. Regul., Title 21, Parts 175.105, 176.170, 176.180, pp. 138–165, 186–211.
- Fox J.G., MacGregor J.A., Shen Z., Li X., Lewis R., Dangler Ch.A. Comparison of methods of identifying Helicobacter hepaticus in B6C3F1 mice used in a carcinogenesis bioassay. J. clin. Microbiol. 1998;36:1382–1387. [PMC free article: PMC104833] [PubMed: 9574710]
- Fukui M., Konishi H., Ohta K., Tanaka K. [Ion–exclusion chromatography with UV detection for the determination of alkanolamines in cosmetics using water–glycerine as an eluent]. Bunseki Kagaku. 1992;41:T27–T31. (in Japanese)
- Galloway S.M., Armstrong M.J., Reuben C., Colman S., Brown B., Cannon C., Bloom A.D., Nakamura F., Ahmed M., Duk S., Rimpo J., Margolin B.H., Resnick M.A., Anderson B., Zeiger E. Chromosomal aberrations and sister chromatid exchanges in Chinese hamster ovary cells: evaluations of 108 chemicals. Environ. mol. Mutag. 1987;10 Suppl. 10:1–175. [PubMed: 3319609]
- Gillner M., Loeper I. Health effects of selected chemicals. 2. Triethanolamine. Nord. 1993;29:235–260.
- Hamilton T.K., Zug K.A. Triethanolamine allergy inadvertently discovered from a fluorescent marking pen. Am. J. Contact Derm. 1996;7:164–165. [PubMed: 8957332]
- Hammer, H., Kornig, W., Weber, T. & Kieczka, H. (1987) Ethanolamines and propanolamines. In: Gerhartz, W., Yamamoto, Y.S., Kaudy, L., Rounsaville, J.F. & Schulz, G., eds, Ullmann's Encyclopedia of Chemical Technology, 5th rev. Ed., Vol. A10, New York, VCH Publishers, pp. 1–19.
- Herman J.J. Intractable sneezing due to IgE mediated triethanolamine sensitivity. J. Allergy clin. Immun. 1983;71:339–344. [PubMed: 6186715]
- Hoshino H., Tanooka H. Carcinogenicity of triethanolamine in mice and its mutagenicity after reaction with sodium nitrite in bacteria. Cancer Res. 1978;38:3918–3921. [PubMed: 100210]
- Hüner A., Fartasch M., Hornstein O.P., Diepgen T.L. The irritant effect of different metalworking fluids. Contact Derm. 1994;31:220–225. [PubMed: 7842676]
- IARC (1994) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 60, Some Industrial Chemicals, Lyon, IARCPress, pp. 73–159. [PMC free article: PMC7681578] [PubMed: 7869582]
- Inoue K., Sunakawa T., Okamoto K., Tanaka Y. Mutagenicity tests and in vitro transformation assays on triethanolamine. Mutat. Res. 1982;101:305–313. [PubMed: 6810163]
- Jones S.T., Kennedy C.T.C. Contact dermatitis from triethanolamine in E45 cream (Short communication). Contact Derm. 1988;19:230. [PubMed: 3191690]
- Kenyon E.M., Hammond S.K., Shatkin J., Woskie S.R., Hallock M.F., Smith T.J. Ethanolamine exposures of workers using machining fluids in the automotive parts manufacturing industry. Appl. occup. environ. Hyg. 1993;8:655–661.
- Kindsvatter V.H. Acute and chronic toxicity of triethanolamine. J. ind. Hyg. Toxicol. 1940;22:206–212.
