NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee on Policies for Allocating Health Sciences Research Funds; Bloom FE, Randolph MA, editors. Funding Health Sciences Research: A Strategy to Restore Balance. Washington (DC): National Academies Press (US); 1990.

Funding Health Sciences Research: A Strategy to Restore Balance.
Show detailsThe United States is widely recognized as the world's greatest investor in health sciences research. Of the estimated $132 billion invested in all research and development (R&D) in the United States in 1989, $20.6 billion was health related (Figure 2-1).,1,2 In this country health research is funded by three autonomous yet interlocking sectors: (1) federal, state, and local governments; (2) industry; and (3) private nonprofit organizations. In 1988 the federal, state, and local governments supported slightly more than half (51 percent) of all health-related R&D in the United States. Of the remainder, industry supported about 45 percent, and private nonprofit organizations supported about 4 to 5 percent (Figure 2-1). This ratio has changed slightly over the past decade, while the nation's investment in health research has tripled in current dollars (Figure 2-2). In inflation-adjusted dollars the investment has grown by 65 percent during this time (Figure 2-3).

Figure 2-1
Estimated U.S. research and development expenditures for 1989. ,

Figure 2-2
Source of U.S. support for health research and development from 1977 to 1989.

Figure 2-3
Total U.S. support for health research and development from 1977 to 1989. (Appendix Table A-l)
Before World War II, however, the federal government did not invest heavily in life sciences R&D. Most federal support for biological research was sponsored by the Department of Agriculture through block grants to the land-grant colleges. Projects sponsored by these funds were targeted toward the applied life sciences of agriculture and forestry, with few provisions for basic biological research. Additionally, geographical criteria were employed as the primary means to disburse these funds.
During this same period, health research was sponsored primarily by industry, academic institutions, and private individuals.3 In fact, of the estimated $45 million spent on biomedical research in 1940, industry contributed 55 percent or about $25 million. Approximately 26 percent ($17 million) came from philanthropy, either through earnings on institutional endowments or grants from foundations. The federal government's investment that year totaled $3 million—about 15 percent of the total, most of which was spent in its own laboratories. Some university-based investigators eschewed governmental support, fearing the loss of intellectual freedom and undue influence on their research. 4
During World War II, basic research in the sciences made significant contributions to the success of the war effort. In 1945 Vannevar Bush, then head of the Office of Scientific Research and Development (OSRD), formulated a set of proposals intended to sustain the nation's war-time research momentum and direct it toward civilian goals. His report to the President, entitled "Science, the Endless Frontier," proposed a coordinated federal policy of investing in research and training new researchers.5 The policy was to be driven by scientific merit rather than by political or geographical interest. Subsequently, Bush and his colleagues in OSRD established a system by which grants and contracts were awarded to institutions based on scientific merit, and this approach became the cornerstone of the peer-reviewed, academically based system now in place for federally sponsored, competitive extramural research grant programs.
In the two decades following the war, several pieces of legislation changed the organization and conduct of scientific research in the United States. The federal government became the largest single sponsor of health research and about three fifths of these funds now come from programs in the Department of Health and Human Services (DHHS), namely those in the Public Health Service (PHS).2 Within the PHS the National Institutes of Health (NIH) and the Alcohol, Drug Abuse, and Mental Health Administration (ADAMHA) allocate the largest percentage of federal funds for health-related research (Figure 2-4). Research funds in DHHS also are allocated to the Centers for Disease Control; the Health Care Financing Administration; the Health Resources and Services Administration; the Food and Drug Administration; the Office of Health Research, Statistics, and Technology; and the Office of the Assistant Secretary for Health in the PHS. Other federal departments and agencies have budgets for health sciences research as well—most notably, the Departments of Defense, Energy, and Veterans Affairs, and the National Science Foundation (NSF) (Figure 2-4).

Figure 2-4
Source of federal support for health research and development for 1989.
Unlike most other countries where government-sponsored research is conducted in government laboratories, two-thirds of federally sponsored health sciences research in the United States is conducted in institutions of higher education (colleges and universities), research organizations, and hospitals, and approximately one-quarter is performed in federal laboratories (Figure 2-5). Whereas the majority of industrial health sciences research is performed within corporate facilities, only a small fraction of federally sponsored research is performed in private industrial laboratories2

Figure 2-5
Distribution of federal health research funds for 1989.
The broad array of research sponsors and the decentralized nature of research efforts by thousands of individual health researchers are recognized widely as the key advantages to the U.S. approach to health research and as the means by which it has flourished over the past four decades. In this type of system the individual scientist is recognized as the most important element in determining scientific priorities. This has been accomplished primarily by scientists by serving on merit review panels and advisory groups. Currently, there are more than 2,000 nonfederal scientists serving on peer review study sections and advisory groups in the NIH alone.6 Whereas the federal government along with other sponsors of research has been highly supportive of these peer review mechanisms and has provided financial resources for performing the research, many of the benefits of health research could not have been realized without a well-trained cadre of scientists.
Despite the success of the health research enterprise, the system has become stressed increasingly in recent years for many reasons. Most significant is the concern over growing federal debt and recent legislation attempting to reduce the huge annual federal budget outlays. Recent attempts to reduce federal deficits have increased the competition for scarce funds for all federally financed programs. Unfortunately, funds from states and private sector sources have been unable to compensate for the slower growth of available federal funds, especially support for fundamental health sciences research. The increasing competition among worthy projects has required making difficult choices, often resulting in concessions to short-term needs rather than longer-term investments.
This study committee was created out of a concern that these short-term choices have helped create an imbalance in the support of research projects, personnel, and the facilities and equipment needed for research. The committee examined the allocation policies of the primary sponsors of health research and the contributions to the scientific decision-making process by all concerned parties. This chapter overviews the funding of health research by the various sponsors and reviews policies affecting the allocation of these resources. The subsequent chapters examine more closely the sources and uses of funds for talent development, research projects, facilities and equipment, and processes for matching scientific priorities with political and fiscal realities.
Federal Support for Health Sciences R&D
NIH and ADAMHA
Before World War II nearly all federally sponsored health research was conducted in the government's own laboratories. The precursor to NIH, the Laboratory of Hygiene (later renamed the Hygienic Laboratory), was established out of the Marine Hospital Service in 1887 and was designated the National Institute of Health in 1930. Its role was expanded over the following decade to include public health advisory functions. The National Cancer Act of 1937 empowered the Surgeon General to administer extramural grants-in-aid for cancer research and provide fellowships to train scientific personnel. The Public Health Service Act of 1944 made NIH a separate entity in the PHS and empowered NIH to support research on diseases other than cancer through extramural grant and fellowship programs. The NIH Research Grants Office, forerunner of the Division of Research Grants, was created in 1946 to administer a program of extramural awards.
