NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Bermúdez-Rattoni F, editor. Neural Plasticity and Memory: From Genes to Brain Imaging. Boca Raton (FL): CRC Press/Taylor & Francis; 2007.
13.1. INTRODUCTION
Emotionally significant experiences tend to be well remembered.1,2 We know this from personal experiences as well as from extensive research findings. Significant experiences such as birthdays, graduation ceremonies, or the loss of a loved one typically leave lasting and vivid memories. Findings of experimental studies indicate that people have good recollections of where they were and what they were doing when they experienced earthquakes3 or witnessed accidents.4 Similarly, a rat remembers the place in an apparatus where it received a footshock or the location of an escape platform in a tank filled with water.5,6 Such memory enhancement is not limited to experiences that are unpleasant or aversive. Pleasurable events also tend to be well remembered. Our research focuses on understanding the role of emotional responses induced by such arousing experiences in enabling the significance of events to regulate their remembrance.
Extensive evidence indicates that stress hormones released from the adrenal glands are critically involved in memory consolidation of emotionally arousing experiences. Epinephrine, glucocorticoids, and specific agonists for their receptors administered after exposure to emotionally arousing experiences enhance the consolidation of long-term memories of these experiences.7–10
Do stress hormones also enhance memories of experiences that are not emotionally arousing? The findings of recent experiments suggest that this may not be the case. As discussed below, we recently reported that the endogenous glucocorticoid corticosterone enhanced memory consolidation of object recognition training when administered to rats that were emotionally aroused by an unfamiliar training apparatus. However, the treatment had no effect when administered to rats that had extensive prior habituation to the training context in order to reduce novelty-induced arousal.11 In studies of human memory, epinephrine or cortisol treatment also appear to selectively enhance memory for emotionally arousing material.12–15
These findings thus provide some important clues concerning the neurobiological mechanism(s) underlying adrenal hormone effects on memory consolidation and suggest that at least some degree of training-associated endogenous emotional arousal is essential for enabling their effects on memory consolidation. Our findings indicate that adrenal stress hormones influence memory consolidation of emotional experiences via interactions with arousal-induced activation of noradrenergic mechanisms within the amygdala.
13.2. STRESS HORMONE EFFECTS ON MEMORY CONSOLIDATION
It is well established that hormones of the adrenal medulla (epinephrine) and adrenal cortex (corticosterone, cortisol in humans) are released during and immediately after stressful stimulation of the kind used in emotionally arousing learning tasks. The degree to which these hormonal systems are activated depends on the severity as well as type of stressor employed.16 As removal of endogenous hormones by adrenalectomy impairs memory consolidation for emotionally arousing experiences,5,17–19 such evidence indicates that stress hormones released by the training experience may act as endogenous modulators of memory consolidation.
In support of this view, single injections of epinephrine or glucocorticoids administered after training enhance the long-term retention of many different kinds of training experiences typically used in animal memory studies including inhibitory avoidance, active avoidance, contextual and cued fear conditioning, spatial discrimination, conditioned taste aversion, object recognition, and appetitively motivated tasks.11,20–23 Further, antagonists of adrenoceptors or adrenal steroid receptors as well as drugs that disrupt glucocorticoid functioning (i.e., metyrapone) impair memory consolidation.5,17,24,25 Injections of stress hormones at doses that enhance memory when administered shortly after training are generally ineffective when administered several hours after training.26,27 Such findings indicate that the hormones affect memory by modulating the storage or “consolidation” phase. Extensive evidence also indicates that epinephrine and glucocorticoids or stressful conditions that stimulate their release enhance memory consolidation in human subjects when administered shortly before or after learning.12,13,28,29
Although epinephrine and glucocorticoids interact in influencing memory consolidation,24,30,31 their effects are initiated through different mechanisms. Because epinephrine does not readily cross the blood–brain barrier, a peripheral central pathway must be involved in mediating epinephrine effects on brain activity in modulating memory consolidation. The findings of many experiments indicate that epinephrine effects on memory consolidation are initiated by activation of peripheral β-adrenoceptors located on vagal afferents that project to the nucleus of the solitary tract (NTS) in the brain stem. Noradrenergic projections originating in the NTS innervate forebrain structures involved in learning and memory, including the amygdala,32,33 but may also influence norepinephrine release via projections to the nucleus paragigantocellularis in the lower medulla, which projects to the locus coeruleus. The locus coeruleus noradrenergic system is viewed as a broad system with projections to many areas involved in memory processing including the amygdala, hippocampus, and prefrontal cortex.34–36
Glucocorticoids are highly lipophilic and readily enter the brain to bind to mineralocorticoid receptors (MRs) and glucocorticoid receptors (GRs). These two receptor types differ in their affinities for corticosterone and synthetic ligands. MRs have a high affinity for the natural steroids and are almost saturated during basal levels of corticosterone and cortisol, whereas GRs have a high affinity for synthetic ligands such as dexamethasone and RU 28362.37 In contrast to MRs, GRs become occupied only during stress and at the circadian peak. Several studies using pharmacological and genetic techniques indicate that the memory–modulatory effects of glucocorticoids selectively involve activation of GRs.17,38–41 GRs are considered classical intra-somatic receptors that, after their activation, translocate to the nucleus and regulate gene transcription by binding of receptor homodimers to DNA or other nuclear proteins.42–45 However, glucocorticoids or specific GR agonists also have rapid (milliseconds to minutes) effects on the brain and behavior, suggesting that they may also produce fast-acting, nongenomic effects, presumably involving an activation of membrane receptors.46–48
13.3. STRESS HORMONES SELECTIVELY ENHANCE MEMORY CONSOLIDATION OF EMOTIONALLY AROUSING EXPERIENCES
Stress hormone effects on memory consolidation follow an inverted-U shaped doseresponse effect. Moderate doses of epinephrine or glucocorticoids enhance memory consolidation but lower or higher doses are less effective or may even impair memory consolidation.49,50 Other variables such as gender and age may also influence the direction of the effects of stress hormones on memory consolidation due to differences in stress responses and vulnerability across populations.51–53
Stress hormone effects on memory consolidation depend further on the level of emotional arousal induced by the training experience. For example, posttraining injections of moderate doses of corticosterone or dexamethasone, a synthetic ligand, enhance memory consolidation in a water-maze spatial task.54 However, the same glucocorticoid treatment impairs memory consolidation when the task becomes more aversive by lowering the water temperature.54,55 Similarly, epinephrine and glucocorticoids, as well as drugs affecting many other neurotransmitter systems, are known to enhance memory of inhibitory avoidance training when administered after a mild, low-arousing foot shock, but to impair memory consolidation when given after a strong, highly aversive foot shock that produces robust memory in control animals.56 Thus, these findings indicate that the efficacy and even the direction of the effects of exogenous drug administration on memory consolidation depend on the level of endogenous emotional arousal evoked by the training experience.
