NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Committee on the Review of Clinical Guidance for the Care of Health Conditions Identified by the Camp Lejeune Legislation; Board on the Health of Select Populations; Institute of Medicine. Review of VA Clinical Guidance for the Health Conditions Identified by the Camp Lejeune Legislation. Washington (DC): National Academies Press (US); 2015 Mar 26.

Review of VA Clinical Guidance for the Health Conditions Identified by the Camp Lejeune Legislation.
Show detailsThe 2009 National Research Council (NRC) report on contaminated drinking water at Camp Lejeune found that there was limited/suggestive evidence of an association between exposure to mixed solvents and neurobehavioral effects. This conclusion was based on the 2003 Institute of Medicine (IOM) report that had similarly found that there was limited/suggestive evidence of an association between “solvents and neurobehavioral effects (that is, abnormal results on neurobehavioral test batteries and symptom findings).” However, the term “neurobehavioral effects” was used in the NRC report (2009) to include such neurobehavioral symptoms as fatigue, lack of coordination, sensory disturbances, confusion, depression, tension, trouble concentrating, and headache; alterations in neurobehavioral tests that indicate deficits in attention, reaction time, visuomotor coordination, motor function, digit symbol, and contrast sensitivity; and certain neuropsychological disorders such as learning or behavioral disorders. That report separated neurologic diseases, such as Alzheimer's disease and Parkinson's disease, from neurobehavioral effects, which left unanswered what those effects indicated in terms of diagnostic entities. Because of the lack of diagnostic specificity, this committee chose to broadly define neurobehavioral effects to include all neurologic and behavioral effects (diseases, disorders, symptoms and deficits) because neurobehavioral symptoms or test findings can be indicative of neurologic or behavioral problems. The 1996 IOM report Veterans and Agent Orange stated:
The central nervous system (CNS) includes the brain and spinal cord, and CNS dysfunction can be subdivided into two general categories, neurobehavioral and motor/sensory. Neurobehavioral difficulties involve two primary categories: cognitive decline, including memory problems and dementia; and neuropsychiatric disorders, including neurasthenia (a collection of symptoms including difficulty concentrating, headache, insomnia, and fatigue), depression, posttraumatic stress disorder (PTSD), and suicide. Other CNS problems can be associated with motor difficulties, characterized by problems such as weakness, tremors, involuntary movements, incoordination, and gait/walking abnormalities.
NEUROBEHAVIORAL AND RELATED EFFECTS
To better define the potential long-term neurobehavioral effects in the U.S. Department of Veterans Affairs (VA) guidance that are associated with an exposure to solvents at Camp Lejeune, this committee reviewed the evidence gathered and synthesized by the 2009 NRC and the 2003 IOM committees and also identified new evidence. Current literature defined the neurobehavioral effects discussed in this chapter; outcomes from the 2009 NRC report without new epidemiologic evidence were not revisited in this report. Literature searches identified new studies published since 2008 in which neurologic and behavioral (including psychological) outcomes were associated with exposure to solvent mixtures, trichloroethylene (TCE), or perchloroethylene (PCE). Those new studies are discussed in conjunction with the evidence proposed in the NRC 2009 report, then synthesized by the committee to determine what neurobehavioral effects might result from exposure to contaminated water at Camp Lejeune. The committee specifically identified neurobehavioral effects solely on the basis of the available literature, using statistically significant findings, the weight of evidence, and the strengths and weaknesses within each key study to determine whether an identified condition should be eligible for coverage under the law. Effects seen in animal studies published since 2008 were not specifically reviewed because the goal of the committee was to identify clinical outcomes in humans.
2009 NRC Report
The NRC (2009) reviewed the scientific evidence on associations between prenatal, childhood, and adult exposures to contaminated water at Camp Lejeune and adverse health effects. Data on accidental and controlled human inhalation and oral exposures and on experimental animal exposures were available and formed the basis for the conclusions in that report.
Neurologic Symptoms, Motor Function, and Sensory Deficits
The NRC report found that most human studies indicate effects on visuomotor and motor function, fatigue, headache, and deficits in concentration, primarily resulting from acute exposures to solvents. Acute inhalation and oral exposure to PCE can induce symptoms of CNS depression (dizziness and drowsiness), electroencephalograpic changes, and neurobehavioral changes such as alterations in flash-evoked visual potentials, deficits in vigilance, and deficits in eye–hand coordination. The effects of long-term occupational exposure to TCE include memory loss, mood swings, the impairment of cognitive function, and olfactory and trigeminal neuropathy (NRC, 2009). Oral doses of PCE given as an anthelminthic (de-wormer) resulted in narcotic effects and various associated changes, such as inebriation, perceptual distortion, and exhilaration (ATSDR, 1997). The NRC report also cited a study (Reif et al., 2003) that evaluated neurobehavioral function in 184 adults who had been exposed to TCE-contaminated drinking water many years before testing. Higher exposures were associated with poorer performance on several tests (such as digit symbol substitution test, contrast sensitivity C test, and contrast sensitivity D test) and with increased neurobehavioral symptoms (such as confusion, depression, and tension).
Amyotrophic Lateral Sclerosis, Alzheimer's Disease, and Multiple Sclerosis
In the NRC report, several neurologic diseases and endpoints were assessed to determine if they were associated with exposure to TCE, PCE, or solvent mixtures. Specifically, amyotrophic lateral sclerosis (ALS), Parkinson's disease, multiple sclerosis (MS), and Alzheimer's disease were considered. The report concluded that there was inadequate/insufficient evidence to determine whether an association exists between exposure to solvents and ALS, MS, or Alzheimer's disease (NRC, 2009).
Parkinson's Disease
The NRC report concluded that there was inadequate/insufficient evidence to determine whether an association exists between solvent exposure and Parkinson's disease (NRC, 2009). Two case-control studies (Dick et al., 2007; McDonnell et al., 2003) and one occupational cohort (Gash et al., 2008) were evaluated by the NRC committee to assess the relationship between exposure to solvents and Parkinson's disease. The first case-control study showed a trend of increasing odds of developing the disease with increasing duration of occupational exposure; however, the study did not account for other possible risk factors or confounders. The second study did not show any association between solvent exposure and the risk of developing Parkinson's disease, but this study relied on individual recall regarding occupational and hobby-related exposures to solvents. The final study was an occupational cluster investigation that showed that three workers diagnosed with Parkinson's disease had workstations adjacent to a TCE source.