In 1953 the PHS was reassigned to the newly created Department of Health, Education, and Welfare, and the scope of NIH's responsibilities began to change.7 In 1956 the National Library of Medicine Act created the National Library of Medicine (NLM) out of the Armed Forces Medical Library, and the Health Research Facilities Act was passed the same year, authorizing a program of matching funds to be administered through NIH for constructing health sciences research facilities. Growing appropriations under this new NIH construction authority were responsible for the major research building projects that expanded the research infrastructure in the United States from the late 1950s to 1970.
In response to new scientific opportunities in the health sciences, Congress increased funding for scientific research dramatically between 1945 and 1970, when appropriations for NIH rose from $26 million to $4.8 billion in constant 1988 dollars* (Figure 2-6).7 Congress also added numerous categorical institutes to NIH during that time, reflecting efforts of special interest groups to target research on specific organ groups and illnesses. However, the rate of growth in funding for health sciences research slowed after 1965 in the wake of increased expenditures for the domestic human service initiatives of the Johnson administration and the Vietnam War.8
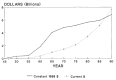
Figure 2-6
NIH appropriations from 1945 to 1990. Note: Constant dollars are calculated using the Biomedical Research and Development Price Index (BRDPI). (Appendix Table A-2)
Despite this declining rate of budgetary growth, NIH continued to expand its role in the health sciences and underwent various reorganizations during the 1960s and 1970s.7 In 1967 the National Institute of Mental Health (NIMH) was removed from NIH and established as a separate bureau within the PHS. Following this, in 1973, the recently created National Institute of Alcohol Abuse and Alcoholism (NIAAA) and the National Institute of Drug Abuse (NIDA) were merged with NIMH to form the ADAMHA. Also, during the 1970s, two institutes were elevated to bureau status within NIH, reflecting congressional emphasis on cancer and heart disease, and the National Institute of Aging was created because of an increasing desire to understand the aging process.
Inflationary pressures in the 1970s reduced the purchasing power of research funds, fostering the academic community's perception that the financial base of federal research support was eroding.9 In response, the director of NIH advised Congress to stipulate the minimum number of new and competing research project grants that NIH would be required to support with its annual appropriations. This policy became known as ''stabilization.'' Beginning in fiscal year 1981, NIH and ADAMHA were required to support 5,000 and 569 research project grants, respectively. Increasing target numbers were proposed for subsequent years but were negotiated between the administration and Congress during the annual federal budget process. Nonetheless, the appropriations for NIH grew steadily over the past decade (Figure 2-7). With the exception of 1982, NIH has realized a growth, after adjustments for inflation, of about 2 percent per year. Appropriations for ADAMHA, although they dropped in the early 1980s, had real growth in the research portion of the budget throughout the 1980s (Figure 2-8).10 The research budgets for NIH and ADAMHA are covered in more detail in Chapter 4.
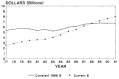
Figure 2-7
NIH appropriations from 1977 to 1991. Note: Figures for 1991 are derived from the President's proposed 1991 budget. (Appendix Table A-2)

Figure 2-8
ADAMHA appropriations from 1977 to 1991. Note: Figures for 1991 are derived from the President's proposed 1991 budget. (Appendix Table A-2)
Over the past decade a variety of new laws and regulations have been enacted, affecting how federal research agencies carry out their missions and how they interact with industry, universities, and other extramural research institutions. For example, the Stevenson-Wydler Act (P.L. 96-480), passed in 1980, mandated that all agencies with R&D budgets allocate 0.5 percent of their research funds to industry or universities for technology transfer. In 1980 the Small Business Patent and Procedure Act (P.L. 96-517) made it possible to transfer patent rights derived from federally supported research to small businesses, universities, and certain nonprofit organizations.
The Small Business Innovation Development Act of 1982 established a program to grant federal research funds to for-profit businesses by all federal agencies with more than $100 million budgets for R&D. 7 This legislation called for a phase-in of the program over 4 subsequent fiscal years—from 1983 to 1986. Currently, all federal agencies awarding extramural research funds must allocate 1.25 percent of their annual R&D appropriations through this program.
The Federal Technology Transfer Act (P.L. 99-502) of 1986 encouraged additional government-industry collaboration. This legislation promotes technology transfer by authorizing government laboratories to enter into cooperative research and economic development agreements with other federal agencies, state and local governments, and for-profit and nonprofit organizations. Thus, companies now have unprecedented access to the research results from government laboratories upon which they can obtain exclusive licensing rights for development.11
Centers for Disease Control
The primary mission of the Centers of Disease Control (CDC) is to assist state and local health authorities and other health-related organizations in stemming the spread of communicable diseases, protecting the public from other diseases or conditions amenable to reductions, providing protection from certain environmental hazards, and improving occupational safety and health. Additionally, the CDC is responsible for licensing of clinical laboratories engaged in interstate commerce, for conducting foreign quarantine activities aimed at preventing the introduction of disease into the United States, and for developing scientific criteria for occupational health hazards. About nine-tenths of CDC's budget is allocated to the nonresearch portion of its mission, predominantly through block grants to states (Figure 2-9).

Figure 2-9
Distribution of budget for the Centers for Disease Control for 1989. (Key: IP = immunization program; STD = sexually transmitted diseases; PHSBG = preventive health services block grant; ED = environmental diseases; ES = epidemic services; NCHS = National (more...)
Of the $982 million appropriated to CDC in fiscal year 1989, only about 10 percent ($100.6 million) was obligated for health research. In constant 1988 dollars, research funds at CDC grew from $56.6 million to $95.5 million between 1984 and 1989 (Figure 2-10). Increases were greatest in fiscal years 1987 and 1988, when research funds grew by 18.8 and 26.8 percent, respectively, in constant dollars. These increases coincided directly with the increasing national emphasis on research into human immunodeficiency virus (HIV) infection.
