NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
| Chemical name: | 16α-[18F]Fluoro-17β-estradiol |
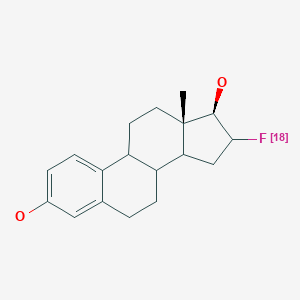
|
| Abbreviated name: | [18F]FES | |
| Synonym: | 16-Fluoroestradiol, fluoroestradiol | |
| Agent category: | Compound | |
| Target: | Estrogen receptor | |
| Target category: | Receptor | |
| Method of detection: | PET | |
| Source of signal: | 18F | |
| Activation: | No | |
| Studies: |
| Click on the above structure for additional information in PubChem. |
Background
[PubMed]
Estrogens and progestins are endogenous hormones that produce many physiological effects. Estrogens act primarily by regulating gene expression. Estrogen receptors are found in the cell nucleus of the female reproductive tract, breast, pituitary, hypothalamus, bone, liver, and other tissues, and also in various tissues in men. Estrogens are lipophilic that they enter the cell passively by diffusion through the cellular membrane. They bind to estrogen receptors that are present in the nucleus.
Breast cancer is the most common malignancy in women. About 33% of women who have this disease will die of disseminated breast cancer. The growth of breast epithelial cells is dependent on estrogen stimulation to induce progestin receptor expression. Two-thirds of breast carcinomas express ERs. It has also been established that the ER status of the tumor is an important prognostic indicator in breast cancer (1). Women with ER-positive breast tumors have a better prognosis than women with ER-negative tumors in terms of responsiveness to anti-estrogen treatment. ER content in breast cancer was assessed by receptor binding assays, which suffer from inter-assay variability and are also limited by intrinsic receptor heterogeneity of the tumor. 16α-[18F]Fluoro-17β-estradiol ([18F]FES) was proven to be a valuable tracer for the studies of the ER status of primary and metastatic breast cancer (2).
Synthesis
[PubMed]
[18F]FES was first synthesized by nucleophilic displacement of the aliphatic triflate of 3,16β-bis(trifluoromethenesulfonyloxy)estrone using tetrabutylammonium [18F]fluoride, followed by hydrolysis and ketone reduction. High-performance liquid chromatography (HPLC) purification provided a 30% radiochemical yield of [18F]FES in about 90 min (3). On the basis of this method, an automated robotic synthesis was developed to give an overall yield of 6% in 80 min (4). A one-pot synthesis of [18F]FES was synthesized by nucleophilic fluorination of 3-methoxymethyl-16β,17β-epiestriol-O-cyclic sulfone. [18F]FES was isolated to provide radiochemical yields of 30-45% with a specific activity of about 37 GBq/µmol (1 Ci/µmol) in 60-120 min (5). Automated modules were developed for the synthesis of [18F]FES to give ~50% radiochemical yields in 50-80 min (6, 7). Recently, automated synthesis procedure has been developed for clinical large-scale production of [18F]FES in good yields (15-35%) with high radiochemical purity (>99%) (8).
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
ER binding affinity was assessed by competitive binding with [3H]estradiol in MCF-7 cells. The binding affinity of FES was about 57% of estradiol (9). FES showed little binding to sex hormone-binding globulin as compared to estradiol.
Animal Studies
Rodents
[PubMed]
It was demonstrated that [18F]FES uptake by target tissues (uterus and ovary) in immature female rats is highly specific (10). The uptake in liver and kidneys was lower than the uterus and nonspecific. There was also a significant depression of [18F]FES uptake by the target tissues after pretreatment with the anti-estrogen, tamoxifen. [18F]FES uptake by rat 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast tumors correlated well with the ER concentration (11). In another study, it was found that metabolism of [18F]FES was rapid in rats bearing DMBA-induced breast tumors within 1-2 h after injection. Most of the radioactivity in the blood and non-target tissues was [18F]FES metabolites, which did not bind to the target tissues and breast tumors (12).
