NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Ashley EA, Niebauer J. Cardiology Explained. London: Remedica; 2004.
Introduction
Arrhythmia is an area of cardiology often feared by generalists. This might be related to the inconsistent terminology used in different centers and countries; or perhaps because the most basic tool of diagnosis, the electrocardiogram (ECG), can at times seem the most esoteric. Whatever the reason, few feel completely comfortable when confronted with a patient with ongoing arrhythmia. Despite this, arrhythmia can be simply managed by asking the following question: is the patient compromised? This is the first and single most important question in the management of your patient. The answer will guide your next steps.
If the patient is not compromised – no pain, no dyspnea, normal blood pressure, and fully alert – you have some time. Take a short history and examination, get the patient monitored (preferably), and acquire a 12-lead ECG. The findings will help to make a diagnosis that will guide treatment. If a 12-lead ECG is not available, immediately refer the patient to somewhere that it can be performed.
If the patient is compromised – with pain, dyspnea, hypotension, and light headedness – this is an emergency and the patient needs an intravenous (IV) cannula inserted and a defibrillator brought in immediately. Treatment then depends on the diagnosis (see Table 1).
Table 1
Possible diagnoses of an arrhythmic patient.
Clinical examination can help, whether an ECG is available or not. The key thing to remember is that there are few components in the electrical "wiring" of the heart (see Figure 1) and the pulse can only be fast or slow, regular or irregular. If an ECG is available, is the QRS complex narrow or broad? Broad (>0.12 seconds) is more likely to be ventricular tachycardia (VT) and will generally be more concerning than narrow, which suggests a high depolarization site and is likely to be supraventricular tachycardia (SVT).