Updated Literature
The committee identified several new epidemiologic studies that looked at the association between exposure to solvents, particularly TCE and PCE, and neurobehavioral effects such as motor function as well as neurobehavioral symptoms resulting from neurological diseases such as Parkinson's disease. The committee did not assess new toxicological studies on these solvents because they currently would not have clinical applications. However, one paper (Bale et al., 2011) reviewed mechanistic studies using TCE, PCE, or dichloromethane and proposed mechanisms of action for the different neurological effects observed in those studies. The authors concluded that cognitive decrements may be due to changes in cholinergic transmission, while visual system changes were mediated by N-methyl-D-aspartic acid (NMDA)-glutamate or the nicotinic acetylcholine receptor pathway. The disruption of sodium channel function may lead to demyelination associated with multiple sclerosis. Data for these solvents were insufficient to propose a mechanism for ototoxicity or sleep-cycle changes.
Neurologic Symptoms, Motor Function, and Sensory Deficits
This committee's updated literature search identified one new study that addressed neurologic effects resulting from solvent exposure. Static postural sway and hand tremor parameters were evaluated in 57 workers occupationally exposed to TCE for 0.1 to 37 years (mean 10.9 years); 60 unexposed workers served as controls (Murata et al., 2010). A cumulative exposure index was calculated by multiplying total urinary trichloro-compound levels by work duration. Neuromotor function tests were conducted on a Friday after the work shift. Maximum ambient TCE was estimated at less than 22 ppm, but air measurements were not taken. Sway area, transversal sways, and sagittal sways with eyes open were all significantly greater in the exposed workers than in the controls (p = < 0.001, 0.012, and 0.029, respectively). Hand tremor intensities in the dominant hand were significantly larger in exposed workers than in the controls (p = 0.038), but there was no significant difference for the non-dominant hand. A trend of greater sway and increased tremor intensity was seen with increasing exposure (as measured by a cumulative exposure index), but the effect was not statistically significant, probably because of the small number of individuals in each exposure category.
This committee concluded, based on the 2009 NRC report and the updated literature, that the best characterized neurologic effects associated with solvent exposure, in particular exposure to TCE and PCE, were deficits in visuomotor function, motor function, memory, and concentration. Based on the evidence, the committee is interpreting “deficits in concentration” to mean attentional disorders.
Amyotrophic Lateral Sclerosis, Alzheimer's Disease, and Multiple Sclerosis
In the 2009 NRC report, several neurologic diseases and endpoints were assessed to determine if they were associated with exposure to TCE, PCE, and solvent mixtures. Specifically, the report considered ALS, Parkinson's disease, MS, and Alzheimer's disease. This committee considers each of these diseases to have neurobehavioral effects that could lead to their diagnosis and therefore looked at any new literature since the 2009 report on exposure to solvents and the development of these neurologic diseases.
No new evidence provided additional support for a relationship between exposure to solvents and the development of ALS, Alzheimer's disease, or MS. However, two investigations of Camp Lejeune military personnel and civilians compared mortality from ALS and MS with that of military personnel and civilians stationed at U.S. Marine Corps Camp Pendleton in California.
In two retrospective cohort studies (Bove et al., 2014a,b), both civilian employees and military personnel stationed at Camp Lejeune were evaluated for exposure to contaminated drinking water and risk of mortality from cancers and other chronic diseases. It should be noted that only mortality resulting from a disease was examined, not the development or prevalence of the disease itself. Both populations were matched to control cohorts from Camp Pendleton. The exposures of Camp Lejeune employees and military personnel were estimated using average monthly contaminant concentrations in the drinking water during the period of their employment or residence on base. All cohorts were identified through the Defense Manpower Data Center files, with vital status at follow up obtained through the Social Security Administration Death Master File and the National Death Index.
Among military personnel, there were 27 and 21 deaths from ALS at Camp Lejeune and Camp Pendleton, respectively, and 10 and 12 deaths from MS at the two camps. These results are elevated compared to the general population but do not reach statistical significance (Bove et al., 2014a). Among civilians who worked at Camp Lejeune or at Camp Pendleton there were one and four deaths from ALS, respectively, and there was one death from MS at each camp. Because there were so few cases, the authors could not compute a hazard ratio comparing the two camps for either disease (Bove et al., 2014b). Thus, no increase in mortality from ALS or MS was observed in these study populations.
Parkinson's Disease
The committee identified four new studies that address solvent exposure and Parkinson's disease that have been published since the NRC report.
In a case-control study to determine the association between occupational exposure to specific solvents—including TCE and PCE—and the development of Parkinson's disease, 99 all-male twin pairs discordant for Parkinson's disease were identified from the National Academy of Sciences/NRC World War II Veteran Twins Registry (Goldman et al., 2012). Occupational solvent exposure was assessed through a questionnaire. Exposure to TCE was associated with a significantly increased risk of Parkinson's disease (OR [odds ratio] = 6.1, 95% CI [confidence interval] 1.2–33) and tended toward significance for PCE (OR = 10.5, 95% CI 0.97–113). The risk was also significantly increased for the combined variable of TCE or PCE exposure (OR = 8.9, 95% CI 1.7–47). In 48% of the pairs, at least one twin was exposed to one or more of the six solvents studied. The mean duration of exposure to TCE or PCE was 9.0 years in the control twin compared with 18.5 years in the twin diagnosed with Parkinson's disease (p = 0.009), suggesting that the duration of exposure was a factor in the development of the disease.
In the retrospective cohort studies of mortality in the Camp Lejeune and Camp Pendleton populations, the civilian cohort included 4,647 full-time civilian employees with a median age of 58 years who were employed at Camp Lejeune during 1973–1985 (Bove et al., 2014b). Controls were 4,690 full-time civilian employees (median age 60 years) at Camp Pendleton. The standardized mortality ratio (SMR) for Parkinson's disease in the Camp Lejeune cohort was 2.91 (95% CI 0.71–5.11) versus an SMR of 0.88 (95% CI 0.24–2.26) for the Camp Pendleton cohort. There was a nonsignificant increase in the risk of mortality from Parkinson's disease among the Camp Lejeune cohort (HR = 3.13, 95% CI 0.76–12.86) compared with the Camp Pendleton cohort, adjusted for sex, race, occupation, and education. There were five cases of Parkinson's disease in the Camp Lejeune cohort, as compared with four cases from Camp Pendleton. Four of the five Camp Lejeune cases were associated with a cumulative exposure above the median for TCE and PCE as well as for vinyl chloride and benzene resulting in hazard ratios of greater than 2.50 (p ≤ 0.05).