Different animal models of estrogen-positive tumors using [18F]FES were evaluated for their suitability to follow tumor response after various treatment protocols, using small-animal positron emission tomography (PET) (13). ER-positive human breast cancer cell lines MCF-7 and T-47D and murine mammary carcinomas MC4-L2, MC4-L3, and MC7-L1 were compared for their in vivo growth rate and retention of ER-positive status. Tumor metabolic activity was estimated from [18F]fluorodeoxyglucose ([18F]FDG) uptake, whereas ER content was determined from [18F]FES uptake. The human cell lines grew at a slower rate in nude mice and failed to accumulate [18F]FES; in contrast, the MC7-L1 and MC4-L2 carcinomas grew well in mice and showed good uptake of both [18F]FDG and [18F]FES. Chemotherapy and hormone therapy delayed the growth of MC7-L1 and MC4-L2 tumors, confirming their suitability as an ER-positive model for therapeutic interventions. The murine MC7-L1 and MC4-L2 tumors are suitable models for the monitoring of ER-positive breast cancer therapy using small-animal PET imaging.
Human Studies
[PubMed]
Thirteen patients with primary breast lesions were studied with [18F]FES PET (14). PET images demonstrated uptake of the tracer at sites of primary tumors and in several axillary lymph node metastases, as well as in one distant metastatic lesion. There was an excellent correlation between uptake within the primary tumor measured on the PET images and the tumor ER concentration measured by in vitro receptor binding assays after excision (r = 0.96). In a study with 15 breast cancer patients (15), [18F]FES was cleared from the blood and metabolized in 20 min with only 20% of intact [18F]FES, most of which was bound to plasma proteins. Liver uptake of [18F]FES was rapid, and [18F]FES metabolites appeared in the blood as early as 5 min. The major, labeled metabolites in the blood and urine were sulfate and glucuronidate conjugates of [18F]FES, as measured by HPLC.
Human dosimetry of [18F]FES was determined from blood samples and PET images in 49 patients after intravenous injection of [18F]FES (16).The effective dose equivalent was 0.022 mSv/MBq (80 mrem/mCi). The organ that received the highest dose was the liver (0.13 mGy/MBq (470 mrad/mCi)), followed by the gallbladder (0.10 mGy/MBq (380 mrad/mCi)) and the urinary bladder (0.05 mGy/MBq (190 mrad/mCi)).
Uptake of [18F]FES in a series of patients with primary breast lesions and recurrent/metastatic breast tumors was compared with in vitro ER content and tumor uptake of [18F]FDG (17). There was no correlation between tumor ER status and [18F]FDG uptake or between tumor [18F]FES and [18F]FDG uptake in these patients. However, the [18F]FES PET assessments were in agreement with the in vitro ER binding assays. Anti-estrogen therapy decreased [18F]FES uptake in patients with ER-positive lesions (18), and patients with high initial uptake of [18F]FES were more likely to respond favorably to hormonal therapy (19). Peterson et al. (20) studied seventeen patients with primary or metastatic breast cancer with dynamic [18F]FES PET. For each tumor, partial-volume-corrected standardized uptake values (SUVs) of [18F]FES uptake were compared with ER expression measured by 3 different ER scoring methods: qualitative scoring, the Allred score , and a computerized immunohistochemistry (IHC) index. There was excellent agreement (r = 0.99) between observers using IHC as well as the different methods of measuring ER content (P < 0.001) with the best correlation being between the IHC index and [18F]FES SUVs. Tumor imaging with [18F]FES PET is useful in the determination of ER status and prognosis of therapy in breast cancer patients [PubMed].
NIH Support
CA25836, CA42045, CA48286, CA72064, CA72964, DK15556, HL13851, RR17229
References
- 1.
- Vollenweider-Zerargui L., Barrelet L., Wong Y., Lemarchand-Beraud T., Gomez F. The predictive value of estrogen and progesterone receptors' concentrations on the clinical behavior of breast cancer in women. Clinical correlation on 547 patients. Cancer. 1986;57(6):1171–80. [PubMed: 3943040]
- 2.
- Flanagan F.L., Dehdashti F., Siegel B.A. PET in breast cancer. Semin Nucl Med. 1998;28(4):290–302. [PubMed: 9800236]
- 3.