- Knaak J.B., Leung H.-W., Stott W.T., Busch J., Bilsky J. Toxicology of mono-, di-, and triethanolamine. Rev. environ. Contam. Toxicol. 1997;149:1–86. [PubMed: 8956558]
- Kohri N., Matsuda T., Umeniwa K., Miyazaki K., Arita T. [Development of assay method in biological fluids and biological fate of triethanolamine]. Yakuzai Gaku. 1982;42:342–348. (in Japanese)
- Konishi Y., Denda A., Uchida K., Emi Y., Ura H., Yokose Y., Shiraiwa K., Tsutsumi M. Chronic toxicity carcinogenicity studies of triethanolamine in B6C3F1 mice. Fundam. appl. Toxicol. 1992;18:25–29. [PubMed: 1601206]
- Lide, D.R. & Milne, G.W.A. (1996) Properties of Organic Compounds, Version 5.0, Boca Raton, FL, CRC Press [CD-ROM]
- Lijinsky W., Kovatch R.M. Induction of liver tumors in rats by nitrosodiethanolamine at low doses. Carcinogenesis. 1985;6:1679–1681. [PubMed: 4064244]
- Lijinsky W., Reuber M.D., Manning W.B. Potent carcinogenicity of nitrosodiethanolamine. Nature. 1980;288:589–590. [PubMed: 7442802]
- Litton Bionetics (1982) Evaluation of Triethanolamine No. 80/175 in the Primary Rat Hepatocyte Unscheduled DNA Synthesis Assay [cited in Knaak et al., 1997]
- Maekawa A., Onodera H., Tanigawa H., Furuta K., Kanno J., Matsuoka C., Ogiu T., Hayashi Y. Lack of carcinogenicity of triethanolamine in F344 rats. J. Toxicol. environ. Health. 1986;19:345–357. [PubMed: 3772984]
- Maurer W., Hohaus E., Schubert B. [Analysis of alkanolamines in cosmetics and pharmaceuticals]. Parfüm. Kosmet. 1996;77:262–266. (in German)
- Melnick, R.L. & Tomaszewski, K.E. (1990) Triethanolamine. In: Buhler, D.R. & Reed, D.J., eds, Ethel Browning's Toxicity and Metabolism of Industrial Solvents, Vol. II, Nitrogen and Phosphorus Solvents, Amsterdam, Elsevier, pp. 441–450.
- Morin R.J., Lim C.T. Inhibition in vitro of incorporation of [32P]-phosphate into rabbit and human endometrial phospholipids. J. reprod. Fert. 1970;23:456–462. [PubMed: 5492028]
- Mortelmans K., Haworth S., Lawlor T., Speck W., Tainer B., Zeiger E. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ. mol. Mutag. 1986;8 Suppl. 7:1–119. [PubMed: 3516675]
- Müller-Decker K., Fürstenberger G., Marks F. Keratinocyte-derived pro-inflammatory key mediators and cell viability as in vitro parameters of irritancy: a possible alternative to the Draize skin irritation test. Toxicol. appl. Pharmacol. 1994;127:99–108. [PubMed: 8048060]
- Müller-Decker K., Heinzelmann T., Fürstenberger G., Kecskes A., Lehmann W.-D., Marks F. Arachidonic acid metabolism in primary irritant dermatitis produced by patch testing of human skin with surfactants. Toxicol. appl. Pharmacol. 1998;153:59–67. [PubMed: 9875300]
- National Toxicology Program (1999)Toxicology and Carcinogenesis Studies of Triethanolamine (CAS No. 102-71-6) in F344/N Rats and B6C3F1 Mice (Dermal Studies) (NTP TR 449; NIH Publ. No 00-3365), Research Triangle Park, NC.
- NOES (1999) National Occupational Exposure Survey 1981-83. Unpublished data as of July 1999. Cincinnati, OH, Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health.
- Pfeiffer, W., Breuer, D., Blome, H., Deininger, C., Hahn, J.-U., Kleine, H., Nies, E., Pflaumbaum, W., Stockmann, R., Willert, G. & Sonnenschein, G. (1996) Kühlschmierstoffe [Cutting Oils], Sankt Augustin, Germany, Hauptverband der gewerblichen Berufsgenossenschaften.
- Sadtler Research Laboratories (1980)Sadtler Standard Spectra, 1980 Cumulative Index (Molecular Formula Index), Philadelphia, PA, p. 134.