To address the question raised earlier in this chapter of whether stress hormone effects on memory consolidation require emotional arousal, we recently investigated the importance of emotional arousal in influencing stress hormone effects on memory consolidation in rats trained on an object recognition task.11 Learning tasks in animal experiments are often emotionally arousing because of the punishment or reward necessary to elicit changes in behavior. It is obvious that with the use of such experimental conditions, it is not possible to determine whether emotional arousal is a prerequisite in regulating stress hormone influences on memory processes. Although no rewarding or aversive stimulation is used during object recognition training,57 such training induces modest novelty-induced stress or arousal. However, extensive habituation of rats to the training apparatus (in the absence of any objects) prior to the training reduces the arousal level induced by object recognition training. Thus, object recognition training may be performed under two distinct conditions in which rats are either exposed to the objects while in a state of heightened arousal or in a less aroused state. We found that corticosterone administered systemically immediately after training enhanced 24-hour retention performance of rats that were not previously habituated to the experimental context (i.e., emotionally aroused rats). In contrast, corticosterone did not affect 24-hour retention of rats that received extensive prior habituation to the experimental context and thus had decreased novelty-induced emotional arousal during training.11 Clearly, these findings indicate that at least some degree of training-associated endogenous emotional arousal is essential for enabling stress hormone effects on memory consolidation.
Recent studies of human memory have also investigated interactions of stress hormones with training-associated emotional arousal. Decreasing glucocorticoid levels below baseline with the cortisol synthesis inhibitor metyrapone impaired long-term memory for both emotionally arousing and emotionally neutral information,58 presumably involving a reduced MR occupancy. However, cortisol administration selectively enhance long-term memory of emotionally arousing, but not emotionally neutral, pictures.12,15 Consistent with these findings, Abercrombie and colleagues14 reported that levels of endogenous cortisol correlated with enhanced memory consolidation only in individuals who were emotionally aroused.
The memory-enhancing effects of epinephrine administration or stress exposure immediately after learning also appear to depend on the arousal level.13,29 For example, Cahill and Alkire13 recorded electrodermal skin responses in human subjects as they viewed standard nonarousing slides, followed by infusions of epinephrine or saline. They reported that epinephrine-treated subjects showed enhanced memory for the first slides (i.e., primacy effect). Likewise, electrodermal skin responses to those slides were significantly greater than responses to slides shown at a later time. Therefore, the authors concluded that epinephrine effects on memory depend on the level of arousal at the time of encoding.
13.4. INVOLVEMENT OF AMYGDALA IN MEDIATING STRESS HORMONE EFFECTS ON MEMORY CONSOLIDATION
Why do stress hormones selectively enhance memory for emotionally arousing experiences? The findings described above suggest that stress hormones must interact with some other component of emotional arousal in mediating memory enhancement. Our findings indicate that stress hormone effects on memory consolidation require amygdala activity. It is well established that emotional experiences that induce the release of adrenal stress hormones also activate the amygdala.59
Lesions or temporary inactivation of the amygdala block the memory-modulatory effects induced by posttraining systemic injections of drugs affecting a variety of neuromodulatory systems including norepinephrine, opioid peptides, GABA, vasopressin, and ACTH.9,60 Furthermore, as noted above, the amygdala mediates epinephrine as well as glucocorticoid effects on memory consolidation.20,61 Selective NMDA-induced lesions of the amygdala restricted to the basolateral complex (BLA; consisting of the lateral, basal, and accessory basal nuclei) block inhibitory avoidance memory enhancement induced by posttraining systemic injections of the synthetic glucocorticoid dexamethasone.39 In contrast, lesions of the adjacent central nucleus (CEA) do not block the dexamethasone-induced memory enhancement. Moreover, posttraining infusions of the specific GR agonist RU 28362 administered into the BLA, but not the CEA, enhanced memory consolidation in a dose-dependent fashion, whereas intra-BLA infusions of the GR antagonist RU 38486 impaired memory consolidation.39 Corticotropin-releasing hormone (CRH) is another neurotransmitter that is released into the amygdala as well as several other brain regions in response to arousing or stressful stimulation. Blockade of endogenous CRH in the BLA with infusions of a CRH receptor antagonist impaired memory for emotionally arousing training,62 whereas preliminary findings indicate that infusions of CRH into the BLA dose-dependently enhance memory consolidation. This evidence indicates that BLA activation by emotional arousal is a general gateway in mediating stress hormone and neurotransmitter effects on memory consolidation.
13.4.1. Amygdala Interacts With Other Brain Regions
Other evidence indicates that the BLA is not a site of permanent storage of the enhanced memory trace but rather is involved in strengthening consolidation processes in other brain regions.9,60 The evidence that lesions of the stria terminalis, a major amygdala input–output pathway, block the memory-modulatory effects of systemic drug infusions and of drugs infused directly into the amygdala63–65 strongly suggests that the amygdala regulates memory consolidation by influencing the storage of information in efferent brain regions. The BLA interacts with many brain regions, including the hippocampus, caudate nucleus and insular, entorhinal, and anterior cingulate cortices in regulating the consolidation of different types of information.60 It also interacts with the hippocampus in regulating stress (hormone) effects on memory consolidation of contextual/spatial components of training. Hippocampal GRs play a role in neuroplasticity66–69 and posttraining activation of hippocampal GRs enhances memory consolidation for both appetitive and aversive training.40,70,71 However, BLA lesions block the memory enhancement produced by posttraining intra-hippocampal infusions of a GR agonist.40 Similarly, electrophysiological findings indicate that BLA lesions or temporary blockade of BLA functioning impair stress- or perforant path stimulation-induced long-term potentiation in the dentate gyrus.72,73 These findings indicate that BLA neuronal activity is required for enabling memory modulation induced by local GR activation in the hippocampus. Since the BLA is normally activated by emotional arousal, such evidence may provide an explanation for the findings that stress hormones selectively influence memory consolidation of emotionally arousing experiences.