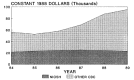
Figure 2-10
Research allocations for the Centers for Disease Control from 1984 to 1989. (Appendix Table A-3)
The National Institute of Occupational Safety and Health (NIOSH) is the primary research arm of the CDC. NIOSH conducts research; develops criteria for occupational safety and health standards; and provides technical services to government, labor, and industry, including training in the recognition, avoidance, and prevention of unsafe or unhealthful working conditions and the proper use of adequate safety and health equipment. Through these various mechanisms, NIOSH tries to reduce the high economic and social costs associated with occupational illness and injury. Obligations for research funded by NIOSH grew only slightly between 1984 and 1987, and declined in the following 2 years (Figure 2-10). Of the $70.4 million appropriated to NIOSH for fiscal year 1989, $24.7 million was committed for research and about $10.1 million was obligated for training.*
The CDC has been a leader in the nation's efforts to prevent and control the spread of HIV infection, managing a comprehensive HIV prevention program that includes surveillance; epidemiologic and laboratory studies; and prevention through information, education, and risk reduction. Appropriations for AIDS activities for fiscal year 1989 were $382.3 million—39 percent of the CDC budget. The research portion of this allocation was $44.6 million for epidemiologic and laboratory studies to determine the natural history of the disease and to gain more knowledge about transmission of HIV. In fact, research funds allocated to other parts of CDC have grown much faster than those in NIOSH (Figure 2-10).
Another part of the CDC, the National Center for Health Statistics (NCHS), is responsible for collecting, maintaining, analyzing, and disseminating statistics on the health, illness, and disability of the U.S. population and on the impacts of these factors on the economy. Although this function is not classified under research, it is an ancillary service for epidemiological studies utilizing the data base. NCHS also is responsible for collecting nonhealth data on births, deaths, marriages, and divorces. For fiscal year 1989, $49 million dollars was appropriated to NCHS.
Office of the Assistant Secretary for Health
In the past, appropriations for the Office of Assistant Secretary for Health included funds for the National Center for Health Services Research and Health Care Technology Assessment (NCHSR). The center was the focal point within the federal government for research on the health care delivery system and examined problems in the organization, delivery, and financing of health care services. It was also within the center's purview to coordinate health services research in the PHS and to disseminate the findings of health services research to policy and decision makers in the public and private sectors.
The Reconciliation Act of 1986 established a program of medical care outcomes research to evaluate the appropriateness, necessity, and effectiveness of selected medical treatments and surgical procedures. Thus, Congress made available in the NCHSR's 1989 allocation $5.9 million from the Medicare trust funds, $3.9 million from the Federal Hospital Insurance Trust Fund, and $2.1 million from the Federal Supplementary Medical Insurance Trust Fund to fund outcomes research—funds that will support extramural research projects based on competitive peer review by NCHSR. These responsibilities have been transferred to the newly created Agency for Health Care Policy and Research (AHCPR).
Department of Veterans Affairs
Historically, the Department of Veterans Affairs (VA), previously known as the Veterans Administration, has provided health care to veterans through a network of 172 hospitals and centers nationwide. Approximately 130 of these units have medical trainees and about 100 have formal agreements with medical schools. The VA provides financial support for 8,350 residents and interns—nearly 13 percent of the trainees in the United States. Additionally, Congress appropriates R&D funds to the VA to conduct studies pertaining to veteran health or using veteran patient populations.
The VA R&D budget is a separate line item in the federal budget. In fiscal year 1989 the VA was appropriated $207.5 million for health sciences research; however, this does not reflect any increase over the last decade when measured in constant 1988 dollars (Figure 2-11). The VA research budget is divided into three major categories: (1) medical research, (2) rehabilitation research, and (3) health services R&D. The distribution among these categories for fiscal year 1988 was 85 percent, 11 percent, and 4 percent, respectively.
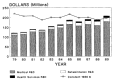
Figure 2-11
Research allocations for the Department of Veterans Affairs from 1979 to 1989. (Appendix Table A-4)
According to the VA, all R&D funds are peer reviewed, and 75 percent undergo a peer review process similar to investigator-initiated research project grants (R01) in the PHS. In 1981 the VA awarded 100 percent of its approved grant applications. However, the number of awards fell from more than 700 in 1985 to 386 in 1988. For 1989 the VA was able to fund approximately 500 meritorious research projects—an award rate of about 41 percent (Richard J. Greene, personal communication).
Approximately 10 percent of the VA research budget is allocated for career development at all levels. This includes limited salary support for some levels of training for young physician investigators. Generally, salary support for established VA investigators is covered with nonresearch funds.
Eight percent of the research budget is directed toward VA Cooperative Studies (multihospital clinical trials).
The VA has several attributes that make it a good resource base for clinical research. First, patient recruitment for clinical investigations is easier for the VA than for NIH. Second, the costs for the standard medical care portion of clinical investigations are charged to health care delivery funds rather than research dollars; thus, only the marginal costs of the research consume research appropriations. The clinical trials conducted by the VA may have a far-reaching impact on research performed by other federal agencies. The VA also is exploring ways to enhance its position as a resource base for clinical investigations by more open cooperation with private industry.12
National Science Foundation
The NSF was founded as an independent government agency in 1950 to promote scientific progress through basic research in all fields of science and engineering. The NSF thus supports a broad spectrum of fields of science and has an equally broad portfolio of research support mechanisms. NSF awards comprise 28 percent of federal funding for basic research in academic institutions. Although its budget is only one-fourth that of NIH, the NSF plays an important role in setting science policy for the nation through the National Science Board.
As with NIH and ADAMHA, budget levels for NSF have risen steadily since its creation. However, in 1988 constant dollars NSF appropriations declined in the early 1980s and have returned to 1979 levels only recently (Figure 2-12).13 The Reagan administration realized that basic research contributes significantly to U.S. competitiveness and therefore promised to commit the resources to NSF in order to double its budget in 5 years, but despite NSF budget requests of 19 percent increases for fiscal years 1988 and 1989, Congress increased appropriations by only 6 and 10 percent, respectively. NSF appropriations for fiscal year 1990 grew only modestly again to nearly $2.1 billion. Approximately $300 million, or 14 percent, of the 1989 appropriations were allocated to research and training related to the health sciences. Most of these funds are distributed through the Directorate of Biological, Behavioral, and Social Sciences (BBSS).

Figure 2-12
Appropriations for the National Science Foundation from 1977 to 1991. (Appendix Table A-5)
NSF's mission specifically excludes disease-related clinical research, which falls under the purview of NIH; therefore, NSF funds primarily are used for investigating basic biological processes that help shape the foundation for biomedical research. However, some funds are available for applied research, conferences and workshops, publication expenses, scientific equipment, libraries, and operation expenses of specialized research facilities. The current emphasis of NSF support is threefold: (1) continuing core support for basic research in all fields, (2) encouraging multidisciplinary projects, and (3) improving cooperation between academia and industry.