- Kiesewetter D.O., Kilbourn M.R., Landvatter S.W., Heiman D.F., Katzenellenbogen J.A., Welch M.J. Preparation of four fluorine- 18-labeled estrogens and their selective uptakes in target tissues of immature rats. J Nucl Med. 1984;25(11):1212–21. [PubMed: 6092569]
- 4.
- Brodack J.W., Kilbourn M.R., Welch M.J., Katzenellenbogen J.A. Application of robotics to radiopharmaceutical preparation: controlled synthesis of fluorine-18 16 alpha-fluoroestradiol-17 beta. J Nucl Med. 1986;27(5):714–21. [PubMed: 3486960]
- 5.
- Lim J.L., Zheng L., Berridge M.S., Tewson T.J. The use of 3-methoxymethyl-16 beta, 17 beta-epiestriol-O-cyclic sulfone as the precursor in the synthesis of F-18 16 alpha-fluoroestradiol. Nucl Med Biol. 1996;23(7):911–5. [PubMed: 8971859]
- 6.
- Oh S.J., Chi D.Y., Mosdzianowski C., Kil H.S., Ryu J.S., Moon D.H. The automatic production of 16alpha-[18F]fluoroestradiol using a conventional [18F]FDG module with a disposable cassette system. Appl Radiat Isot. 2007;65(6):676–81. [PubMed: 16963265]
- 7.
- Romer J., Fuchtner F., Steinbach J., Kasch H. Automated synthesis of 16alpha-[18F]fluoroestradiol-3,17beta-disulphamate. Appl Radiat Isot. 2001;55(5):631–9. [PubMed: 11573796]
- 8.
- Kumar P., Mercer J., Doerkson C., Tonkin K., McEwan A.J. Clinical production, stability studies and PET imaging with 16-alpha-[18F]fluoroestradiol ([18F]FES) in ER positive breast cancer patients. J Pharm Pharm Sci. 2007;10(2):256s–265s. [PubMed: 17718929]
- 9.
- Seimbille Y., Rousseau J., Benard F., Morin C., Ali H., Avvakumov G., Hammond G.L., van Lier J.E. 18F-labeled difluoroestradiols: preparation and preclinical evaluation as estrogen receptor-binding radiopharmaceuticals. Steroids. 2002;67(9):765–75. [PubMed: 12123788]
- 10.
- Katzenellenbogen J.A., Mathias C.J., VanBrocklin H.F., Brodack J.W., Welch M.J. Titration of the in vivo uptake of 16 alpha-[18F]fluoroestradiol by target tissues in the rat: competition by tamoxifen, and implications for quantitating estrogen receptors in vivo and the use of animal models in receptor-binding radiopharmaceutical development. Nucl Med Biol. 1993;20(6):735–45. [PubMed: 8401374]
- 11.
- Sasaki M., Fukumura T., Kuwabara Y., Yoshida T., Nakagawa M., Ichiya Y., Masuda K. Biodistribution and breast tumor uptake of 16alpha-[18F]-fluoro-17beta-estradiol in rat. Ann Nucl Med. 2000;14(2):127–30. [PubMed: 10830531]
- 12.
- Mathias C.J., Welch M.J., Katzenellenbogen J.A., Brodack J.W., Kilbourn M.R., Carlson K.E., Kiesewetter D.O. Characterization of the uptake of 16 alpha-([18F]fluoro)-17 beta-estradiol in DMBA-induced mammary tumors. Int J Rad Appl Instrum B. 1987;14(1):15–25. [PubMed: 3108199]
- 13.
- Aliaga A., Rousseau J.A., Ouellette R., Cadorette J., van Lier J.E., Lecomte R., Benard F. Breast cancer models to study the expression of estrogen receptors with small animal PET imaging. Nucl Med Biol. 2004;31(6):761–70. [PubMed: 15246367]
- 14.
- Mintun M.A., Welch M.J., Siegel B.A., Mathias C.J., Brodack J.W., McGuire A.H., Katzenellenbogen J.A. Breast cancer: PET imaging of estrogen receptors. Radiology. 1988;169(1):45–8. [PubMed: 3262228]
- 15.