Other findings indicate the existence of interactions between the BLA and medial prefrontal cortex (mPFC). The mPFC is implicated in higher cognitive functions such as thought, decision-making, and working memory74,75 and also plays a role in memory consolidation.76 The BLA interacts with the mPFC via reciprocal inhibitory connections.77,78 In a recent study, we examined whether the BLA and mPFC interact in regulating glucocorticoid effects on memory consolidation.79 A GR agonist infused posttraining into either the mPFC or the BLA enhanced memory consolidation of inhibitory avoidance training. The same GR agonist administered into the mPFC also increased BLA neuronal activity, as assessed by elevated phosphorylation levels of the transcription factor mitogen-activated protein kinase (MAPK) in the BLA. Importantly, the GR agonist infused into the mPFCs of animals that had not received inhibitory avoidance training did not increase MAPK levels in the BLA, supporting the hypothesis that glucocorticoid effects on memory consolidation and brain activity require training-associated emotional arousal. Because the inhibition of this MAPK activation in the BLA with infusions of a MEK inhibitor blocked the memory enhancement induced by intra-mPFC infusions of the GR agonist,79 these findings further indicate that BLA activity is essential in regulating stress hormone effects on the consolidation of emotionally arousing memories.
13.4.2. Amygdala Involvement In Human Studies
Considerable evidence from human studies indicates that the enhancing influence of emotional arousal on memory involves activation of the amygdala. Human studies, however, have not yet investigated a possible selective involvement of the BLA. The evidence that emotionally arousing stimulation does not enhance long-term memory in human subjects with amygdala lesions supports the view that amygdala activation may be critical for emotionally enhanced memory.80 Interestingly, the reactions of amygdala-damaged subjects to the emotional material in these studies appeared normal, suggesting that the amygdala in humans may not be as critical for the production of emotional reactions per se.
The involvement of amygdala activation in emotionally influenced memory has also been investigated in many studies using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) in healthy humans.81–84 These studies reported that activity of the amygdala assessed during the presentation of emotionally arousing stimuli correlated highly with memory of the stimuli tested weeks later. Further, the relationship between amygdala activity during encoding and subsequent long-term memory was greatest for the most emotionally arousing stimuli. Human studies indicate that the enhanced memory for emotionally arousing events (versus non-arousing events) involves amygdala modulation of the hippocampal formation.85–87 Collectively, these studies of emotionally influenced memory in human subjects are consistent with findings of animal experiments and indicate that emotional arousal-induced amygdala (BLA) activation may be a critical step in enabling stress hormone effects in modulating memory processes involving other brain regions including hippocampus-dependent explicit/declarative memory.
13.5. ROLE OF EMOTIONAL AROUSAL-INDUCED NORADRENERGIC ACTIVATION WITHIN AMYGDALA IN ENABLING EPINEPHRINE AND GLUCOCORTICOID EFFECTS ON MEMORY CONSOLIDATION
The enhancing effects of adrenal stress hormones on memory consolidation depend on the integrity of the amygdala noradrenergic system. Infusions of β-adrenoceptor antagonists administered into the amygdala block the memory-enhancing effects of peripherally administered epinephrine that, as discussed above, are known to be mediated by activation of the noradrenergic cells of the NTS and locus coeruleus.61 Glucocorticoids also require noradrenergic activation within the amygdala to influence memory for emotionally arousing training. A β-adrenoceptor antagonist infused into the BLA blocks the memory-enhancing effect of systemically administered glucocorticoids.88,89 Furthermore, a β-adrenoceptor antagonist infused into the BLA blocked memory enhancement induced by a GR agonist infused into the hippocampus.90
Studies using in vivo microdialysis indicate that stress induced by prolonged immobilization or tail pinch increased amygdala norepinephrine levels.91,92 Even a single mild foot shock of the kind typically used in inhibitory avoidance training increased amygdala norepinephrine levels93 and the increase in norepinephrine varied with footshock intensity.94 Furthermore, as shown in Figure 13.1, amygdala norepinephrine levels assessed following inhibitory avoidance training correlated with retention latencies tested 24 hours later,95 whereas posttraining infusions of norepinephrine or β-adrenoceptor agonists administered into the BLA enhanced memory consolidation.96,97

FIGURE 13.1
Norepinephrine levels in the amygdala are significantly correlated with retention latency scores. Each line represents norepinephrine levels as a percentage of baseline for an individual rat. Latency to enter the dark compartment on the retention test (more...)
Such findings suggest that systemically administered stress hormones may influence noradrenergic function by altering the synthesis, release, and/or reuptake of norepinephrine. In accord with this hypothesis, Williams and colleagues33 showed that epinephrine administered immediately after inhibitory avoidance training increased norepinephrine levels in the amygdala.
Brainstem noradrenergic cells in the locus coeruleus and NTS are involved not only in mediating epinephrine effects on memory consolidation but also express high levels of GRs.98 Posttraining infusions of a GR agonist into the NTS dose-dependently enhanced memory consolidation of inhibitory avoidance training and the memory enhancement was blocked by intra-BLA infusions of the β-adrenoceptor antagonist atenolol.50 The findings of a recent in vivo microdialysis experiment support the view that glucocorticoids may influence norepinephrine release in the BLA. Corticosterone administration after inhibitory avoidance training increased norepinephrine levels in the amygdala.99 In contrast, corticosterone did not increase norepinephrine levels in the amygdala when administered to naive rats that did not receive inhibitory avoidance training, indicating that glucocorticoids facilitate, but cannot initiate, the release of norepinephrine in the amygdala.
At a postsynaptic level, glucocorticoids may enhance memory consolidation by potentiating β-adrenoceptor-cAMP/PKA efficacy in the BLA.100 Activation of β-adrenoceptors in the BLA enhanced memory consolidation via stimulation of the cAMP/PKA pathway.101,102 We found that intra-BLA infusions of a GR antagonist attenuated the dose-response effects of a β-adrenoceptor agonist on retention enhancement for inhibitory avoidance training. The GR antagonist had no effect on memory enhancement induced by posttraining intra-BLA infusions of the synthetic cAMP analog 8-Br-cAMP.100 These findings suggest that glucocorticoids facilitate the efficacy of noradrenergic stimulation in the BLA on memory consolidation via an interaction with the β-adrenoceptor-cAMP cascade. A model of this interaction is illustrated in Figure 13.2.