The military cohort included 154,932 marine and Navy personnel with a median age of 49 years who began active-duty service between April 1975 and December 1985, and who were stationed at Camp Lejeune at some time during that period. The controls were 154,969 personnel stationed at Camp Pendleton at any time during the same active-duty interval. SMRs were not calculated for Parkinson's disease because there were fewer than five cases in each cohort. The committee notes that the military Camp Lejeune cohort is still too young to be informative about the risk of death from Parkinson's disease. At the end of the 2008 mortality follow up, the median age was 49, and only 2.7% of cohort members were 55 or older (Bove et al., 2014a). Because Parkinson's disease is a rare condition before age 50 (National Parkinson Foundation, 2014) and mortality occurs several years after diagnosis, a longer follow up is needed to provide meaningful results on this disease.
In a recent review, Lock et al. (2013) concluded that neither toxicologic nor epidemiologic studies present clear evidence that any specific solvent or class of solvents is an established cause of Parkinson's disease. However, based on the findings of Goldman et al. (2012) and Bove et al. (2014a,b), as well as limited data from the NRC (2009) and the U.S. Environmental Protection Agency (EPA) (2011) assessments, the committee finds that TCE and similar solvents may have potential etiologic relevance in the development of Parkinson's disease.
The committee concludes that Parkinson's disease is a neurobehavioral effect that may have resulted from the consumption of the contaminated drinking water at Camp Lejeune. This conclusion is based on the positive trends of increased risks from occupational and drinking water exposures reported by Goldman et al. (2012), NRC (2009), and Bove et al. (2014b). Despite the limitations of these studies, such as lack of statistical significance, the potential for recall bias, and the lack of incidence data pertaining to Parkinson's disease, the committee recommends including Parkinson's disease as an outcome associated with exposure to TCE and PCE. Because of the slow onset of Parkinson's disease, patients developing it years after their exposure, regardless of their age at exposure, may have not had symptoms at the time of exposure. Patients who have Parkinson's disease now or who develop it in the future and are otherwise eligible for the Camp Lejeune program should be covered by the guidance for neurobehavioral effects even if the symptoms were not apparent during their time at Camp Lejeune.
The committee recommends that VA consider adding Parkinson's disease in the clinical guidance and in algorithm B as a neurobehavioral effect that may result from exposure to contaminated drinking water at Camp Lejeune.
IN UTERO AND CHILDHOOD EXPOSURES
Although the majority of the diseases listed in the Janey Ensminger Act have been associated with adult exposure to solvents, the act also acknowledges that pregnant women who resided at Camp Lejeune may have ingested the contaminated drinking water and by doing so inadvertently exposed their fetuses. In addition, children living on Camp Lejeune also consumed the contaminated water. The committee believes that the health impacts of the consumption of solvent-contaminated drinking water on fetuses, infants, and children need to be considered in the VA guidance (discussed later) and therefore, the effects of solvents on children, who were exposed in utero or during early childhood—and who are now adults—are discussed in this section. Furthermore, because of the neurologic involvement and behavioral effects resulting from some types of birth defects, such as neural tube defects, the committee also reviewed the evidence for these types of outcomes.
2009 NRC REPORT
The 2009 NRC report describes how children are particularly susceptible to contaminants such as solvents by noting the following:
[T]here were “windows of vulnerability” or short periods of early human development when chemical exposures may significantly alter organ function or structure. Potentially vulnerable targets in infants and young children include the endocrine, reproductive, immune, visual, and nervous systems. Little information is available on the effects of TCE, PCE, and other solvents on the development of those organ systems in laboratory animals or humans.
The NRC report found that only a few studies assessed neurotoxic outcomes in rats resulting from the exposure of the fetus to low concentrations of TCE in drinking water during pregnancy and lactation. In all studies, the doses to the animals were below those causing overt maternal or fetal toxicity. Reported neurobehavioral effects included increased activity, reduced 2-deoxyglucose uptake in the brain, learning deficits, and reduced hippocampal myelin. The effects of PCE exposure during development included the neurobehavioral impairment of rats and mice on certain days of testing, reductions in acetylcholine and dopamine, changes in motor activity and attenuation of habituation, and altered pain and seizure thresholds. These studies of behavioral effects in rats and mice exposed to PCE prenatally or postnatally further suggest that there may be sensitive windows for neurobehavioral impairment during development.
There were few epidemiologic data available to characterize the effects of solvents in children exposed in utero or postnatally in the 2009 NRC report. This exposure pathway was not considered in the earlier IOM report that assessed potential health outcomes in veterans of the 1990–1991 Gulf War who were exposed to solvents (IOM, 2003). That report assumed that there were no pregnant female service members deployed in the 1990–1991 Gulf War. In the NRC report, the few studies that were identified focused on childhood cancers that may have resulted from solvent exposure. Only one study available at that time assessed the effects of prenatal exposure to PCE on learning and behavioral disorders; these exposures came from contaminated drinking water in Cape Cod (Janulewicz et al., 2008).
The only conclusion that the 2009 NRC report made regarding adverse outcomes in children was “that there continues to be inadequate/insufficient evidence to determine whether an association exists between chronic exposure to TCE or PCE and congenital malformations.” The congenital malformations assessed included heart defects, neural tube defects, and oral clefts.
Updated Literature
Most of the new literature identified by the committee was the result of a series of epidemiologic studies on a Cape Cod, Massachusetts, population that had been exposed to PCE. From 1968 through 1980, PCE had leached into the drinking water supplies from lined pipes installed in the public water distribution systems of several towns on Cape Cod. Aschengrau et al. published a series of population-based retrospective cohort studies examining the association between prenatal and postnatal drinking water exposure to PCE and a number of adverse neurobehavioral outcomes (Aschengrau et al., 2011, 2012; Getz et al., 2012; Janulewicz et al., 2008, 2012, 2013) and congenital anomalies (Aschengrau et al., 2009). Exposure estimates came from the modeled cumulative mass of PCE entering the homes of study participants and were not a direct measure of PCE intake by the subjects. Cumulative exposure during gestation and early childhood was calculated as the sum of 75% of the estimated mass of PCE delivered to the residence during the birth year plus the estimated mass of PCE from the month and year following birth through the month and year of the fifth birthday. Exposure assessments beyond the fifth birthday could not be conducted because of limitations in the water systems records. Exposed and unexposed populations were cross-matched with a database of all street locations served by the contaminated pipes. Because nearly all subjects with prenatal exposure also had early childhood exposure, the impact of prenatal exposure alone could not be determined in these studies.