Figure 1
A diagrammatic representation of the electrical "wiring" of the heart. AV: atrioventricular; SA: sinoatrial. Reproduced with permission from Elsevier Science (Hampton J. The ECG Made Easy. Churchill Livingstone, 2003).
Bradycardia
Bradycardia is defined as heart rate <60 bpm, regardless of the cause. However, this is not a particularly helpful definition as it is well known that elite athletes can have "normal" heart rates as low as 30 bpm. In general, bradycardia needs attention if it is associated with symptoms, hemodynamic compromise, or is the substrate for escape rhythms. Symptoms associated with bradycardia include shortness of breath, fatigue, lethargy, nausea, mental confusion, dizziness, and presyncope or syncope. If there is hemodynamic compromise and interruption of cerebral perfusion is prolonged, grand mal seizures may result (Stokes–Adams attacks). The causes of bradycardia are outlined in Table 2.
Table 2
Causes of bradycardia.
Treatment
Acute bradycardia only requires treatment if it is associated with hemodynamic compromise or dangerous escape rhythms. This will most often be the case in the setting of inferior myocardial infarction (MI), but check the temperature! IV atropine at a dose of 500 μg can be administered and repeated up to a maximum cumulative dose of 3 mg. If this fails, there are two options.
- External pacing. This is a temporizing measure only. Most defibrillators have detachable modules to facilitate this. Attach electrode gels anteriorly and posteriorly and pace at 80 bpm. The procedure is distressing for the conscious patient and should be used with sedative analgesia.
- IV isoproterenol (isoprenaline) infusion. If the parasympathetic system is entirely blocked, the only alternative is to stimulate the sympathetic pathway. However, this is proarrhythmic and not without risk.
Sinus node dysfunction
Sinus node dysfunction (SND) is also known as tachycardia–bradycardia syndrome and sick sinus syndrome. These terms encompass a spectrum of disorders of cardiac conduction tissue that are not necessarily confined to the sinus node (eg, atrial conducting tissue and even the atrioventricular [AV] node). The cause is not entirely clear, but inflammation, degeneration, and fibrosis of the conducting tissue are characteristic. SND is most common in the elderly.
The spectrum of associated arrhythmias is diverse, but usually includes inappropriate sinus bradycardia, sinus pauses (>3 seconds) with junctional escape, sinus arrest (asystolic pause), atrial tachycardia, atrial flutter, and atrial fibrillation (AF). Commonly, there is alternating bradycardia–tachycardia with a normal sinus rate or bradycardia between attacks. Hence, the diagnostic landmarks are palpitations (tachycardia), dizziness, and syncope (bradycardia), although the disease is often asymptomatic.
SND is diagnosed by a 12-lead ECG and Holter monitoring. Although there is no evidence of mortality benefit (SND does not seem to cause fatal asystolic arrest), consensus exists that a pacemaker (AAI, DDD – see p.135 for a description of the pacemaker codes) should be implanted if there is a clear relationship between bradycardia and cerebral symptoms in the absence of drugs – a diagnosis that may be difficult to secure. In addition, a pacemaker may be considered (AAI, DDD) when bradycardia is secondary to drug therapy necessary to limit tachycardia.
Carotid sinus syndrome
This term refers to hypersensitivity of the baroreceptor reflex, leading to bradycardia and hypotension. It is usually caused when pressure is applied to the neck around the area of the carotid sinus, eg, when shaving or by wearing a tight shirt collar. It can be predominantly cardioinhibitory (bradycardia, AV block) or vasodepressive (hypotension). Diagnosis is by ECG monitoring during carotid sinus massage. Patients with >3 seconds of asystole benefit from permanent dual-chamber pacing (DDI). Single-lead atrial pacing is contraindicated because it offers no protection against AV block. In patients with predominant vasodepressor syncope or mixed forms of the condition, pacing only prevents symptoms due to asystole or bradyarrhythmia; it does not prevent neurologic symptoms due to reflex hypotension.
Syncope
Syncope is defined as a sudden transient loss of consciousness and postural tone with spontaneous recovery. A careful diagnostic evaluation, though often difficult, is imperative in all patients. A vast variety of conditions may result in syncope, including any condition causing relative cerebral hypoxia for ≥10 seconds. It occurs as a result of low cardiac output and can be a consequence of mechanical, rhythm, and vascular disturbances, and noncardiac causes (see Table 3).
Table 3
Disturbances that can lead to syncope.