- Santa María A., Pozuelo J.M., López A., Sanz F. Toxicity of potential irritants in mammalian cells in vitro. Ecotoxicol. environ. Saf. 1996;34:56–58. [PubMed: 8793320]
- Scheuer B. [Contact allergy with triethanolamine.] Der Hautarzt. 1983;34:126–129. (in German) [PubMed: 6222016]
- Schubert B.A., Hohaus E., Dengel H.S., Riepe W., Maurer W. [Determination of alkanolamines in water-miscible cooling lubricants by capillary zone electrophoresis.] Gefahrstoffe—Reinhaltung der Luft. 1996;56:393–399. (in German)
- Shrank A.B. Allergy to cutting oil (Short communication). Contact Derm. 1985;12:229. [PubMed: 3160536]
- Sollenberg J. Isotachophoretic determination of ethanolamines in metalworking fluids and in air samples from workrooms. Proc. Cont. Quality. 1997;10:313–317.
- Spalding J.W., French J.E., Stasiewicz S., Furefi-Machacek M., Conner F., Tice R.R., Tennant R.W. Responses of transgenic mouse lines p53± and Tg-AC to agents tested in conventional carcinogenicity bioassays. Toxicol. Sci. 2000;53:213–223. [PubMed: 10696769]
- Tosti A., Guerra L., Morelli R., Bardazzi F. Prevalence and sources of sensitization to emulsifiers: A clinical study. Contact Derm. 1990;23:68–72. [PubMed: 2145129]
- United Nations Environment Programme (1999)Recommendations and Legal Mechanisms [http://dbserver
.irptc .unep.ch:8887/irptc/owa/Ig.get_search] - United Nations Environment Programme Chemicals (2000)OECD High Production Volume Chemicals Programme. Phase 3. SIDS (Screening Information Data Set), initial assessment report [http://irptc
.unep.ch], Geneva. - Verschueren, K. (1996) Handbook of Environmental Data on Organic Chemicals, 3rd Ed., New York, Van Nostrand Reinhold, pp. 1823–1825.
- Waechter, J.M. & Rick, D.L. (1988) Triethanolamine: Pharmacokinetics in C3H/HeJ Mice and Fischer-344 Rats Following Dermal Administration, Midland, MI, Dow Chemical Company.
- West R.J., Gonsior S.J. Biodegradation of triethanolamine. Environ. Toxicol. Chem. 1996;15:472–480.
- Yoon J.S., Mason J.M., Valencia R., Woodruff R.C., Zimmering S. Chemical mutagenesis testing in Drosophila. IV. Results of 45 coded compounds tested for the National Toxicology Program. Environ. mol. Mutag. 1985;7:349–367. [PubMed: 3930235]
Footnotes
- 1
Calculated from: mg/m3 = (relative molecular mass/24.45) × ppm, assuming a temperature of 25 °C and a pressure of 101 kPa
- Review Diethanolamine.[IARC Monogr Eval Carcinog Risk...]Review Diethanolamine.. IARC Monogr Eval Carcinog Risks Hum. 2000; 77:349-79.
- Review Ethylbenzene.[IARC Monogr Eval Carcinog Risk...]Review Ethylbenzene.. IARC Monogr Eval Carcinog Risks Hum. 2000; 77:227-66.
- Review Di(2-ethylhexyl) phthalate.[IARC Monogr Eval Carcinog Risk...]Review Di(2-ethylhexyl) phthalate.. IARC Monogr Eval Carcinog Risks Hum. 2000; 77:41-148.
- Review Nitromethane.[IARC Monogr Eval Carcinog Risk...]Review Nitromethane.. IARC Monogr Eval Carcinog Risks Hum. 2000; 77:487-501.
- Review Pyridine.[IARC Monogr Eval Carcinog Risk...]Review Pyridine.. IARC Monogr Eval Carcinog Risks Hum. 2000; 77:503-28.
- Triethanolamine - Some Industrial ChemicalsTriethanolamine - Some Industrial Chemicals
Your browsing activity is empty.
Activity recording is turned off.
See more...