Other studies of in utero or childhood exposure to solvents include a case control study of mothers from Camp Lejeune during the time of the water contamination (Ruckart et al., 2013), a population-based National Birth Defects Prevention Study (Desrosiers et al., 2012), and two studies of environmental exposures (Storm et al., 2011; Till et al., 2005). In the sections below, the committee considers the neurobehavioral effects that have been reported in the children exposed to solvents in utero or in childhood at Cape Cod, Camp Lejeune, and elsewhere.
Birth Defects Affecting the Nervous System
A case-control study was conducted to determine whether children born to mothers with residential exposure to contaminated drinking water at Camp Lejeune during pregnancy were more likely to have childhood hematopoietic cancers, neural tube defects, or oral clefts (Ruckart et al., 2013). The exposed population included live births between 1968 and 1985 to mothers who resided on base at any time during their pregnancy. Parents of 12,598 children were asked during a telephone interview if their child had a birth defect. The risk for neural tube defects associated with average first-trimester exposures were increased nonsignificantly for TCE above 5 parts per billion (ppb) (OR = 2.4, 95% CI 0.6–9.6). A monotonic exposure response relationship was observed in those exposed to less than 5 ppb (OR = 1.1, 95% CI 0.3–3.5) and greater than 5 ppb (OR = 2.4, 95% CI 0.6–9.6) compared with those who were unexposed. (Five ppb is the maximum contaminant level; see Chapter 1 for a description.)
A Cape Cod population cohort of 1,658 children exposed prenatally to PCE and 2,999 unexposed children was also examined for risks of congenital anomalies (Aschengrau et al., 2009). No meaningful increases in ORs were seen for cardiac and musculoskeletal malformations, and there were too few exposed cases to estimate ORs for eye; ear, face, and neck; respiratory; and other anomalies. Among children with any prenatal exposure, there was a nonsignificant increase in the ORs for neural tube defects (OR = 3.5, 95% CI 0.8–14.0). The neural tube defects observed in the cohort included four cases of anencephaly among exposed children versus none in the unexposed children, one case of spina bifida among the exposed children versus three cases among unexposed children, and one case of Arnold-Chiari malformation among exposed versus none among unexposed children.
Risks of neural tube defects from a maternal occupational exposure to organic solvents were assessed using data from the population-based National Birth Defects Prevention Study (Desrosiers et al., 2012). The maternal occupational exposure period was restricted to 1 month prior to the estimated date of conception through the end of the first trimester; jobs were coded by occupation and industry and assessed for exposure to 10 organic solvents, including TCE and PCE. Regression analyses were used to determine associations between solvent class (chlorinated, Stoddard, aromatic) and outcome. A total of 511 neural tube defect cases with 2,972 controls were included in the analyses; exposure to chlorinated solvents was associated with a statistically significant increased risk of neural tube defects (OR = 1.96, 95% CI 1.34–2.87).
Risks of congenital anomalies in children born to mothers exposed to TCE, PCE, or other solvents during pregnancy have been evaluated in several studies. An association between neural tube defects and drinking water exposure (Aschengrau et al., 2009; Ruckart et al., 2013) or occupational exposure (Desrosiers et al., 2012) has been shown. The committee concludes that neural tube defects may have resulted from in utero exposures to these solvents in the contaminated drinking water at Camp Lejeune.
The committee recommends that VA consider including neurobehavioral effects as a result of neural tube defects to the Camp Lejeune clinical guidance and in algorithm B-1.
Neuropsychological Performance
Performance on a battery of neuropsychological tests was assessed in a small cohort of 35 adults who had been exposed to PCE at Cape Cod in utero or during early childhood between 1969 and 1983 and also in 28 unexposed subjects (Janulewicz et al., 2012). No associations were found between prenatal and early postnatal exposure to PCE and decrements on tests that assess abilities in the domains of omnibus intelligence, academic achievement, and language. Trends were found among exposed individuals both for mood alterations and for slightly worse performances in various domains, including visuospatial, learning and memory, attention, fine motor speed, and executive function, but the effects were not statistically significant, most likely because of the small sample size.
A sample of 1,349 exposed and 737 unexposed children was evaluated for risk of learning and behavioral disorders following prenatal and early postnatal exposure to PCE (Janulewicz et al., 2008). All the children were born between 1969 and 1983 to mothers who lived on Cape Cod at the time of birth; enrollment occurred during 2002–2003. The measures of learning, attention, and behavior used in the study included whether the child ever had a diagnosis of attention deficit disorder or hyperactive disorder, ever received tutoring for reading or math, ever had special class placement for academic or behavioral problems, ever had an individual education plan, or ever repeated a school grade, as well as the child's highest level of education achieved. No associations were found between exposures and the maternal reports of any measured outcome.
Psychiatric Disorders
Another cohort of the Cape Cod population with 831 exposed and 547 unexposed children enrolled during 2006–2008 was evaluated for an affinity for risky behaviors and for the occurrence of mental illness. Risky behaviors included in the study were smoking, drinking, or illicit drug use as a teen or adult (Aschengrau et al., 2011). Individuals with any level of exposure during gestation and early childhood were more likely than unexposed subjects to have used two or more major illicit drugs as a teenager (risk ratio [RR] = 1.4, 95% CI 1.0–1.8) or as an adult (RR = 1.3, 95% CI 1.0–1.6). Individuals in the highest tertile of exposure (that is, greater than the 67th percentile) during gestation and early childhood were 50 to 60% more likely to have used two or more major illicit drugs as a teenager (RR = 1.6, 95% CI 1.2–2.2) or as an adult (RR = 1.5, 95% CI 1.2–1.9). The specific drugs for which increased risks were observed included cocaine and crack cocaine, psychedelics and hallucinogens, and club and designer drugs, Ritalin without a prescription, and heroin (RRs = 1.4–2.1). Increases in the risk of certain drinking behaviors were seen only among highly exposed subjects, with no evidence of a dose–response relationship. For example, only individuals in the highest tertile of exposure during gestation and early childhood experienced increases in the risk of drinking more than 8 days/month as a teenager (RR = 1.6, 95% CI 1.1–2.3).