- Mankoff D.A., Tewson T.J., Eary J.F. Analysis of blood clearance and labeled metabolites for the estrogen receptor tracer [F-18]-16 alpha-fluoroestradiol (FES). Nucl Med Biol. 1997;24(4):341–8. [PubMed: 9257333]
- 16.
- Mankoff D.A., Peterson L.M., Tewson T.J., Link J.M., Gralow J.R., Graham M.M., Krohn K.A. [18F]fluoroestradiol radiation dosimetry in human PET studies. J Nucl Med. 2001;42(4):679–84. [PubMed: 11337559]
- 17.
- Dehdashti F., Mortimer J.E., Siegel B.A., Griffeth L.K., Bonasera T.J., Fusselman M.J., Detert D.D., Cutler P.D., Katzenellenbogen J.A., Welch M.J. Positron tomographic assessment of estrogen receptors in breast cancer: comparison with FDG-PET and in vitro receptor assays. J Nucl Med. 1995;36(10):1766–74. [PubMed: 7562040]
- 18.
- McGuire A.H., Dehdashti F., Siegel B.A., Lyss A.P., Brodack J.W., Mathias C.J., Mintun M.A., Katzenellenbogen J.A., Welch M.J. Positron tomographic assessment of 16 alpha-[18F] fluoro-17 beta-estradiol uptake in metastatic breast carcinoma. J Nucl Med. 1991;32(8):1526–31. [PubMed: 1869973]
- 19.
- Dehdashti F., Flanagan F.L., Mortimer J.E., Katzenellenbogen J.A., Welch M.J., Siegel B.A. Positron emission tomographic assessment of "metabolic flare" to predict response of metastatic breast cancer to antiestrogen therapy. Eur J Nucl Med. 1999;26(1):51–6. [PubMed: 9933662]
- 20.
- Peterson L.M., Mankoff D.A., Lawton T., Yagle K., Schubert E.K., Stekhova S., Gown A., Link J.M., Tewson T., Krohn K.A. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J Nucl Med. 2008;49(3):367–74. [PubMed: 18287268]
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review 4,16α-[16α-(18)F]Difluoro-11β-methoxyestradiol.[Molecular Imaging and Contrast...]Review 4,16α-[16α-(18)F]Difluoro-11β-methoxyestradiol.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review [(18)F]Fluoropropyl-Tanaproget.[Molecular Imaging and Contrast...]Review [(18)F]Fluoropropyl-Tanaproget.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (99m)Tc-(3Z)-4-{4-[2-(Dimethylamino)ethoxy]phenyl}-3,4-diphenylbut-3-en-1-ylN,N-bis[2-(2,6-dioxomorpholin-4-yl)ethyl]glycinate.[Molecular Imaging and Contrast...]Review (99m)Tc-(3Z)-4-{4-[2-(Dimethylamino)ethoxy]phenyl}-3,4-diphenylbut-3-en-1-ylN,N-bis[2-(2,6-dioxomorpholin-4-yl)ethyl]glycinate.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review Gadolinium-Hexyl-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid-progesterone.[Molecular Imaging and Contrast...]Review Gadolinium-Hexyl-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid-progesterone.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review Whole-Body Characterization of Estrogen Receptor Status in Metastatic Breast Cancer with 16α-18F-Fluoro-17β-Estradiol Positron Emission Tomography: Meta-Analysis and Recommendations for Integration into Clinical Applications.[Oncologist. 2020]Review Whole-Body Characterization of Estrogen Receptor Status in Metastatic Breast Cancer with 16α-18F-Fluoro-17β-Estradiol Positron Emission Tomography: Meta-Analysis and Recommendations for Integration into Clinical Applications.Kurland BF, Wiggins JR, Coche A, Fontan C, Bouvet Y, Webner P, Divgi C, Linden HM. Oncologist. 2020 Oct; 25(10):835-844. Epub 2020 May 15.
- 16α-[18F]Fluoro-17β-estradiol - Molecular Imaging and Contrast Agent Database (M...16α-[18F]Fluoro-17β-estradiol - Molecular Imaging and Contrast Agent Database (MICAD)
Your browsing activity is empty.
Activity recording is turned off.
See more...

 In vitro
In vitro