It should be kept in mind that generalized tonic/clonic movements can be the result of syncope, rather than the cause of it.
Evaluation
It can be difficult to identify the cause of syncope. Initial clinical evaluation should include supine and erect blood pressures and heart rate measurements. Blood glucose, serum electrolytes, hematocrit, and drug levels (if appropriate) should also be obtained. Assessment of a syncopal event requires information from both the patient and a witness; the pattern can be helpful (see Table 4). A baseline 12-lead ECG may suggest possible causes such as ischemia, Wolff–Parkinson–White (WPW) syndrome, or a prolonged QT interval. In most cases, it will be necessary to refer the patient for additional testing (see Figure 2). Additional testing may include the following.
Table 4
Patterns of syncope.
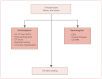
Figure 2
Evaluation of syncope. CT: computed tomography; ECG: electrocardiogram; EEG: electroencephalogram; EP: electrophysiology; MRI: magnetic resonance imaging.
- Documentation of a symptomatic arrhythmia may be achieved by Holter monitoring. However, if syncope is infrequent, event-recorder monitoring may be more helpful. These devices allow patients to turn the device on when the episode begins (or ends, facilitated by the use of a buffer), and thus record over a much greater period of time.
- An echocardiogram should be used to detect major structural abnormalities potentially associated with syncope (aortic stenosis, hypertrophic cardiomyopathy, ischemia). Carotid sinus massage should be performed if bruits are absent.
- Before invasive electrophysiological testing, coronary artery disease should be excluded or treated. Electrophysiological testing is indicated whenever an arrhythmia is considered as a probable cause of syncope.
- Exercise testing may contribute to establishing a basis for syncope by revealing myocardial ischemia (substrate for ventricular tachyarrhythmias), catecholamine-sensitive tachycardias, exercise-induced AV block, and chronotropic incompetence (SND).
Malignant vasovagal syncope
In malignant vasovagal syncope, the sympathetic system is activated via a trigger, usually venous pooling, which forces vigorous contraction of a poorly filled ventricle. This stimulates the mechanoreceptors in the ventricular wall, which lead (via C fibers and the brainstem) to overactivation of the parasympathetic system, and thus bradycardia and hypotension. This reflex is named Bezold–Jarisch after those who first described it (see Figure 3).
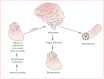
Figure 3
The Bezold–Jarisch reflex.
Tilt-table testing
If malignant vasovagal syncope is suspected, the key investigation is upright tilt-table testing. This test is performed on a specially designed table with the patient initially in a supine position. He/she is then tilted upright to a maximum of 60°–80° in such a way as to avoid recruiting the postural muscles. This maximizes venous pooling. The patient is left in this position for up to 40 minutes.
The normal tilt-table response is a baroreceptor-mediated decrease in inhibitory drive to the vasomotor center, ie, vasoconstriction and an increase in heart rate and ventricular contractility. The test result is considered abnormal when symptomatic hypotension is reproduced (Bezold–Jarisch reflex).
Treatment
Therapy to prevent recurrent vasovagal syncope has included the use of β-blockers and vagolytics (eg, disopyramide).
Atrioventricular block
AV block occurs when the electrical impulse from the atria to the ventricles is delayed or blocked.
First-degree AV block
This is where there is a prolonged PR interval of >200 milliseconds (5 small squares; see Figure 4). No specific therapy is required and the prognosis is excellent. However, it can be a marker for an underlying problem such as myocarditis, MI, degenerative disease, or, most commonly, a drug effect (eg, tricyclic antidepressants).
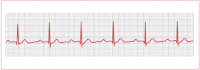
Figure 4
Electrocardiogram of first-degree atrioventricular block.
Second-degree AV block
This is divided into two types:
- type I (Mobitz I or Wenckebach AV block)
- type II (Mobitz II AV block)
Type I occurs when there is a repeated pattern of progressive prolongation of the PR interval, which eventually results in the failure of conduction of one atrial beat (see Figure 5). The cause is usually benign, but it can be a marker for the same underlying cardiac problems as first-degree AV block. In most cases, treatment is unnecessary. Routine prophylactic permanent pacing is not recommended unless the patient is symptomatic with presyncope, recurrent syncope, or bradycardia that exacerbates congestive heart failure or angina.