In 1988 the BBSS Directorate created the Division of Instrumentation and Resources to centralize its support for infrastructure and research resources. With regard to the health sciences, this division oversees the development of necessary biological software and data bases, genetic stock centers, and the acquisition of major specialized equipment for groups of investigators.
National Aeronautics and Space Administration
The National Aeronautics and Space Administration (NASA) has a small but highly specialized life sciences research program. The Office of Space Science and Applications at NASA spends approximately $75 million annually in its Life Sciences Division, of which about $37 million could be classified as health-related. This accounts for 0.4 percent of the total NASA budget, a level that has been maintained or lowered for the past decade.
The NASA biomedical research program is intended to support NASA's manned space programs. As the agency shifts from short-term space flights to more extended missions aboard the Space Station Freedom or to Mars, NASA will need to address specific questions relating to a microgravity environment, but because NASA has a very small life sciences budget, it must rely heavily on programs funded by other federal agencies.
Most of NASA's life science expenditures support intramural programs tailored to meet specific agency objectives. The agency does award small grants ($50 to $60 thousand) to investigators in the academic community. This, in effect, provides only partial support to extramural investigators but keeps an active community of scientists focusing on the problems associated with space travel.
Health Care Financing Administration
The primary mission of the Health Care Financing Administration (HCFA) is to manage the Medicare and Medicaid programs for health care payments, but the agency has a small research budget as well. Congress allocated $30 million to HCFA for research and demonstrations in fiscal year 1989.14 These funds support a variety of studies on the Medicare and Medicaid populations and the health industry providing services to these populations. Issues that Congress wants HCFA to focus on include quality and access to health care; in-home and ambulatory care; special population needs, including those of minorities; and long-term care.
Department of Defense
The Department of Defense (DOD) conducts health research vital to national security. Three branches conduct intramural and extramural health research: (1) the U.S. Army Medical Research and Development Command (USAMRDC), (2) the Directorate of Life Sciences in the Air Force Office of Scientific Research, and (3) the Life Sciences Programs Directorate of the Office of Naval Research.
Of the three branches, the USAMRDC receives the largest allocation of DOD funds for military health sciences research—about 80 percent of the total DOD health sciences research budget. In fiscal year 1989, $252 million was appropriated. When corrected to constant 1988 dollars, the USAMRDC budget grew from $136 million in 1980 to more than $318 million by 1987. However, this growth trend was reversed in 1988 and 1989, when the budget declined by 18 and 9 percent, respectively (Figure 2-13).
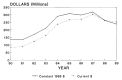
Figure 2-13
Research allocations for the U.S. Army Medical Research and Development Command from 1980 to 1989. (Appendix Table A-6)
The USAMRDC conducts mission-oriented medical R&D designed to support the soldier in the field. More specifically, this program supports research on increasing manpower efficiency by improving instrumentation and new medical knowledge in the following areas: (1) military disease hazards, including infectious diseases, biological warfare defense, and AIDS; (2) combat casualty care, including shock, wound healing, and craniofacial injuries; (3) medical chemical defense; and (4) army systems hazards.
The Directorate of Life Sciences in the Air Force Office of Scientific Research has a much smaller health-related research budget than the USAMRDC. In 1989, allocations for health research were only $17.1 million. These funds support research in several areas of neuroscience, experimental psychology, toxicology, visual and auditory psychophysics, radiation biology, and cardiovascular physiology.
The Office of Naval Research funds health research through the Life Sciences Programs Directorate. In fiscal year 1989 $24.4 million was allocated to biological and medical sciences and $11.5 million to cognitive and neural sciences. The 1990 budget request shows only slight growth for the biological and medical sciences—to $25.3 million—and $13.7 million for the cognitive and neural sciences.
Department of Energy
The Department of Energy (DOE) sponsors research related to the health effects of exposure to radiation and hazardous substances and has been a pioneer in the efforts to map the human genome. Most of the health research sponsored by DOE is conducted in the network of national laboratories under its direction. In 1989 and 1990, DOE allocated $218 million and $275 million to programs in biological and environmental research. However, its general life sciences program was allocated $45 million and $56 million for these past two years.
The largest portion of the life sciences program is mapping the human genome. Since both NIH and DOE have expertise in the necessary technology, a joint leadership plan is being implemented. DOE will develop the engineering technology and instrumentation crucial to the early stages of the project, and NIH will contribute through individual investigator work later. DOE allocations for this research endeavor have grown $18 million in 1989 to a proposed $46 million for fiscal year 1991. Both NIH and DOE have set up planning offices to coordinate the resources and efforts within the agencies. Additionally, the White House has created an interagency genome-coordination panel, under the authority of the White House Office of Science and Technology Policy, to work with NIH and DOE on project coordination. This precedent-setting interdepartmental effort will use the mechanisms outlined by the Federal Coordinating Council for Science, Engineering, and Technology (FCCSET).
Industry
Before World War II, industry funded more than half of all health sciences research in the United States.3,4 After the war, industry's support, although still increasing, was outpaced by the investment of the federal government. Industry again is playing an increasingly important role in health sciences research, focusing primarily on product development. The types of industries engaged in health sciences R&D include biotechnology firms and manufacturers of pharmaceuticals, medical devices, and instrumentation. These industries tend to be much more research intensive than other U.S. corporations. Development and testing requirements for investigative new drugs or devices probably account for these larger R&D expenditures. Also, high levels of investment have been attributed in part to the commercial potential for genetically engineered products such as insulin.15
Individual corporations are reluctant to release proprietary data on their research programs. However, three aggregate measures of industrial investment related to biomedicine are available: (1) the NSF's Survey of Biotechnology Research and Development Activities in Industry, (2) the Annual Survey of the Pharmaceutical Manufacturers Association (PMA), and (3) a subset of companies included in Business Week's ''R&D Scoreboard.'' The criteria used to select companies for inclusion differ among surveys, and it is likely that some companies are included in more than one survey.