Mental illnesses (depression, bipolar disorder, PTSD, and schizophrenia) were also assessed in the Cape Cod cohort (Aschengrau et al., 2012). Subjects with any exposure during gestation and childhood were 1.8 times more likely to have developed bipolar disorder (95% CI 0.9–3.5), although the effect was not statistically significant. However, when the analysis was restricted to subjects in the highest tertile of exposure, a significantly increased risk for bipolar disorder was observed (RR = 2.7, 95% CI 1.3–5.6). The risk of PTSD was greater for subjects with any exposure during gestation and childhood (RR = 1.5, 95% CI 0.9–2.5), but the effect was not statistically significant. While there were too few cases of schizophrenia to examine a dose–response relationship, three of the four schizophrenia cases were in the exposed group (RR = 2.1, 95% CI 0.2–20.0 for any exposure). No associations were found between PCE exposure and an increased risk of depression among exposed subjects.
The available studies of the effects of solvents in children and adults who were exposed in utero or in childhood in Cape Cod found a number of neurobehavioral effects. The limitations of these studies include their retrospective nature, modeled exposure estimates, and self-reported mental illnesses. However, the major strengths of the studies were that exposed and unexposed groups were from the same geographic location, population characteristics were similar between the two groups, and similar proportions of participants and nonparticipants were exposed to PCE, which reduced selection bias. No other studies were identified that examined these psychological and psychosocial outcomes in association with in utero or childhood exposure to PCE, TCE, or other solvents. Thus, although the positive findings reported for the Cape Cod cohorts for illicit drug use and bipolar disorder associated with in utero and early childhood exposures to PCE have not been confirmed by research in other populations, the committee agreed that the studies provide important information on such exposures and warrant further research.
Committee members were not in agreement on whether the two studies on illicit drug use and bipolar disorder (Aschengrau et al., 2011, 2012) provided enough evidence to warrant a recommendation on the inclusion of these two neurobehavioral effects in the guidance and in the algorithms.
Nevertheless, in keeping with the VA policy that “in cases where there is reasonable doubt as to the diagnosis or primary cause for the diagnosis, clinicians should resolve in favor of the Camp Lejeune veteran or family member,” the committee recommends that VA consider including adolescent and adult illicit drug use and bipolar disorder as neurobehavioral effects in the Camp Lejeune clinical guidance and in algorithm B-1.
ADDITIONAL ENDPOINTS
The literature identifies a number of endpoints of concern, other than those identified in the preceding sections, but the evidence precluded the IOM committee from making a recommendation on the findings at this time. Trends for problem drinking and alcoholism, PTSD, and schizophrenia were found in some populations (Aschengrau et al., 2009, 2011, 2012). However, the strength of these results was limited by the small numbers of cases observed in the study populations, a lack of dose–response effects, and failures to reach statistical significance.
Other endpoints of concern are discussed in the following sections.
Structural Brain Changes
The final neurobehavioral-related outcome examined in a cohort from Cape Cod was overt structural brain changes as detected with structural magnetic resonance imaging (MRI) (Janulewicz et al., 2013). Brain imaging was performed on 26 exposed and 16 unexposed subjects in order to obtain measurements of specific brain regions. No statistically significant differences were found between exposed and unexposed subjects on the measures of white matter hypointensities, white matter volumes, or gray matter volumes.
Vision
The 2003 IOM and the 2009 NRC reports found that there was inadequate/insufficient evidence to determine whether an association existed between exposures to solvents and long-term reduction in color discrimination. Peer consultants for the 2004 EPA draft Summary Report of the Peer Review Workshop on the Neurotoxicity of Tetrachloroethylene (Perchloroethylene) Discussion Paper suggested that:
contrast sensitivity loss may reflect impaired function throughout the brain, because contrast sensitivity is affected by retinal, optic nerve, or central brain dysfunction (EPA, 2004). Nonetheless, drawing strong conclusions from these studies is difficult, particularly in light of the paucity of data on this test in occupational populations with higher exposure concentrations and in animal studies. (EPA, 2012)
The committee identified three additional studies of vision effects resulting from PCE exposure (two of which included children) and one study of the effects of organic solvent exposure on vision. In the first study, deficits in color vision and contrast sensitivity were assessed in a small cohort of 29 exposed and 25 unexposed Cape Cod adults who were about 30 years of age at testing, and all of whom had been exposed to PCE during the prenatal and early postnatal period (Getz et al., 2012). None of the participants had subjective visual complaints. However, the participants in the higher PCE exposure group exhibited lower contrast sensitivity at intermediate and high spatial frequencies than did the unexposed participants, although the differences were generally not statistically significant. The exposed participants also exhibited poorer color discrimination than the unexposed participants; the mean difference in the Farnsworth color confusion index between PCE-exposed and unexposed participants was 0.05 (95% CI 0.003–0.10; p = 0.04).
Storm et al. (2011) evaluated the effects of current exposure to PCE on visual contrast sensitivity in 54 adults and 50 children residing in buildings co-located with a dry cleaner using PCE. Increases in PCE levels in indoor air, breath, and blood were significantly (p = 0.02) associated with decreased visual contrast sensitivity in the children's worse-performing eyes at the specific spatial frequency of 12 cycles per degree. Adult visual contrast sensitivity was not affected.
Till et al. (2005) examined visual abnormalities in 21 infants (mean age 12 months) exposed prenatally to organic solvents and compared them with 27 unexposed age-matched infants; the specific chemicals were not identified. Exposed infants showed a significant reduction in contrast sensitivity (p < 0.001) as well as abnormal visual evoked potential responses to the red–green onset stimulus (p < 0.01) but not to either blue–yellow or achromatic stimuli.
Vision abnormalities, including diffuse color vision losses (p < 0.01), have been reported for 25 adults occupationally exposed (gas station workers) to mixed solvents (Costa et al., 2012). Gobba and Cavalleri (2000)1 found that in 33 PCE-exposed dry cleaners who had occupationally induced color vision loss, no change in color perception was observed for those workers whose exposure decreased, while in others a rise in PCE levels was followed by a significant worsening of color vision.
The committee acknowledges that the visual deficits found in these studies are subclinical, that several studies had very small samples sizes, and that the evidence necessary to assess whether the effects are short term or long term is lacking. Nevertheless, the committee finds that the weight of evidence indicates deficits in contrast sensitivity and color discrimination may result from exposure to TCE, PCE, or solvents and are neurobehavioral effects that may result from prenatal, childhood, and adult exposures to solvents in the contaminated drinking water at Camp Lejeune.