FIGURE 13.2
Summary of interactions of glucocorticoids with the noradrenergic system of the basolateral amygdala at both presynaptic and post-synaptic sites as suggested by the findings of our experiments. α1 = α1-adrenoceptor. β =β-adrenoceptor. (more...)
13.5.1. Role Of Emotional Arousal-Induced Noradrenergic Activation
The findings summarized above indicate that emotional arousal induces the release of norepinephrine in the BLA and that adrenal stress hormones may facilitate this training-induced noradrenergic activation. Such findings suggest that emotional arousal-induced noradrenergic activation within the BLA may be essential in enabling stress hormone effects on memory consolidation.
In a recent experiment we investigated this hypothesis.89 As addressed above, corticosterone enhanced memory consolidation of object recognition training only in emotionally aroused rats that were not previously habituated to the context. Object recognition training in these rats also induced marked increases in noradrenergic activity within the BLA, as assessed by immunoreactivity for phosphorylated tyrosine hydroxylase (the rate-limiting enzyme in the biosynthesis of norepinephrine). As shown in Figure 13.3, a β-adrenoceptor antagonist administered either systemically or into the BLA blocked this corticosterone-induced memory enhancement. In contrast, infusion of a β-adrenoceptor antagonist into the hippocampus did not prevent the corticosterone-induced memory enhancement of object recognition training. These findings further indicate that glucocorticoids require noradrenergic activity in the BLA in regulating memory consolidation.
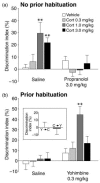
FIGURE 13.3
Glucocorticoid effects on memory consolidation for object recognition training require noradrenergic activation. Data represent discrimination index (%) on a 24-hour retention trial, expressed as mean ± SEM. (A) Effects of immediate posttraining (more...)
Importantly, training of context-habituated rats on the object recognition task did not induce significant increases in noradrenergic activation within the BLA.89 If the failure of corticosterone to enhance memory consolidation in context-habituated rats is due selectively to insufficient arousal-induced noradrenergic activation, then posttraining pharmacological augmentation of noradrenergic activity should provide the activation normally produced by novelty stress and enable glucocorticoid enhancement of memory consolidation. To examine this implication, a low dose of the α2-adrenoceptor antagonist yohimbine, which increases norepinephrine levels in the brain, was administered to habituated rats either alone or together with corticosterone immediately after object recognition training. Yohimbine administered alone did not affect retention performance. However, as shown in Figure 13.3, corticosterone administered concurrently with yohimbine induced dose-dependent retention enhancement. Posttraining injections of the two drugs separated by a 4-hour delay did not induce a preference for the novel object on the retention. These findings thus indicate that arousal-induced noradrenergic activation is necessary to mediate glucocorticoid effects on memory consolidation but that pharmacologically stimulated noradrenergic activity mimics the effects of emotional arousal in enabling glucocorticoid enhancement of memory consolidation under low-arousing training conditions.89 These finding support the notion that the noradrenergic component of emotional arousal is critical for memory enhancement induced by glucocorticoids and possibly by epinephrine.
13.5.2. Interactions At Cellular Level
Synergistic effects of glucocorticoids and the noradrenergic system in peripheral tissues including the lungs and liver have been implicated in the regulation of several cellular functions.103 Is there molecular evidence for interactions between these two systems in regulating memory consolidation? We recently reported that corticoster-one interacts with emotion-induced noradrenergic activation in activating the cAMP response-element binding (CREB) pathway in the BLA.89 Several findings have implicated CREB phosphorylation in the BLA in the modulation of memory consolidation.104,105 We found that corticosterone administered immediately after object recognition training significantly increased the number of pCREB-positive neurons in the BLA. Corticosterone did not alter the number of pCREB-positive BLA neurons in rats that received prior habituation to the training context. Importantly, however, corticosterone administered together with the noradrenergic stimulant yohimbine after object recognition training significantly increased pCREB immunoreactivity in the BLA. Thus, these findings are in accord with behavioral studies and indicate that corticosterone activates the CREB pathway in the BLA only with training conditions that induce sufficient noradrenergic activation.
Other studies have indicated that glucocorticoids may interact with noradrenergic mechanisms in increasing the expression and enzymatic activity of the MAPK pathway, leading to an increased expression of the immediate early gene Egr1 (early growth response-1).106 Like CREB, phosphorylation of MAPKs is considered critical for memory consolidation and long-term neuronal plasticity.107,108 Blockade of MAPK signaling in the hippocampus abolishes the enhancing effect of systemically administered corticosterone on contextual fear conditioning.106 Glucocorticoid effects on MAPK activation may be modulated by β-adrenoceptor activation. Activation of β-adrenoceptors by epinephrine and norepinephrine leads to the dissociation of G protein subunits β and γ. It has been demonstrated that β G protein subunits interact with phosphoinositide-3 kinase to stimulate the MAPK pathway.109,110 Further, epinephrine and norepinephrine were found to potentiate ligand-dependent GR transactivation in cultured hippocampal cells via β2-adrenoceptors.111
13.6. CONCLUSIONS
The evidence summarized in this chapter indicates that adrenal stress hormones influence memory processes in various animal and human memory tasks. Acutely administered or released epinephrine or glucocorticoids dose-dependently enhance the consolidation of long-term memory. However, the effects of stress hormones on the storage of long-term memories depend critically on the arousal state and noradrenergic activation of the BLA. These findings may help to explain why stress hormones do not uniformly modulate memory for all kinds of information but rather, preferentially influence the consolidation of emotionally arousing information. As adrenal stress hormones also play a critical role in the development of traumatic memories and posttraumatic stress disorder (PTSD),112–114 these findings may provide some understanding of the neurobiological processes that underlie the development of PTSD as well as some possible implications for therapeutic intervention (see Reference 115) to ensure that significant events are well remembered, but do not turn into pathophysiological conditions.
ACKNOWLEDGMENT
We sincerely appreciate Gabriel Hui’s assistance with the figures.
REFERENCES
- 1.
- Kleinsmith LJ, Kaplan S. Paired-associate learning as a function of arousal and interpolated interval. J. Exp. Psych. 1963;65:190. [PubMed: 14033436]
- 2.