Although most corporate R&D is done "in house," industry relies heavily on university research programs for basic knowledge and scientific talent. However, pharmaceutical firms generally contract with clinicians in academic centers to test compounds in all phases of clinical trials. Corporate research focuses mainly on applied and developmental research rather than on disease-oriented research or fundamental basic biology. Shared interests in specific problems have helped create some industry-sponsored cooperative basic research programs located in universities.16
From the 1950s to the mid 1970s, industry focused its research programs on product development and relied largely on universities for basic research. By the 1970s policymakers and business people alike grew concerned that U.S. industry was losing its competitive edge in world markets, and this neglect of basic research was cited as a leading cause. Concern over foreign competition prompted U.S. industries to increase their investment in R&D markedly in the past decade. In 1977 industry spent approximately $20 billion on corporate R&D and somewhat over $100 million on research within universities. By 1986 their total R&D investment rose to nearly $60 billion internally and $600 million in university research.14 In 1989 industry contributed about $9.3 billion to health sciences research, amounting to about 45 percent of the total national investment (Figure 2-2). 2
Pharmaceutical Industry
The pharmaceutical industry increased expenditures for R&D by 16 percent in 1986 and 13 percent in 1987. Although the rate of increase slowed, it still exceeded the average industrial investment for R&D of 6 percent of gross income. Pharmaceutical firms also boast a high level of R&D expenditures in relation to sales, increasing from 11.6 percent in 1983 to 13.0 percent in 1987.
The distribution of R&D expenditures varies by company and type of research. The NSF reports that nearly 80 percent of industrial R&D is development, whereas basic research accounts for only 5 percent. The remaining 15 percent is categorized as applied research.17 These estimates may not reflect R&D investment by the pharmaceutical industry correctly. However, according to one committee member, approximately one-third of a pharmaceutical firm's R&D investment is devoted to discovery and new product development, one-third is spent on existing product improvement and expansion of current business, and one-third is directed toward process improvement for defending current market shares of products. A large portion of pharmaceutical R&D is spent on clinical evaluation of drugs in phases I through IV (Table 2-1).
TABLE 2-1
Distribution of U.S. R&D Expenditures for Ethical Pharmaceuticals by Function, 1987 (dollars in millions).
The pharmaceutical industry relies heavily on academia to provide new scientific talent. Scientific employment at U.S. pharmaceutical R&D facilities increased approximately 7 percent per year from 1983 to 1986. In 1986 the U.S. work force was 38,270 for PMA member firms, of which 24,500 were classified as scientific or professional.18 While few companies provide training funds for predoctorates, several sponsor postdoctoral fellowships in their own research facilities. Also, the Pharmaceutical Manufacturers Association Foundation (PMAF), which is supported by dues from member firms, provides fellowships in pharmacology and related fields for postdoctoral trainees studying at academic institutions.
Biotechnology
Biotechnology is one subcategory of industrial biomedical R&D of particular importance to this committee. According to the Office of Technology Assessment (OTA), biotechnology is defined broadly as "any technique that uses living organisms (or parts of organisms) to make or modify products, to improve plants or animals, or to develop micro-organisms for specific uses."19 However, traditional biotechnology, which has been employed throughout history for improving products, such as fermentation and animal husbandry, can be referred to as "old biotechnology." With the more recent understanding of genetics, recombinant DNA, cell fusion, and novel bioprocessing techniques have become known as "new biotechnology." Although the demarcation between old and new is somewhat cloudy, the committee focused only on the latter. (It also should be noted that not all biotechnology is in the realm of biomedical science.)
The federal government is the primary source of R&D funds for biotechnology; most funds come from NIH. NIH reported that nearly 22 percent or $1.02 billion of its 1988 R&D budget was allocated to research on developing biotechnology techniques or employing the technology. The size of the NIH investment in biotechnology reflects the importance of molecular genetics in biomedicine.
The OTA conducted two surveys of biotechnology firms in 1987. Of the 296 dedicated biotechnology firms contacted in the first survey, 63 (21 percent) were involved with human therapeutics and 52 (18 percent) were conducting R&D in diagnostics. In the second survey of 53 large, diversified companies investing in biotechnology, 20 were performing R&D in human therapeutics and diagnostics. Overall; OTA estimated that, as of January 1988, 403 dedicated biotechnology firms and more than 70 major corporations were investing in biotechnology. OTA estimated further that the total investment by industry was between $1.5 and $2.0 billion per year.
The NSF surveyed corporations engaged in biotechnology research as a pilot study for future investigation of industrial R&D in emerging technologies.20 In 1986 and 1987 the NSF sent questionnaires to firms expected to spend at least $1 million annually on biotechnology R&D. A total of 54 firms responded to both surveys—a total estimated to account for half of all industrial investment in biotechnology R&D. 21 These 54 companies increased their R&D investments by 20 percent in 1985 but by only 16 and 12 percent, respectively, in 1986 and 1987. Although these firms showed a slowing rate of growth for R&D investment, their expenditures as a percent of sales continued to surpass those of industry overall. The NSF estimated that industry invested $1.4 billion in biotechnology R&D in 1987.
General Trends
From 1985 through 1987, Business Week reported both sales and R&D expenditures for 38 health care companies in its R&D scoreboard. These firms have continued to increase their rate of investment in R&D—from 12 percent in 1986 to 16 percent in 1987. These rates exceeded industrial averages by 2 percent in 1986 and 9 percent in 1987. In addition, the health care firms' ratio of R&D investment to sales surpassed the average industrial ratio by more than 4 percent.
The NSF survey estimated that the biotechnology industry spent $1.4 billion on R&D in 1987 and that pharmaceutical manufacturers invested nearly $5.4 billion in the same year.11 This suggests that industry's contribution to biomedical R&D is comparable to the total NIH budget. NIH staff members have estimated that industry is the most rapidly growing sector of health R&D and that the aggregate industrial investment in biomedical R&D has exceeded the NIH budget since 1982. In fact, the PMA has reported that the combined total R&D expenditures of its member firms exceeded the NIH budget in 1989.18
Recently, it appears that the growth of industrial investment in R&D has begun to level off. This slower growth has been attributed to mergers that force corporations to cut costs, to economic troubles in some industries, and to other pressures to show short-term profits. 14 Additionally, a reduction in tax credits for incremental increases in R&D investment may have caused some firms to trim their R&D expenditures. The NSF and PMA surveys suggest that growth of industrial investment in biomedical R&D has plateaued. This could indicate that the field of biotechnology has begun to mature or that firms engaged in biomedical R&D are not immune to the economic pressures facing all U.S. corporations.
Legislation Affecting Corporate R&D
In the past decade the federal government helped industry strengthen its associations with universities. For example, the NSF developed special research centers to foster collaboration between universities and corporations.11 In addition, the antitrust law was relaxed so as to permit companies within the same industry to form nonprofit research consortia, such as Sematech.
The 1981 Economic Recovery Tax Act provided a tax credit for incremental increases in R&D spending to foster additional investment and stimulate technology transfer. In a recent report, the General Accounting Office estimates that the tax credit stimulated between $1 billion and $2.5 billion of additional R&D between 1981 and 1985. However, the cost was estimated to be $7 billion in foregone tax revenues.22 While these costs seem high, the societal benefits derived from the research may be much higher.