The committee recommends that problems with contrast sensitivity and color discrimination be included in the clinical guidance and in algorithm B as neurobehavioral effects that may result from exposure to contaminated drinking water at Camp Lejeune, although it recognizes that these are typically subclinical (that is, they are not detectable upon routine examination), and no treatments for them are currently available. Given their subclinical nature, the committee further recommends that patients not be screened for these conditions unless there is a clear reason to do so (for example, the patient reports visual problems), and that the results of any screening or testing for visual problems should be noted in the patient's record.
DISCUSSION OF THE GUIDANCE AND ALGORITHM
In this section, the committee considers the text of the VA guidance and discusses the accuracy and clarity of the guidance and algorithm B as well as inconsistencies within and between the guidance and algorithm B. The committee also suggests specific language for revising the original algorithm B and proposes a new algorithm B-1 for neurobehavioral effects associated with solvent exposures in utero and in childhood. Algorithm B applies to exposures for all ages.
The guidance currently has a short section for clinicians on what is meant by neurobehavioral effects, which conditions qualify as covered conditions, and what signs or symptoms should be determined to have been present when veterans or family members were exposed to contaminated drinking water during or shortly after their time at Camp Lejeune. The committee notes that the neurobehavioral effects presented in the text (pp. 8–9) are not entirely consistent with those given in algorithm B, nor do they reflect the supporting data on those effects as presented in the 2009 NRC report (see Table 3-1).
TABLE 3-1
Neurobehavioral Effects as Given in Algorithm B and the Revised Algorithm B.
For example, in the text of the guidance, deficits in color vision are not mentioned; however, algorithm B specifically lists problems with color vision as one of the few effects that have been identified as a neurobehavioral symptom for the Camp Lejeune program.
In Box 4 of the original algorithm B, “Reductions in color discrimination” is also listed as a diagnosed (and therefore exclusionary) neurologic condition that may cause the neurobehavioral symptoms otherwise associated with residence at Camp Lejeune. This is confusing and should be clarified and made consistent both in the guidance text and the algorithm B.
The guidance states on page 8 that neurobehavioral effects “would likely have been manifest at the time of exposure or shortly thereafter” (emphasis in guidance) and further cautions clinicians that neurobehavioral effects that first occur after a long asymptomatic period are “not likely to be secondary to the contaminated water at Camp Lejeune,” nor are they likely to have persisted and to require treatment at this time. In essence, the guidance suggests that neurobehavioral effects in adults resulting from exposure to contaminated water at Camp Lejeune are unlikely to require treatment at the current time; impacts on children exposed in utero or in childhood are not discussed. It might be helpful for clinicians if the guidance text clarified that for residents who have neurobehavioral symptoms that have persisted since their time at Camp Lejeune, those symptoms are indeed eligible to be covered; this is clear in the algorithm but not in the text.
The guidance contains a paragraph on page 9 that discusses neurobehavioral effects that are associated with long-term exposure to mixed solvents, occupational exposures, and chronic low-level exposures. One citation is given for each statement. The committee finds that this paragraph may present an incomplete picture of the neurobehavioral effects that have been associated with solvent exposure in the recent literature (e.g., EPA, 2011, 2012). It is unclear what criteria VA used to select the studies mentioned in the paragraph (that is, Chen et al., 2001; Dick et al., 2010; Flodin et al., 1984; and van Valen et al. 2009) as they are not necessarily representative of the epidemiological literature on TCE, PCE, and other solvents.
The classification scheme for neurobehavioral effects is taken from the National Institute for Occupational Safety and Health (NIOSH) bulletin on organic solvent neurotoxicity (NIOSH, 1987). The committee notes that this document is for occupational exposures and that although it includes chronic exposure and solvent abuse situations, these are not representative of the exposures at Camp Lejeune. The neurobehavioral effects discussed in the NIOSH bulletin do not parallel those in the guidance or the algorithm. Should the NIOSH classification scheme be retained in the guidance, the committee suggests that the guidance explain to clinicians how they should use the algorithm when evaluating Camp Lejeune program participants.
Given the inconsistencies between the guidance and algorithm B for neurobehavioral effects in adults following exposure to Camp Lejeune drinking water, the committee recommends that the VA clinical guidance and algorithm B be revised to be consistent and to reflect recent literature.
Finally, the committee notes that although the Janey Ensminger Act specifically states that family member includes those who were in utero while at Camp Lejeune, the guidance does not address prenatal exposure and possible subsequent neurobehavioral effects. Based on the evidence considered in the earlier section “In Utero and Childhood Exposures,” the committee believes it is important that the guidance address prenatal and childhood exposure to Camp Lejeune contaminants as the outcomes differ and are not captured in the current guidance or in algorithm B.
The committee has determined that it is reasonable to expect that the exposure period relevant for the “prenatal and adolescence exposure algorithm” should encompass the time period from conception through the age of 18. Although the Cape Cod studies modeled exposure only through the age of 5 years because of data limitations, the human brain continues to develop after age 5 through multiple cellular processes (e.g., gliogenesis, synaptic pruning, synaptic remodeling, and apoptosis). This continued brain development has been captured and analyzed using MRI technology with individuals from the ages of 2 weeks though 18 years (Brain Development Cooperative Group, 2012; Sanchez et al., 2012). If the exposure occurred after the age of 18, the individual would be evaluated using the adult exposure algorithm.
Thus, the committee recommends that VA consider including in the clinical guidance a new algorithm B-1 for neurobehavioral effects specific to prenatal and childhood exposure at Camp Lejeune.
Algorithm B: Adult Exposure
Algorithm B is for neurobehavioral symptoms seen in Camp Lejeune residents who were exposed at any age to the contaminated drinking water. The symptoms must have begun while the patient was at Camp Lejeune and have continued through to the present. The committee finds that this accurately reflects what the evidence says about the development of these conditions, with the exception of Parkinson's disease, which may have developed at any time from the point of exposure to the present; the revised algorithm accounts for this difference. The original algorithm and guidance specify that the symptoms must not be associated with more common neurological or psychiatric conditions not known to be related to exposure to contaminated drinking water at Camp Lejeune. Because different clinicians may be diagnosing the conditions in Box 4 (assessed by a neurologist) and Box 5 (assessed by a psychiatrist), it is helpful to differentiate the conditions in each one. Table 3-1 presents the neurobehavioral symptoms and exclusionary conditions presented in algorithm B and in the guidance compared to the committee's suggested changes to that algorithm. A revised algorithm B is presented in Figure 3-1.
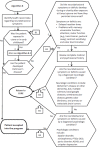
FIGURE 3-1
Algorithm B—Neurobehavioral outcomes. ANNOTATIONS FOR REVISED ALGORITHM B: ADHD = attention deficit hyperactivity disorder; ALS = amyotrophic lateral sclerosis; OCD = obsessive compulsive disorder; PTSD = posttraumatic stress disorder.