- Bradley MM, Greenwald MK, Petry MC, Lang PJ. Remembering pictures: pleasure and arousal in memory. J. Exp. Psych. Learn. Mem Cognition. 1992;18:379. [PubMed: 1532823]
- 3.
- Neisser U, Winograd E, Bergman ET, Schreiber CA, Palmer SE, Weldon MS. Remembering the earthquake: direct experience vs. hearing the news. Memory. 1996;4:337. [PubMed: 8817459]
- 4.
- Bohannon JN. Flashbulb memories for the space shuttle disaster: a tale of two theories. Cognition. 1988;2:179. [PubMed: 3168421]
- 5.
- Roozendaal B, Bohus B, McGaugh JL. Dose-dependent suppression of adreno-cortical activity with metyrapone: effects on emotion learning. Psychoneuroendocrinology. 1996;21:681. [PubMed: 9247987]
- 6.
- Vazdarjanova A, McGaugh JL. Basolateral amygdala is not critical for cognitive memory of contextual fear conditioning. Proc. Nat. Acad. Sci. USA. 1998;95:15003. [PMC free article: PMC24565] [PubMed: 9844005]
- 7.
- de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: are glucocorticoids good or bad guys? Trends Neurosci. 1999;22:422. [PubMed: 10481183]
- 8.
- McGaugh JL. Memory: a century of consolidation. Science. 2000;287:248. [PubMed: 10634773]
- 9.
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27:1. [PubMed: 15217324]
- 10.
- Roozendaal B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213. [PubMed: 10737694]
- 11.
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc. Natl. Acad. Sci USA. 2004;101:853. [PMC free article: PMC321770] [PubMed: 14711996]
- 12.
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendochrinology. 2001;26:307. [PubMed: 11166493]
- 13.
- Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiol. Learn. Mem. 2003;79:194. [PubMed: 12591227]
- 14.
- Abercrombie HC, Speck NS, Monticelli RM. Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. Psychoneuroendocrinology. 2006;31:187. [PubMed: 16225997]
- 15.
- Kuhlmann S, Wolf OT. Arousal and cortisol interact in modulating memory consolidation in healthy young men. Behav. Neurosci. 2006;120:217. [PubMed: 16492134]
- 16.
- Korte SM. Corticosteriods in relation to fear anxiety and psychopathology. Neurosci. Biobehav. Rev. 2001;25:117. [PubMed: 11323078]
- 17.
- Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav. Neurosci. 1992;106:62. [PubMed: 1313244]
- 18.
- Beylin AV, Shors TJ. Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience. Horm. Behav. 2003;43:124. [PMC free article: PMC3363955] [PubMed: 12614642]
- 19.
- Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav. Neurosci. 1997;111:503. [PubMed: 9189265]
- 20.
- Roozendaal B, McGaugh JL. Amygdaloid nuclei lesions differentially affect glucocorticoid-induced memory enhancement in an inhibitory avoidance task. Neurobiol. Learn. Mem. 1996;65:1. [PubMed: 8673403]
- 21.
- Cordero MI, Sandi C. A role for brain glucocorticoid receptors in contextual fear conditioning: dependence upon training intensity. Brain Res. 1998;786:11. [PubMed: 9554934]
- 22.
- Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by posttraining injections of corticosterone in rats. Neurobiol. Learn. Mem. 2004;81:67. [PubMed: 14670360]
- 23.
- Smotherman WP, Burt G, Kimble DP, Strickrod G, BreMiller R, Levine S. Behavioral and corticosterone effects in conditioned taste aversion following hippocampal lesions. Physiol. Behav. 1981;27:569. [PubMed: 7323158]
- 24.
- Roozendaal B, Carmi O, McGaugh JL. Adrenocortical suppression blocks the memory-enhancing effects of amphetamine and epinephrine. Proc. Natl. Acad. Sci USA. 1996;93:1429. [PMC free article: PMC39955] [PubMed: 8643648]
- 25.
- Cahill L, Pham CA, Setlow B. Impaired memory consolidation in rats produced with beta-adrenergic blockade. Neurobiol. Learn. Mem. 2000;74:259. [PubMed: 11031131]
- 26.
- Flood JF, Vidal D, Bennett EL, Orme AE, Vasquez S, Jarvik ME. Memory facilitating and anti-amnestic effects of corticosteroids. Pharmacol. Biochem. Behav. 1978;8:81. [PubMed: 625481]
- 27.
- Sandi C, Rose SPR. Training-dependent biphasic effects of corticosterone in memory formation for a passive avoidance task in chicks. Psychopharmacology. 1997;133:152. [PubMed: 9342781]
- 28.
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionally laden and neutral information. Behav. Neurosci. 2003;117:505. [PubMed: 12802879]
- 29.
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learn. Mem. 2003;10:270. [PMC free article: PMC202317] [PubMed: 12888545]
- 30.
- Borrell J, de Kloet ER, Versteeg DH, Bohus B. Inhibitory avoidance deficit following short-term adrenalectomy in the rat: the role of adrenal catecholamines. Behav. Neural Biol. 1983;39:241. [PubMed: 6670974]
- 31.
- Borrell J, de Kloet ER, Bohus B. Corticosterone decreases the efficacy of adrenaline to affect passive avoidance retention of adrenalectomized rats. Life Sci. 1984;34:99. [PubMed: 6694514]
- 32.
- Fallon JH, Coifi P. Distribution of monoamines within the amygdala. In: Aggleton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss; New York: 1992. p. 84.
- 33.
- Williams CL, Men D, Clayton EC, Gold PE. Norepinephrine release in the amygdala following systemic injection of epinephrine or escapable foot shock: contribution of the nucleus of the solitary tract. Behav. Neurosci. 1998;112:1414. [PubMed: 9926823]
- 34.
- Loy R, Koziell DA, Lindsey JD, Moore RY. Noradrenergic innervation of the adult rat hippocampal formation. J. Comp. Neurol. 1980;189:699. [PubMed: 7381046]
- 35.
- Devoto P, Flore G, Saba P, Fa M, Gessa GL. Stimulation of the locus coeruleus elicits noradrenaline and dopamine release in the medial prefrontal and parietal cortex. J. Neurochem. 2005;92:368. [PubMed: 15663484]
- 36.
- Petrov T, Krukoff T, Jhamandas J. Branching projections of catecholaminergic brainstem neurons to paraventricular hypothalamic nucleus and central nucleus of the amygdala in the rat. Brain Res. 1993;609:81. [PubMed: 8099526]
- 37.