Figure 5
Electrocardiogram of type I second-degree atrioventricular block (Wenckebach).
In type II, most beats are conducted with a constant PR interval, but occasionally atrial depolarization is not followed by ventricular depolarization (see Figure 6). Type II is pathological and indicates disease of the conduction system distal to the AV node. It can frequently lead to complete AV block, causing Stokes–Adams attacks. Therefore, temporary and then permanent pacing (DDD) is indicated in most patients, even those who initially present without symptoms.

Figure 6
Electrocardiogram of type II second-degree atrioventricular block (Mobitz II).
Third-degree AV block (complete heart block)
With complete heart block, there is complete dissociation of the P waves and QRS complexes (see Figure 7). The ventricular escape complexes are usually wide and occur at around 30–40 bpm. There is a significant risk of asystole and thus permanent pacing (DDD) is indicated, regardless of symptoms. Acquired AV block is most commonly due to ischemic heart disease or drug toxicity (in particular β-blockers, digitalis, and calcium-channel blockers).

Figure 7
Electrocardiogram of third-degree atrioventricular block.
Bradyarrhythmia in atrial fibrillation
In patients with intermittent or chronic AF, AV node dysfunction is not an uncommon finding. Clearly, this will be aggravated by many of the rate-limiting drugs given to control the ventricular rate. In these cases, consideration should be given to the implantation of a pacemaker to protect against bradycardia while still allowing pharmacological control of a rapid ventricular rate.
Bundle branch block
A problem in the bundle of His presents in an identical fashion to a combined block of both bundles, ie, complete heart block. However, a more common occurrence is an isolated left or right bundle branch block. These are usually distinct from any problem with AV conduction (ie, they usually coexist with normal sinus rhythm [SR]). The patterns of the ECG are characteristic, but highly variable; the hallmark is a wide QRS complex.
- In left bundle branch block (LBBB), the pattern is best detected in V6 where there is an "M" pattern, while in V1 there is a "W" pattern (see Figure 8).
- In right bundle branch block (RBBB), the pattern is best detected in V1 where there is an RSR complex, while in V6 there is a QRS complex (see Figure 8).

Figure 8
Electrocardiogram patterns of left bundle branch block and right bundle branch block.
In fact, both LBBB and RBBB are found in the "normal" population. New LBBB is cause for concern, and if it can clearly be related to an acute episode of chest pain then it probably indicates MI. Both RBBB and LBBB probably indicate increased risk for cardiovascular disease; however, neither on its own is an indication for pacing.
Fascicular block
One confusing aspect of electrocardiology is the terminology used to describe blocks of the fascicles (see Figure 9). The confusion arises from the fact that the right bundle is included in the list of three fascicles:

Figure 9
Fascicular block.
- left anterior fascicle
- left posterior fascicle
- right bundle branch
Left anterior and left posterior fascicular block
Fascicular block causes axis deviation on the ECG. Therefore, left anterior hemiblock causes left axis deviation (see Figure 10), while left posterior hemiblock causes right axis deviation (see Figure 11).

Figure 10
Left axis deviation.

Figure 11
Right axis deviation.
Bifascicular block
The term "bifascicular block" refers to a block of any two of the three fascicles. Clearly, this should include LBBB (left anterior + left posterior); however, the term is usually reserved for:
- RBBB + left anterior hemiblock, ie, RBBB + left axis deviation
- RBBB + left posterior hemiblock, ie, RBBB + right axis deviation (the axis is usually normal in RBBB)
Bifascicular block is not in itself an indication for pacing. However, when combined with intermittent second- or third-degree block, a DDD pacemaker should be fitted.
Trifascicular block
Trifascicular block refers to a block of all three fascicles (but with intact AV conduction). It usually refers to LBBB + a long PR interval. Although trifascicular block is not strictly speaking an indication for permanent pacing, some centers carry this out on the basis that it must reflect extensive conducting tissue damage.
Nonspecific intraventricular conduction defect
Another term that is sometimes used is "nonspecific intraventricular conduction defect". This usually refers to an abnormal ECG that does not clearly fit any of the patterns described above. The QRS complex will generally not be wide, but the waveform will be atypical. It is of unknown significance, but is likely to be benign.
Tachyarrhythmia
The general principle of patient management outlined at the beginning of this chapter holds true for patients suffering from tachyarrhythmia: is the patient compromised? And, as above, the use of an ECG will be critical when making the diagnosis. However, you will often be called to see a patient in the community where no ECG is available. In this situation, try to make a diagnosis based on the defibrillator monitor. Most patients with tachyarrhythmia will require referral, but making your own diagnosis can only help the situation. The key questions are:
- do the results show broad (>0.12 seconds) or narrow complexes?
- are they regular or irregular?
More sophisticated interpretation of the ECG is helpful, but the answers to these key questions will be sufficient to guide the immediate management (see Table 5).
Table 5
Possible diagnoses of tachyarrhythmias based on the electrocardiogram.
Sinus tachycardia
Sinus tachycardia is usually a response to physiological stress such as exercise or anxiety, and it may be the result of an abnormally heightened sympathetic tone. Abnormal pathological causes include fever, hypotension, anemia, thyrotoxicosis, hypovolemia, pulmonary emboli, myocardial ischemia, and shock. Nicotine, caffeine, alcohol, and some medications (sympathetic agonists or parasympatholytic agents) are frequently the underlying cause of sinus tachycardia. The QRS complexes are preceded by P waves of normal morphology, duration, and axis. Sinus tachycardia alone does not require any treatment, but the underlying cause should be determined.
Atrial tachycardia
Atrial tachycardia can occur in the presence of cardiac or pulmonary disease at a rate varying from 140 to 240 bpm. P-wave morphology is generally different from that during SR, but the P–QRS relationship remains 1:1 (see Figure 12). Some atrial tachycardias are catecholamine sensitive; in this case, a β-blocker is appropriate therapy. Curative radiofrequency ablation of atrial tachycardia is effective in 70% of cases. For refractory cases, creation of complete heart block by radiofrequency catheter ablation with implantation of a permanent dual-chamber pacemaker provides control of the rate and avoids drug toxicity.
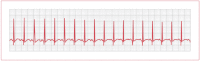
Figure 12
Electrocardiogram of atrial tachycardia.
Atrioventricular nodal re-entrant tachycardia
AV nodal re-entrant tachycardia (AVNRT) accounts for more than 70% of cases of paroxysmal SVT (see Figures 13 and 14). This is also termed "classic" SVT with fast (140–250 bpm) narrow complexes and no P waves. Initial management involves interventions to increase vagal tone. These should only be carried out on a monitored patient, and include:

Figure 13
Electrocardiogram of atrioventricular nodal re-entrant tachycardia.

Figure 14
Mechanisms of re-entrant tachycardia. AVN: atrioventricular node; AVNRT: atrioventricular nodal re-entrant tachycardia; SR: sinus rhythm; VT: ventricular tachycardia.
- carotid sinus massage – apply firm pressure to one carotid artery at the level of the upper thyroid cartilage and move a small distance back and forth for up to 5 seconds (check for bruits first)
- Valsalva maneuver – the patient should take a deep breath, then attempt to exhale forcefully against a closed glottis for up to 15 seconds
- diving reflex – this vestigial reflex, which allows marine animals to lower their metabolism when diving underwater, exists in humans and can increase vagal tone. Suddenly immerse the patient's face in very cold water
In a number of patients, the tachycardia (ie, AVNRT) terminates spontaneously with these maneuvers. However, if it does not, you should consider an adenosine challenge. This procedure involves rapid IV injections of increasing doses of adenosine (3–16 mg). Adenosine has a very short half-life (10 seconds) and produces temporary AV block, which can interrupt re-entry. This procedure is also very useful diagnostically, as the transient AV block can unmask an underlying atrial rhythm.
The use of a short-acting β-blocker (eg, esmolol) or a calcium-channel blocker (eg, verapamil) has also been found to be safe and effective in terminating AVNRT. Verapamil is particularly useful in patients with asthma, in whom adenosine is contraindicated.
Symptomatic patients with frequent episodes of AVNRT can be considered for radiofrequency catheter ablation of the "slow" pathway; this can be successfully ablated in more than 95% of cases.
Ventricular pre-excitation
Pre-excitation is defined as an early depolarization of the ventricular myocardium that occurs prior to any conduction through the AV node. The most common condition in which this is seen is WPW syndrome, where there is an accessory AV pathway called the bundle of Kent. The anomalous conducting system can be located anywhere around the mitral or tricuspid rings. Most WPW patients have no evidence of structural heart disease. In the majority of cases, there is only a single accessory connection and the electrophysiological properties of the anomalous pathway differ from those of the AV node. Conduction through the accessory connection is faster and is independent of the heart rate. Consequently, the ventricular myocardium is activated from two directions: through the normal system and through the accessory pathway. The resulting QRS complex is a product of fusion of the two distinct activation wavefronts. Since conduction over the accessory pathway is faster, the initial part of the QRS complex represents ventricular activation through this route (delta wave – see Figure 15).
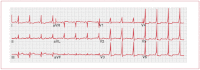
Figure 15
Electrocardiogram of Wolff–Parkinson–White syndrome.
The medical treatment of acute arrhythmias in WPW syndrome depends on the type of tachycardia. ECG results can help to determine this. A narrow complex indicates that accessory pathway re-entry is occurring. Treatment should include vagal maneuvers and adenosine as above. A broad complex is likely to be seen when AF is present, and can be particularly dangerous in WPW syndrome. It is characterized by rapid, irregular, wide complexes and should be treated by immediate direct current (DC) cardioversion. Adenosine, verapamil, and digoxin are not appropriate treatments as they increase the possibility of VT.
Following acute therapy, radiofrequency catheter ablation can be used as a curative treatment for symptomatic patients with an accessory pathway. Pathways can be successfully ablated in more than 90% of all cases and the recurrence rate after successful ablation is approximately 8%–10%. Severe complications are rare, occurring in 2% of all cases.
In the Beginning…
Wolff–Parkinson–White syndrome was first described by Frank Norman Wilson in 1915. Another case in a 19-year-old student was reported by Wedd in 1921, and Louis Wolff, John Parkinson, and Paul Dudley White alluded to these two descriptions when they described the disorder in 1930. The original article by these three authors contained an account of a form of bundle branch block in 11 healthy young adults who were subject to episodes of paroxysmal tachycardia. The relative contributions of the authors are uncertain. Wolff (1898–1972) and White (1886–1973) were both US cardiologists, while Parkinson (1885–1976) was an English physician: one patient had been seen at the London Hospital, while most of the others were examined in Boston. White was a giant of cardiology – he studied the ECG with Thomas Lewis and was a strong advocate of heart disease prevention through exercise, especially bicycle exercise. A bike path in Boston is named after him.
Ventricular tachycardia
In the acute situation, broad complex tachycardias present a diagnostic challenge because SVT with aberrant conduction can be difficult to distinguish from VT. As mentioned previously, the key question remains – is the patient compromised? Certain features on the ECG can help to distinguish VT from SVT (see Figure 16):