The law expired in 1985 but was renewed in 1986. However, the renewal trimmed the tax credit from 25 to 20 percent of investment and added restrictions to the types of research that qualified for the credit. Also, the 1986 renewal included a 20 percent credit for industry-supported research conducted at universities and other academic institutions.
The credits, set to expire in 1988, again were extended through 1989 and although companies still could receive a 20 percent tax credit, they had to reduce the R&D expenses they deducted on their tax returns by an amount equal to half of the earned credit.23 New bills introduced into the House and Senate continue this provision. President Bush, who favors making the tax credit permanent, is supporting a provision for companies to subtract 100 percent of the tax credit value from their declared R&D expenses. The administration also would like to allow start-up companies to carry earned credits forward 15 years, for these companies generally do not earn taxable profits in their early years and therefore cannot benefit from the present law.
The Technology Transfer Act of 1986 was intended to facilitate more active collaboration between industry and federal agencies involved in R&D. Although this legislation was intended in part to respond to the steadily rising costs of health care, the legislation actually dampened enthusiasm for these collaborations between some corporations and NIH. Pharmaceutical firms are displeased particularly because of the government's insistence on imposing price controls for 10 years after development on new drugs developed cooperatively.
University-Industry Cooperation
In 1986 industry contributed approximately 5 percent to overall support for academic research. Despite increasing academic research funding from industry since then, industry investment is not expected to exceed 7 to 8 percent of university research budgets. The mechanisms of this industrial support for academic research span the spectrum, from small, unrestricted gifts and contract research to highly organized cooperative ventures.
Many issues are involved when cooperative ventures between universities (or government) and industry are established. Differences exist between the cultures of corporations and universities, the most notable being freedom of information. For instance, in-house corporate research is proprietary information, but similar secrecy and publication constraints in a university setting can threaten the very essence of university freedom. Despite these differences, however, a number of cooperative ventures have succeeded in the past decade. Reconciliation between the goals and expectations of industry and academe has been and remains crucial to their success. When successful, these cooperatives provide a unique technology transfer mechanism, one of the federal government's key policies for increasing U.S. economic competitiveness.
An example of successful industrial support of university research is the Monsanto Corporation's collaborative research effort with Washington University on the peptides and proteins that regulate cellular function and communication. Monsanto initiated the arrangement in 1982 to support research in an area in which it did not have in-house expertise. The firm provides a pool of funds for grants to Washington University faculty, with 30 percent allocated to basic research and 70 percent to projects that may result eventually in the development of commercial products. Research results are made public, and Washington University holds patents on products created by the research. Monsanto reserves both the right to view results for 30 days before submission for publication and the right of first refusal for exclusive licensing to develop products. Under this arrangement, Monsanto is expected to have provided the university with $62 million for research by 1990.16
Nonprofit Organizations
During the nineteenth and the first half of the twentieth centuries, private nonprofit foundations constituted a primary source of funds for health sciences research. Many early foundations were established to benefit particular institutions or to address specific social or health problems. These foundations' assets were derived generally from an individual's or family's gifts. During the twentieth century, voluntary health agencies, which are referred to also as operating foundations, have proliferated. Additionally, a special type of nonprofit organization—the medical research organization— has developed, such as the Howard Hughes Medical Institute.
Each of these types of organizations differs in its mission, governance, and mechanisms of support. Although these organizations comprise a limited portion of health sciences research support, they are vital to the nation's research enterprise because of their flexibility and their dedication to curing human disease and suffering. The NIH estimated that private nonprofit organizations contributed about $700 million (or about 4.3 percent of the total), to health R&D in 1988.2 However, this figure probably underestimates the role of philanthropy in health sciences research by excluding endowed professorships and donations for facilities and equipment. Another estimate has placed philanthropy at nearly one-quarter of a typical institution's budget for biomedical R&D.4
Foundations
In the early 1900s the philosophy of foundation philanthropy began to change, becoming less restrictive as broad charters were given to the boards of directors of such newly formed foundations as the Rockefeller and Russell Sage Foundations and the Carnegie Corporation of New York.4 These charters allowed the directors to focus their foundation's philanthropy in ways they believed would provide the greatest social benefit rather than at specific problems. At the same time, community foundations were beginning to form in cities around the United States. Unlike independent foundations, these community foundations relied (and continue to rely) on charitable contributions.
Tax law changes in the mid 1930s allowed corporations to deduct charitable contributions and fostered the formation of corporate foundations to serve as the primary philanthropic arm of companies. Presently, there are more than 400 company-sponsored foundations actively involved in grant support, and they provide more than $2 billion per year to all scientific areas, including the health sciences.
Since World War II, federal investment in health sciences research has eclipsed that of foundations, but foundations still play a vital role in the research enterprise, augmenting federal funding for health sciences research. However, some foundations that support health-related activities may not support research directly; rather, they support talent development or facilities. Also, some foundations that previously supported research no longer do so. Nonetheless, foundations, in general, have provided crucial support in filling gaps in the research agenda that have not been addressed appropriately or profitably by government or industry.
Currently, private foundations provide a great variety of support mechanisms for health sciences research. Few of these foundations conduct in-house research, most believing that extramural research provides the most efficient use of funds. Common types of foundation support include individual research project grants, predoctoral and postdoctoral fellowships, equipment grants, publication expenses, special library collections grants, and sponsorship of conferences or workshops. Large, independent foundations contributing to health sciences research include but are not limited to the following: the Lucille P. Markey Trust, the Pew Charitable Trusts, the Duke Endowment, the Commonwealth Fund, the Alfred P. Sloan Foundation, the John A. Hartford Foundation, the Henry J. Kaiser Family Foundation, the Robert Wood Johnson Foundation, the John D. and Catherine T. MacArthur Foundation, and the Andrew W. Mellon Foundation.4
The mechanisms for priority setting vary among foundations—company sponsored as well as independent. In some instances, funding decisions are made through personal contacts or because of interest in a specific disorder. Large, independent foundations may form advisory committees to determine areas of emphasis; proposals also may be subjected to a peer review process similar to that used by NIH. Smaller foundations may not plan program initiatives but rather may fund the best unsolicited proposals received in a given time period. The extent of foundation support for health sciences research varies from year to year, depending on the relative timing of costly initiatives. Also, company-sponsored foundations frequently restrict support in communities in which the company has operations and in programs that may affect its employees directly. Several committee members believe that corporate charity is becoming more closely tied to individual employee charitable giving, with corporate donations often matching the employee's contributions. This diminishes the size of corporate gifts to academic institutions for research purposes.