- Reul JMHM, de Kloet ER. Two receptor systems for corticosterone in the rat brain: Microdistribution and differential occupation. Endocrinology. 1985;117:2505. [PubMed: 2998738]
- 38.
- Roozendaal B, Portillo-Marquez G, McGaugh JL. Basolateral amygdala lesions block glucocorticoid-induced modulation of memory for spatial learning. Behav. Neurosci. 1996;110:1074. [PubMed: 8919010]
- 39.
- Roozendaal B, McGaugh JL. Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiol. Learn. Mem. 1997;67:176. [PubMed: 9075247]
- 40.
- Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur. J. Neurosci. 1997;9:76. [PubMed: 9042571]
- 41.
- Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav. Neurosci. 1992;108:62. [PubMed: 1313244]
- 42.
- McEwen BS, Brinton RE, Sapolsky RM. Glucocorticoid receptors and behavior: Implications for the stress response. Adv. Exp. Med. Biol. 1988;245:35. [PubMed: 3067561]
- 43.
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive suppressive and stimulatory and preparative actions. Endocr. Rev. 2000;21:55. [PubMed: 10696570]
- 44.
- Beato M, Herrlich P, Schultz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851. [PubMed: 8521509]
- 45.
- Datson NA, van der Perk J, de Kloet ER, Vreugdenhil E. Identification of corticosteroid-responsive genes in rat hippocampus using serial analysis of gene expression. Eur. J. Neurosci. 2001;14:675. [PubMed: 11556892]
- 46.
- Orchinik M, Murray TF, Moore FL. A corticosteroid receptor in neuronal membranes. Science. 1991;252:1848. [PubMed: 2063198]
- 47.
- Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones: a focus on rapid nongenomic effects. Pharmacol. Rev. 2000;52:513. [PubMed: 11121509]
- 48.
- Dallman MF. Fast glucocorticoid actions on brain: back to the future. Front. Neuroendocrinol. 2005;26:103. [PubMed: 16242180]
- 49.
- Gold PE, van Buskirk R. Facilitation of time-dependent memory processes with post-trial epinephrine injections. Behav. Biol. 1975;13:145. [PubMed: 1122202]
- 50.
- Roozendaal B, Williams CL, McGaugh JL. Glucocorticoid receptor activation in the rat nucleus of the solitary tract facilitates memory consolidation: involvement of the basolateral amygdala. Eur. J. Neurosci. 1999;11:1317. [PubMed: 10103127]
- 51.
- Yang Y, Cao J, Xiong W, Zhang J, Zhou Q, Wei H, Liang C, Deng J, Li T, Yang S, Xu L, Xu L. Both stress experience and age determine the impairment or enhancement effect of stress on spatial memory retrieval. J. Endocrinol. 2003;178:45. [PubMed: 12844335]
- 52.
- Mabry TR, Gold PE, McCarty R. Age related changes in plasma catecholamine responses to acute swim stress. Neurobiol. Learn. Mem. 1995;63:260. [PubMed: 7670839]
- 53.
- Wolf OT, Dziobec I, McHugh P, Sweat V, de Leon MJ, Javier E, Convit A. Selective memory complaints in aging are associated with elevated cortisol levels. Neurobiol Aging. 2005;26:1357. [PubMed: 16243606]
- 54.
- Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur. J. Neurosci. 1997;9:637. [PubMed: 9153570]
- 55.
- Akirav I, Kozenicky M, Tal D, Sandi C, Venero C, Richter-Levin G. A faciltative role for corticosterone in the acquisition of a spatial task under moderate stress. Learn. Mem. 2004;11:188. [PMC free article: PMC379689] [PubMed: 15054134]
- 56.
- Gold PE, Hankins L, Edwards RM, Chester J, McGaugh JL. Memory interference and facilitation with posttrial amygdala stimulation: effect on memory varies with footshock level. Brain Res. 1975;86:509. [PubMed: 1116015]
- 57.
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988;31:47. [PubMed: 3228475]
- 58.
- Maheu FS, Joober R, Beaulieu S, Lupien SJ. Differential effects of adrenergic and corticosteroid hormonal systems on human short- and long-term declarative memory for emotionally arousing material. Behav. Neurosci. 2004;118:420. [PubMed: 15113269]
- 59.
- Pelletier JG, Likhtik E, Filali M, Paré D. Lasting increases in basolateral amygdala activity after emotional arousal: Implications for facilitated consolidation of emotional memories. Learn. Mem. 2005;12:96. [PMC free article: PMC1074326] [PubMed: 15805308]
- 60.
- McGaugh JL. Memory consolidation and the amygdala: A systems perspective. Trends Neurosci. 2002;25:456. [PubMed: 12183206]
- 61.
- Liang JC, Juler R, McGaugh JL. Modulating effects of posttraining epinephrine on memory: Involvement of the amygdala noradrenergic system. Brain Res. 1986;368:125. [PubMed: 3955350]
- 62.
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc. Natl. Acad. Sci USA. 2002;99:13908. [PMC free article: PMC129796] [PubMed: 12361983]
- 63.
- Liang KC, McGaugh JL. Lesions of the stria terminalis attenuate the enhancing effect of posttraining epinephrine on retention of an inhibitory avoidance response. Behav. Brain Res. 1983;9:49. [PubMed: 6882519]
- 64.
- Roozendaal B, McGaugh JL. The memory-modulatory effects of glucocorticoids depend on an intact stria terminalis. Brain Res. 1996;709:243. [PubMed: 8833760]
- 65.
- Roozendaal B, de Quervain DJ, Ferry B, Setlow B, McGaugh JL. Basolateral amygdala–nucleus accumbens interactions in mediating glucocorticoid enhancement of memory consolidation. J. Neurosci. 2001;21:2518. [PMC free article: PMC6762383] [PubMed: 11264325]
- 66.
- Foy MR, Stanton ME, Levine S, Thompson RF. Behavioral stress impairs long-term potentiation in rodent hippocampus. Behav. Neural Biol. 1987;48:138. [PubMed: 2820370]
- 67.
- Diamond DM, Bennet MC, Fleshner M, Rose GM. Inverted U-relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421. [PubMed: 1308198]
- 68.
- Palvides C, Watanabe Y, McEwen BS. Effects of glucocorticoids on hippocampal long-term potentiation. Hippocampus. 1993;3:183. [PubMed: 8353605]
- 69.