Suggested Algorithm B-1 for In Utero and Childhood Exposure
As discussed earlier in this chapter, in utero and childhood exposures were possible at Camp Lejeune. However, the committee finds that if the patient was exposed as a child or fetus the neurobehavioral deficits caused by exposure may not have been manifest at the time of exposure. Therefore, the committee finds that it is prudent to consider including the following neurobehavioral conditions in adults who were exposed in utero or as children (up until age 18 years) to contaminated drinking water at Camp Lejeune, although not all committee members were in agreement on the addition of illicit drug use and bipolar disorder:
- Illicit drug use
- Bipolar disorder
- Neurobehavioral effects caused by neural tube defects
The suggested Algorithm B-1 is presented in Figure 3-2.

FIGURE 3-2
Algorithm B-1—Neurobehavioral outcomes in adults exposed in utero or in early childhood. ANNOTATIONS FOR ALGORITHM B-1: B1—Identified neurobehavioral symptoms include illicit drug use, bipolar disorder, and neurological problems associated (more...)
REFERENCES
- Aschengrau A, Weinberg JM, Janulewicz PA, Gallagher LG, Winter MR, Vieira VM, Webster TF, Ozonoff DM. Prenatal exposure to tetrachloroethylene-contaminated drinking water and the risk of congenital anomalies: A retrospective cohort study. Environmental Health. 2009;8:44. [PMC free article: PMC2761868] [PubMed: 19778411]
- Aschengrau A, Weinberg JM, Janulewicz PA, Romano ME, Gallagher LG, Winter MR, Martin BR, Vieira VM, Webster TF, White RF, Ozonoff DM. Affinity for risky behaviors following prenatal and early childhood exposure to tetrachloroethylene (PCE)-contaminated drinking water: A retrospective cohort study. Environmental Health. 2011;10:102. [PMC free article: PMC3268745] [PubMed: 22136431]
- Aschengrau A, Weinberg JM, Janulewicz PA, Romano ME, Gallagher LG, Winter MR, Martin BR, Vieira VM, Webster TF, White RF, Ozonoff DM. Occurrence of mental illness following prenatal and early childhood exposure to tetrachloroethylene (PCE)-contaminated drinking water: A retrospective cohort study. Environmental Health. 2012;11:2. [PMC free article: PMC3292942] [PubMed: 22264316]
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological profile for tetrachloroethylene. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 1997.
- Bale AS, Barone S Jr, Scott CS, Cooper GS. A review of potential neurotoxic mechanisms among three chlorinated organic solvents. Toxicology and Applied Pharmacology. 2011;255(1):113–126. [PubMed: 21609728]
- Bove FJ, Ruckart PZ, Maslia M, Larson TC. Evaluation of mortality among marines and Navy personnel exposed to contaminated drinking water at USMC Base Camp Lejeune: A retrospective cohort study. Environmental Health. 2014a;13(1):10. [PMC free article: PMC3943370] [PubMed: 24552493]
- Bove FJ, Ruckart PZ, Maslia M, Larson TC. Mortality study of civilian employees exposed to contaminated drinking water at USMC Base Camp Lejeune: A retrospective cohort study. Environmental Health. 2014b;13:68. [PMC free article: PMC4237831] [PubMed: 25115749]
- Brain Development Cooperative Group. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: The NIH MRI Study of Normal Brain Development. Cerebral Cortex. 2012;22(1):1–12. [PMC free article: PMC3236790] [PubMed: 21613470]
- Chen R, Dick F, Semple S, Seaton A, Walker LG. Exposure to organic solvents and personality. Occupational and Environmental Medicine. 2001;58(1):14–18. [PMC free article: PMC1740035] [PubMed: 11119629]
- Costa TL, Barboni MT, Moura AL, Bonci DM, Gualtieri M, de Lima Silveira LC, Ventura DF. Long-term occupational exposure to organic solvents affects color vision, contrast sensitivity and visual fields. PLoS ONE. 2012;7(8):e42961. [PMC free article: PMC3419737] [PubMed: 22916187]
- Desrosiers TA, Lawson CC, Meyer RE, Richardson DB, Daniels JL, Waters MA, van Wijngaarden E, Langlois PH, Romitti PA, Correa A, Olshan AF. Maternal occupational exposure to organic solvents during early pregnancy and risks of neural tube defects and orofacial clefts. Occupational and Environmental Medicine. 2012;69(7):493–499. [PMC free article: PMC3719396] [PubMed: 22447643]
- Dick FD, De Palma G, Ahmadi A, Scott NW, Prescott GJ, Bennett J, Semple S, Dick S, Counsell C, Mozzoni P, Haites N, Wettinger SB, Mutti A, Otelea M, Seaton A, Soderkvist P, Felice A. Environmental risk factors for Parkinson's disease and parkinsonism: The Geoparkinson study. Occupational and Environmental Medicine. 2007;64(10):666–672. [PMC free article: PMC2078401] [PubMed: 17332139]
- Dick FD, Bourne VJ, Semple SE, Fox HC, Miller BG, Deary IJ, Whalley LJ. Solvent exposure and cognitive ability at age 67: A follow-up study of the 1947 Scottish Mental Survey. Occupational and Environmental Medicine. 2010;67(6):401–407. [PubMed: 19910296]
- EPA (U.S. Environmental Protection Agency). Summary report of the peer review workshop on the neurotoxicity of tetrachloroethylene. Washington, DC: National Center for Environmental Assessment, Office of Research and Development; 2004. (EPA/600/R-04/041).
- EPA. Toxicological review of tricholorethylene (CAS No. 79-01-6) in support of summary information on the Integrated Risk Information System (IRIS). U.S. Environmental Protection Agency; 2011. (EPA/635/R-08/011F).
- EPA. Toxicological review of tetracholorethylene (Perchloroethylene) (CAS No. 127-18-4) in support of summary information on the Integrated Risk Information System (IRIS). U.S. Environmental Protection Agency; 2012. (EPA/635/R-08/011F).