Tax laws and the economic environment affect foundation contributions to all areas, including the health sciences. Until 1969 there were few specific federal regulations pertaining to foundations. Modifications to the Internal Revenue Code in that year, however, changed the rules regarding organizations classified as private foundations by federal tax law. Included in the changes were restrictions on self-dealing and limitations on business ownership. Now, all foundations with assets exceeding $5,000 must file an annual report with the IRS, listing all of the principal officers of the foundation, its total assets and investments, and every grant made in that year.
Prior to the Tax Reform Act of 1976, a foundation's annual giving requirements were based on whichever was greater: adjusted net income or a variable percentage of the market value of investment assets. The 1976 act fixed the giving requirements at 5 percent of market value assets or net income, and it eliminated the variable percentage method. Private foundations were being charged a 4 percent excise tax on their net investment until 1978, when the law reduced the tax rate to 2 percent. The Economic Recovery Tax Act of 1981 changed the giving requirements again to equal a flat 5 percent of market value of assets per year. These tax law changes have contributed to the growth of foundation giving in recent years.
Since giving requirements are tied directly to the market value of foundation assets, the economy has a significant effect on total giving. In periods of high inflation, such as that experienced in the late 1970s, foundations actually lost assets when measured in constant dollars. However, the bull markets and low inflation rates of the 1980s helped increase the value of foundation assets and subsequently increased contributions to health research.
Voluntary Health Agencies
Voluntary health agencies (often referred to as operating foundations) are private charities supported primarily by public donations. There are now perhaps as many as 200 national and regional organizations actively supporting health research. Many of these organizations were founded by the families and friends of individuals suffering from a particular disease.
These voluntary health agencies, such as the American Cancer Society and the American Heart Association, play critically important roles in advancing their areas of interest. With activities that include public awareness and education, patient referrals, continuing education for health professionals, grants for research and training, and lobbying to increase federal funding for disease-specific research. However, it should be noted that not all disease-specific organizations support research, and of those that do, most do not conduct in-house research.
The six largest voluntary health agencies (in terms of revenues) are, in descending order, the American Cancer Society, the American Heart Association, the March of Dimes-Birth Defects Foundation, the Muscular Dystrophy Association, the National Easter Seal Society, and the American Lung Association. These six organizations reported combined expenditures for disease-related research of more than $250 million in 1988.* Since these organizations rely on voluntary contributions, they are not able to make long-term commitments to research efforts. However, they are effective in responding rapidly to new research initiatives and in providing resources to scientists to develop new lines of investigation.
The voluntary health agencies also can play a very critical role in the early stages of many individuals' scientific career development. Through funding mechanisms such as fellowships and career development awards, these organizations attract young researchers to a specific field and provide them with research funding before they are able to compete successfully for federal support. Grant awards from these organizations commonly range between $20,000 and $50,000.
Voluntary health agencies also act as lobbyists for increases in disease-specific funds for NIH. These organizations increase public awareness of the need to fight particular diseases and solicit grass-roots support for more federal research funds, and they also have been very influential in establishing new institutes at NIH.
Medical Research Organizations
Medical research organizations (MRO), such as the Howard Hughes Medical Institute (HHMI) and the J. David Gladstone Foundation Laboratories for Cardiovascular Disease, conduct medical research in conjunction with hospitals. By law, these types of organizations must spend 3.5 percent of their endowments on medical research annually. The Gladstone Foundation is a relatively small medical research organization with assets estimated at $118 million and is affiliated with the University of California at San Francisco.
On the other extreme, the largest MRO is HHMI with assets in excess of $6 billion. In recent years HHMI has become the largest single private nonprofit contributor to biomedical research. Currently, HHMI's total investment in biomedical research is comparable to the budget of a small institute within NIH, with expenditures totaling $238.4 million in 1989. The trustees have designed the institute's program to complement NIH activities within a few selected areas of research: cell biology and regulation, genetics, immunology, neuroscience, and structural biology. A 10 member medical advisory board has ultimate responsibility for the quality of the research program, whereas scientific review boards composed of scientists in each of the five areas oversee work in their respective fields. Although the institute is sufficiently large to make a major contribution, it does not seek to replace the central role of NIH in any field.
HHMI traditionally has established large laboratories with a core group of investigators in universities and hospitals around the United States to facilitate interaction with the larger research community. Investigators are appointed for fixed terms of 3 to 7 years, with full funding provided for faculty and technician salaries as well as research expenses. Investigator productivity is evaluated through research conferences, annual progress reports, and site visits.
By mid 1988 HHMI employed approximately 180 investigators and a 1,350-member support staff in 30 sites. In order to expand the number of host institutions, HHMI recently began to support individual investigators rather than multi-investigator laboratories. The institute plans to support approximately 250 investigators and 2,000 support staff in at least 40 sites within a few years.
HHMI has undertaken a broad program to strengthen science education from the precollege to the postdoctoral stages. The Institute is funding a study by the Commission of Life Sciences of the National Academy of Sciences that is examining the curricula and teaching of high school biology. The new HHMI Undergraduate Science Education Program awards grants to strengthen science education and research in private undergraduate colleges. Begun in 1988, the program is intended to increase the number of students, especially minorities and women, pursuing careers in the biomedical sciences. In 1988 HHMI awarded $30.4 million to 44 colleges, including 10 historically black colleges. Expansion of this program in 1989 granted $61 million to 51 undergraduate colleges affiliated with research universities and other doctorate-granting institutions.
The graduate science education program funds several levels of graduate training. For instance, doctoral fellowships in the biological sciences (60 per year) provide predoctoral students with a stipend and cost-of-education allowance for 3 to 5 years; medical Student Research Training Fellowships (up to 60 per year) are modeled after HHMI's Research Scholars Program, supporting students for a year of research training at any U.S. academic or research institution. The Research Resources Program funds development of institutional infrastructures related to graduate research and education. The resources program may provide support in the following areas: courses and symposia concerned directly with HHMI areas of interest, replenishment of biological stocks and materials, and genetic analysis projects that complement the HHMI human genome data base.
Summary and Conclusions
The committee concluded that health research is supported by a diverse, yet interlocking network of federal agencies, industry, and private nonprofit organizations. Of these, the federal government is the single largest sponsor of health research in the U.S. Of the $71 billion the federal government will invest in R&D during fiscal year 1991, nearly $10 billion will be health related. Contributions by health-oriented corporations are roughly equal in magnitude, but devoted largely to product application developments rather than fundamental discovery research. Contributions by private nonprofit sponsors favor fundamental discovery research, generally in somewhat restricted fields of interest, but represent only about 4 to 5 percent of the total U.S. investment in health research.