- Korz V, Frey JU. Stress-related modulation of hippocampal long-term potentiation in rats: Involvement of adrenal steroid receptors. J. Neursci. 2003;23:7281. [PMC free article: PMC6740434] [PubMed: 12917361]
- 70.
- Cottrell GA, Nakajima S. Effect of corticosteroids in the hippocampus on passive avoidance behavior in the rat. Pharmacol. Biochem. Behav. 1977;7:277. [PubMed: 928484]
- 71.
- Micheau J, Destrade C, Soumireu-Mourat B. Time-dependent effects of posttraining intrahippocampal injections of corticosterone on retention of appetitive learning tasks in mice. Eur. J. Pharmacol. 1984;106:39. [PubMed: 6529972]
- 72.
- Ikegaya Y, Saito H, Abe K. High-frequency stimulation of the basolateral amygdala facilitates the induction of long-term potentiation in the dentate gyrus in vivo. Neurosci. Res. 1995;22:203. [PubMed: 7566701]
- 73.
- Ikegaya Y, Nakanishi K, Saito H, Abe K. Amygdala β-noradrenergic influence on hippocampal long-term potentiation in vivo. NeuroReport. 1997;8:3143. [PubMed: 9331930]
- 74.
- Arnsten AFT, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch. Gen. Psychiatr. 1998;55:362. [PubMed: 9554432]
- 75.
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J. Neurosci. 1999;19:5473. [PMC free article: PMC6782338] [PubMed: 10377356]
- 76.
- Runyan JD, Dash PK. Intra-medial prefrontal administration of SCH-23390 attenuates ERK phosphorylation and long-term memory for trace fear conditioning in rats. Neurobiol. Learn. Mem. 2004;82:65. [PubMed: 15341790]
- 77.
- Perez-Jaranay JM, Vives F. Electrophysiological study of the response of medial prefrontal cortex neurons to stimulation of the basolateral nucleus of the amygdala in the rat. Brain Res. 1991;564:97. [PubMed: 1777825]
- 78.
- Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J. Neurosci. 2002;22:324. [PMC free article: PMC6757602] [PubMed: 11756516]
- 79.
- McIntyre CK, Roozendaal B, McGaugh JL. Glucocorticoid-induced inhibition of the medial prefrontal cortex enhances memory consolidation and increases consolidation of the extracellular signal-regulated kinases I/II in the amygdala. Soc. Neurosci. Abstr. 539.3 online, 2005.
- 80.
- Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995;377:295. [PubMed: 7566084]
- 81.
- Adolphs R, Tranel D, Denburg N. Impaired emotional declarative memory following unilateral amygdala damage. Learn. Mem. 2000;7:180. [PMC free article: PMC311327] [PubMed: 10837507]
- 82.
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator K, Wu J, McGaugh JL. Amygdala activity at encoding correlated with long-term free recall of emotional information. Proc. Natl. Acad. Sci USA. 1996;93:8016. [PMC free article: PMC38867] [PubMed: 8755595]
- 83.
- Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experiences. J. Neurosci. 2000;20:RC99. [PMC free article: PMC6772800] [PubMed: 11000199]
- 84.
- van Stegeren AH, Goekoop R, Everaerd W, Scheltens P, Barkhof F, Kuijer JP, Rombouts SA. Noradrenaline mediates amygdala activation in men and women during encoding of emotional material. Neuroimage. 2005;24:898. [PubMed: 15652324]
- 85.
- Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20:2091. [PubMed: 14683713]
- 86.
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: Role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc. Natl. Acad. Sci USA. 2005;102:2626. [PMC free article: PMC548968] [PubMed: 15703295]
- 87.
- Strange BA, Dolan RJ. β-Adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proc. Nat. Acad. Sci USA. 2004;101:11454. [PMC free article: PMC509222] [PubMed: 15269349]
- 88.
- Quirarte GL, Roozendaal B, McGaugh JL. Glucocorticoid enhancenemt of memory storage involves noradrenergic activation in the basolateral amygdala. Proc. Natl. Acad. Sci USA. 1997;94:14148. [PMC free article: PMC28430] [PubMed: 9391150]
- 89.
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc. Natl. Acad. Sci USA. 2006;103:6741. [PMC free article: PMC1458951] [PubMed: 16611726]
- 90.
- Roozendaal B, Nguyen BT, Power AE, McGaugh JL. Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proc. Natl. Acad. Sci. USA. 1999;96:11642. [PMC free article: PMC18087] [PubMed: 10500230]
- 91.
- Tanaka TH, Yokoo K, Mizoguchi, et al. Noradrenaline release in the rat amygdala is increased by stress: studies with intracerebral microdialysis. Brain Res. 1991;544:174. [PubMed: 1855137]
- 92.
- Pacak K, Palkovitz M, Kvetnanasky R, et al. Effects of single or repeated immobilization on release of norepinephrine and its metabolites in the central nucleus of the amygdala in conscious rats. Neuroendocrinology. 1993;57:626. [PubMed: 8367029]
- 93.
- Galvez R, Mesches M, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol. Learn. Mem. 1996;66:253. [PubMed: 8946419]
- 94.
- Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to foot shock and opioid peptidergic drugs. Brain Res. 1998;808:134. [PubMed: 9767150]
- 95.
- McIntyre CK, Hatfield T, McGaugh JL. Norepinephrine levels in the amygdala following inhibitory avoidance training predict retention score. Eur. J. Neurosci. 2002;16:1223. [PubMed: 12405982]
- 96.
- Ferry B, Roozendaal B, McGaugh JL. Involvement of α1-adrenoceptors in the basolateral amygdala in modulation of memory storage. Eur. J. Pharm. 1999;372:9. [PubMed: 10374709]
- 97.
- Hatfield T, McGaugh JL. Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiol. Learn. Mem. 1999;91:232. [PubMed: 10082642]
- 98.
- Härfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikström AC, Okret S, Yu ZY, Goldstein M, Steinbusch H, Verhofstad A, Gustafsson JA. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc. Natl. Acad. Sci USA. 1987;84:9779. [PMC free article: PMC387225] [PubMed: 2879285]
- 99.
- McIntyre CK, Roozendaal B, McGaugh JL. Glucocorticoid treatment enhances training-induced norepinephrine release in the amygdala. Soc. Neurosci. Abstr. 772.12 online, 2004.
- 100.