- Flodin U, Edling C, Axelson O. Clinical-studies of psychoorganic syndromes among workers with exposure to solvents. American Journal of Industrial Medicine. 1984;5(4):287–295. [PubMed: 6720692]
- Gash DM, Rutland K, Hudson NL, Sullivan PG, Bing G, Cass WA, Pandya JD, Liu M, Choi DY, Hunter RL, Gerhardt GA, Smith CD, Slevin JT, Prince TS. Trichloroethylene: Parkinsonism and complex 1 mitochondrial neurotoxicity. Annals of Neurology. 2008;63(2):184–192. [PubMed: 18157908]
- Getz KD, Janulewicz PA, Rowe S, Weinberg JM, Winter MR, Martin BR, Vieira VM, White RF, Aschengrau A. Prenatal and early childhood exposure to tetrachloroethylene and adult vision. Environmental Health Perspectives. 2012;120(9):1327–1332. [PMC free article: PMC3440105] [PubMed: 22784657]
- Gobba F, Cavalleri A. Evolution of color vision loss induced by occupational exposure to chemicals. Neurotoxicology. 2000;21(5):777–781. [PubMed: 11130282]
- Goldman SM, Quinlan PJ, Ross GW, Marras C, Meng C, Bhudhikanok GS, Comyns K, Korell M, Chade AR, Kasten M, Priestley B, Chou KL, Fernandez HH, Cambi F, Langston JW, Tanner CM. Solvent exposures and Parkinson's disease risk in twins. Annals of Neurology. 2012;71(6):776–784. [PMC free article: PMC3366287] [PubMed: 22083847]
- IOM (Institute of Medicine). Veterans and Agent Orange: Update 1996. Washington, DC: National Academy Press; 1996. [PubMed: 25121271]
- IOM. Gulf War and health, Volume 2: Insecticides and solvents. Washington, DC: The National Academies Press; 2003.
- Janulewicz PA, White RF, Winter MR, Weinberg JM, Gallagher LE, Vieira V, Webster TF, Aschengrau A. Risk of learning and behavioral disorders following prenatal and early postnatal exposure to tetrachloroethylene (PCE)-contaminated drinking water. Neurotoxicology and Teratology. 2008;30(3):175–185. [PMC free article: PMC2494864] [PubMed: 18353612]
- Janulewicz PA, White RF, Martin BM, Winter MR, Weinberg JM, Vieira V, Aschengrau A. Adult neuropsychological performance following prenatal and early postnatal exposure to tetrachloroethylene (PCE)-contaminated drinking water. Neurotoxicology and Teratology. 2012;34(3):350–359. [PMC free article: PMC3553661] [PubMed: 22522125]
- Janulewicz PA, Killiany RJ, White RF, Martin BM, Winter MR, Weinberg JM, Aschengrau A. Structural magnetic resonance imaging in an adult cohort following prenatal and early postnatal exposure to tetrachloroethylene (PCE)-contaminated drinking water. Neurotoxicology and Teratology. 2013;38:13–20. [PMC free article: PMC3893699] [PubMed: 23571160]
- Lock EA, Zhang J, Checkoway H. Solvents and Parkinson disease: A systematic review of toxicological and epidemiological evidence. Toxicology and Applied Pharmacology. 2013;266(3):345–355. [PMC free article: PMC3621032] [PubMed: 23220449]
- McDonnell L, Maginnis C, Lewis S, Pickering N, Antoniak M, Hubbard R, Lawson I, Britton J. Occupational exposure to solvents and metals and Parkinson's disease. Neurology. 2003;61(5):716–717. [PubMed: 12963777]
- Murata K, Inoue O, Akutsu M, Iwata T. Neuromotor effects of short-term and long-term exposures to trichloroethylene in workers. American Journal of Industrial Medicine. 2010;53(9):915–921. [PubMed: 20698023]
- National Parkinson Foundation. 2014. [September 11, 2014]. (PD 101 for patients). http://www
.parkinson .org/Patients/PD-101.aspx. - NIOSH (National Institute for Occupational Safety and Health). Organic Solvent Neurotoxicity. Current Intelligence Bulletin 48. 1987. [December 31, 2014]. (DHHS Publication Number 87-104). www
.cdc.gov/niosh/docs/87-104. - NRC (National Research Council). Contaminated water supplies at Camp Lejeune: Assessing potential health effects. Washington, DC: The National Academies Press; 2009. [PubMed: 25009942]
- Reif JS, Burch JB, Nuckols JR, Metzger L, Ellington D, Anger WK. Neurobehavioral effects of exposure to trichloroethylene through a municipal water supply. Environmental Research. 2003;93(3):248–258. [PubMed: 14615234]
- Ruckart PZ, Bove FJ, Maslia M. Evaluation of exposure to contaminated drinking water and specific birth defects and childhood cancers at Marine Corps Base Camp Lejeune, North Carolina: A case-control study. Environmental Health: A Global Access Science Source. 2013;12(1):104. [PMC free article: PMC3880212] [PubMed: 24304547]
- Sanchez CE, Richards JE, Almli CR. Neurodevelopmental MRI brain templates for children from 2 weeks to 4 years of age. Developmental Psychobiology. 2012;54(1):77–91. [PMC free article: PMC3184192] [PubMed: 21688258]
- Storm JE, Mazor KA, Aldous KM, Blount BC, Brodie SE, Serle JB. Visual contrast sensitivity in children exposed to tetrachloroethylene. Archives of Environmental and Occupational Health. 2011;66(3):166–177. [PubMed: 21864105]
- Till C, Westall CA, Koren G, Nulman I, Rovet JF. Vision abnormalities in young children exposed prenatally to organic solvents. Neurotoxicology. 2005;26(4):599–613. [PubMed: 16054697]
- Van Valen E, Wekking E, van der Laan G, Sprangers M, van Dijk F. The course of chronic solvent induced encephalopathy: A systematic review. Neurotoxicology. 2009;30(6):1172–1186. [PubMed: 19538991]
Footnotes
- 1
This study is included here despite the date of publication because it was not cited in the NRC report (2009).
- Characterization of Neurobehavioral Effects - Review of VA Clinical Guidance for...Characterization of Neurobehavioral Effects - Review of VA Clinical Guidance for the Health Conditions Identified by the Camp Lejeune Legislation
- Serratia inhibens PRI-2CSerratia inhibens PRI-2CSerratia inhibens PRI-2C Genome sequencingBioProject
- SRP302932 (10)SRA
- PP_RS03085 [Pseudomonas putida KT2440]PP_RS03085 [Pseudomonas putida KT2440]Gene ID:1044396Gene
- USP17L5 ubiquitin specific peptidase 17 like family member 5 [Homo sapiens]USP17L5 ubiquitin specific peptidase 17 like family member 5 [Homo sapiens]Gene ID:728386Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...