In light of this investment and the continuing budget limitations, the scientific community must reexamine its resource base to improve its effectiveness and efficiency. Federally sponsored health research by the various agencies is generally mission oriented. NIH and ADAMHA are the primary agencies that disburse federal health research funds for investigation into fundamental biological discovery, but the committee emphasizes that all health research expands the boundaries of knowledge.
Although industry has been playing an increasingly important role in health research, focusing primarily on product development, it relies heavily on university research programs for basic scientific knowledge and talent. Cooperative ventures between universities (or government) and industry provide a unique mechanism for sharing knowledge and technology transfer, a central policy of the federal government for increasing U.S. economic competitiveness.
Foundations, voluntary health agencies, and other nonprofit organizations have played a very important role in sponsoring health research. The committee believes that these organizations have been particularly helpful in providing crucial support in filling gaps in the nation's research agenda and sponsoring new initiatives. Although the federal government rapidly eclipsed the investment by these organizations following World War II, they have continued to supply a steady stream of research dollars. These funds are used for individual research projects, supporting career development awards in specific research fields, equipment, facilities, and various programs of knowledge dissemination. The committee anticipates that these organizations will continue to provide support for the health sciences.
References
- 1.
- National Science Foundation. 1989. Science and Technology Resources: Funding and Personnel. Publication No. 89-300. Washington, D.C.
- 2.
- U.S. Department of Health and Human Services; Public Health Service. 1989. NIH Data Book 1989. Publication No. 89-1261. Bethesda, Md.: National Institutes of Health.
- 3.
- Ginzberg, E. and A.B. Dutka. 1989. The Financing of Biomedical Research. Baltimore: The Johns Hopkins University Press.
- 4.
- Boniface, Z.E. and R.W. Rimel. 1987. U.S. Funding of Biomedical Research. Philadelphia: The Pew Charitable Trusts.
- 5.
- Bush, V. 1945. Science-The Endless Frontier, A Report to the President on a Program for Postwar Scientific Research. Washington, D.C.: Office of Scientific Research and Development. (Reprinted by the National Science Foundation, May 1980.)
- 6.
- U.S. Department of Health and Human Services; Public Health Service. 1986. DRG Peer Review Trends; Member Characteristics: DGR Study Sections, Institute Review Groups, Advisory Councils and Boards, 1976-1986. Bethesda, Md.: National Institutes of Health.
- 7.
- U.S. Department of Health and Human Services; Public Health Service. 1989. NIH Almanac. Publication No. 89-5. Bethesda, Md.: National Institutes of Health.
- 8.
- Strickland, S.P. 197Z Politics, Science, and Dread Disease: A Short History of United States Medical Research Policy. Cambridge, Mass.: Harvard University Press.
- 9.
- Seggel, R. L. 1985. Stabilizing the Funding of NIH and ADAMHA Research Project Grants. Washington, D.C.: National Academy Press.
- 10.
- U.S. Department of Health and Human Services; Public Health Service. 1989. ADAMHA Data Source Book 1988. Rockville, Md.: Alcohol, Drug Abuse, and Mental Health Administration.
- 11.
- U.S. Congress; Office of Technology Assessment. 1988. New Developments in Biotechnology: U.S. Investment in Biotechnology-Special Report. OTA-BA-360. Washington, D.C.: U.S. Government Printing Office.
- 12.
- Institute of Medicine. 1989. Government and Industry Collaboration in Biomedical Research and Education. Washington, D.C.: National Academy Press.
- 13.
- National Science Foundation. 1987. Report on Funding Trends and Balance of Activities: National Science Foundation 1951-1988. NSF 88-3. Washington, D.C.
- 14.
- U.S. House of Representatives. 1989. Report of the House of Representatives Appropriations Subcommittee for the Departments of Labor, Health and Human Services, and Education, and Related Agencies Appropriations Bill, 1989, Report No. 100-689. Washington, D.C.
- 15.
- Boniface, Z.E. U.S. Funding for Biomedical Research: An Update. Background paper prepared for this study committee.
- 16.
- National Academy of Sciences; Government-University-Industry Research Roundtable. 1986. New Alliances and Partnerships in American Science and Engineering. Washington, D.C.: National Academy Press.
- 17.
- National Science Foundation. 1988. The Science and Technology Resources of Japan: A Comparison with the United States. NSF 88-318. Washington, D.C.
- 18.
- Pharmaceutical Manufacturers Association. 1989. Annual Survey Report of the U.S. Pharmaceutical Industry, 1987-89. Washington, D.C.
- 19.
- U.S. Congress; Office of Technology Assessment. 1984. Commercial Biotechnology: An International Analysis. OTA-BA-218. Washington, D.C.
- 20.
- National Science Foundation. 1987. Biotechnology Research and Development Activities in Industry: 1984 and 1985. NSF 87-311. Washington, D.C.
- 21.
- National Science Foundation. 1988. Science Resource Highlights: Industrial Biotechnology R&D Increased an Estimated 12 Percent in 1987 to $1.4 Billion. NSF 88-306. Washington, D.C.
- 22.
- U.S. General Accounting Office. 1989. The Research Tax Credit Has Stimulated Some Additional Research Spending. Report number GAO/GGD-89414. Washington, D.C.
- 23.
Footnotes
- *
All constant dollar figures in this text use the biomedical R&D price index developed by the Commerce Department for NIH. Although there are minor differences between the deflators for the intramural and extramural indices, only the combined deflator is used for all calculations.
- *
There is a discrepancy between the NIOSH appropriations for research in the conference report from Congress ($60.5 million for fiscal year 1989) and the information received directly from CDC, which reported only $24.7 million.
- *
Figures were obtained from 1988 annual reports.
- PubMedLinks to PubMed
- Funding for Health Sciences Research - Funding Health Sciences ResearchFunding for Health Sciences Research - Funding Health Sciences Research
- Chain A, Caspase-3Chain A, Caspase-3gi|704360675|pdb|4QTX|AProtein
- Gm12936 predicted gene 12936 [Mus musculus]Gm12936 predicted gene 12936 [Mus musculus]Gene ID:102635893Gene
- DLGAP2-AS1 DLGAP2 antisense RNA 1 [Homo sapiens]DLGAP2-AS1 DLGAP2 antisense RNA 1 [Homo sapiens]Gene ID:100507435Gene
- Gm13889 predicted gene 13889 [Mus musculus]Gm13889 predicted gene 13889 [Mus musculus]Gene ID:620695Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...