- Roozendaal B, Quirarte GL, McGaugh JL. Glucocorticoids interact with the basolateral amygdala β-adrenoceptor-cAMP/PKA system in influencing memory consolidation. Eur. J. Neurosci. 2002;15:553. [PubMed: 11876783]
- 101.
- Liang KC, Chen LL, Huang TE. The role of amygala norepinephrine in memory formation: involvement in memory-enhancing effects of peripheral epinephrine. Chin. J. Physiol. 1995;38:81. [PubMed: 8697902]
- 102.
- Ferry B, Roozendaal B, McGaugh JL. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between beta- and alpha1-adrenoceptors. J. Neurosci. 1999;19:5119. [PMC free article: PMC6782651] [PubMed: 10366644]
- 103.
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: Molecular mechanisms. Clin. Sci. 1998;94:557. [PubMed: 9854452]
- 104.
- Saha S, Datta S. Two-way active avoidance-specific increases in phosphorylated cAMP response element binding protein in the dorsal hippocampus, amygdala, and hypothalamus. Eur. J. Neurosci. 2005;21:3403. [PubMed: 16026478]
- 105.
- Josselyn SA, Kida S, Silva AJ. Inducible repression of CREB function disrupts amygdala-dependent memory. Neurobiol. Learn. Mem. 2004;82:159. [PubMed: 15341801]
- 106.
- Revest RM, Di Blasi F, Kitchener P, Rouge-Pont F, Desmedt A, Turiault M, Tronche F, Piazza PV. The MAPK pathway. Egr-1 mediated stress-related behavioral effects of glucocorticoids. Nat. Neurosci. 2005;8:835. [PubMed: 15834420]
- 107.
- Impey S, Obrietan K, Storm DR. Making new connections: Role of ERK/MAP kinase signaling in neuronal plasticity. Neuron. 1999;23:11. [PubMed: 10402188]
- 108.
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. J. Neurosci. 2000;20:8177. [PMC free article: PMC6772720] [PubMed: 11050141]
- 109.
- Crespo P, Xu N, Simonds WF, Gutkind JS. Ras-dependent activation of MAP kinase pathway mediated by G-protein beta–gamma subunits. Nature. 1994;369:418. [PubMed: 8196770]
- 110.
- Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science. 1997;275:394. [PubMed: 8994038]
- 111.
- Schmidt P, Holsboer F, Spengler D. Beta(2)-adrenergic receptors potentiate glucocorticoids receptor transactivation via G-protein beta gamma-subunits and the phosphoinositide 3-kinase pathway. Mol. Endocrinol. 2005;15:553. [PubMed: 11266507]
- 112.
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP. Pilot study of secondary prevention of post-traumatic stress disorder with propranolol. Biol. Psychiatr. 2002;51:189. [PubMed: 11822998]
- 113.
- Schelling G, Roozendaal B, de Quervain DJ. Can post-traumatic stress disorder be prevented with glucocorticoids? Ann. NY Acad. Sci. 2004;1032:158. [PubMed: 15677403]
- 114.
- Delahanty DL, Nugent NR, Christopher NC, Walsh M. Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinology. 2005;30:121. [PubMed: 15471610]
- 115.
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA 3rd, Arnsten A, Charney DS. Role of norepinephrine in pathophysiology and treatment of posttraumatic stress disorder. Biol. Psychiatr. 1999;46:1192. [PubMed: 10560025]
- INTRODUCTION
- STRESS HORMONE EFFECTS ON MEMORY CONSOLIDATION
- STRESS HORMONES SELECTIVELY ENHANCE MEMORY CONSOLIDATION OF EMOTIONALLY AROUSING EXPERIENCES
- INVOLVEMENT OF AMYGDALA IN MEDIATING STRESS HORMONE EFFECTS ON MEMORY CONSOLIDATION
- ROLE OF EMOTIONAL AROUSAL-INDUCED NORADRENERGIC ACTIVATION WITHIN AMYGDALA IN ENABLING EPINEPHRINE AND GLUCOCORTICOID EFFECTS ON MEMORY CONSOLIDATION
- CONCLUSIONS
- ACKNOWLEDGMENT
- REFERENCES
- Review Adrenal stress hormones, amygdala activation, and memory for emotionally arousing experiences.[Prog Brain Res. 2008]Review Adrenal stress hormones, amygdala activation, and memory for emotionally arousing experiences.Roozendaal B, Barsegyan A, Lee S. Prog Brain Res. 2008; 167:79-97.
- Review The amygdala modulates the consolidation of memories of emotionally arousing experiences.[Annu Rev Neurosci. 2004]Review The amygdala modulates the consolidation of memories of emotionally arousing experiences.McGaugh JL. Annu Rev Neurosci. 2004; 27:1-28.
- Glucocorticoids interact with the noradrenergic arousal system in the nucleus accumbens shell to enhance memory consolidation of both appetitive and aversive taste learning.[Neurobiol Learn Mem. 2012]Glucocorticoids interact with the noradrenergic arousal system in the nucleus accumbens shell to enhance memory consolidation of both appetitive and aversive taste learning.Wichmann R, Fornari RV, Roozendaal B. Neurobiol Learn Mem. 2012 Sep; 98(2):197-205. Epub 2012 Jun 28.
- Consolidating memories.[Annu Rev Psychol. 2015]Consolidating memories.McGaugh JL. Annu Rev Psychol. 2015 Jan 3; 66:1-24.
- Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala.[Proc Natl Acad Sci U S A. 2006]Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala.Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Proc Natl Acad Sci U S A. 2006 Apr 25; 103(17):6741-6. Epub 2006 Apr 12.
- Adrenal Stress Hormones and Enhanced Memory for Emotionally Arousing Experiences...Adrenal Stress Hormones and Enhanced Memory for Emotionally Arousing Experiences - Neural Plasticity and Memory
- LOC652276 [Homo sapiens]LOC652276 [Homo sapiens]Gene ID:652276Gene
- ZSCAN4 zinc finger and SCAN domain containing 4 [Homo sapiens]ZSCAN4 zinc finger and SCAN domain containing 4 [Homo sapiens]Gene ID:201516Gene
- Tlr7 toll-like receptor 7 [Mus musculus]Tlr7 toll-like receptor 7 [Mus musculus]Gene ID:170743Gene
- MGC32805 [Homo sapiens]MGC32805 [Homo sapiens]Gene ID:153163Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...