Figure 16
Electrocardiogram of ventricular tachycardia.
- a very broad QRS complex (>0.14 seconds)
- AV dissociation (but P waves are often difficult to distinguish in broad complex tachycardias)
- concordance (all QRS complexes in V1–V6 are either positive or negative)
- fusion beats
- capture beats
However, the key test is an adenosine challenge, which will interrupt an SVT but have no effect on VT. If VT is clear from the ECG or adenosine challenge has no effect and the patient is compromised, immediate DC cardioversion is needed. If there is time, a short-acting induction agent should be administered (or IV midazolam can be used). If the patient is losing consciousness, even this can be bypassed. Finally, if the patient loses their pulse, the cardiac arrest protocol should be immediately instituted (see Chapter 1, Cardiac arrest).
If the patient is only mildly compromised or suffering recurrent episodes, other therapeutic options are:
- IV amiodarone
- IV magnesium
- IV lidocaine (lignocaine) (no left ventricular [LV] dysfunction)
- overdrive pacing (this needs a temporary wire. The stimulator rate is turned up above the VT rate [usually ×3] to "capture" ventricular depolarization, and then gradually turned down)
The chronic investigation and management of VT will be carried out by a specialist (and probably a cardiologist with a special interest in electrophysiology). Investigation will center on finding a cause of VT. In the acute situation, there is often an obvious precipitating event (eg, MI). However, the most common cause of recurrent VT is ischemic heart disease. Another key aspect of the investigation will be distinguishing between polymorphic and monomorphic VT. The former, in which complexes vary within or between episodes in their pattern, has a stronger association with sudden death. In difficult cases of VT, invasive electrophysiological testing (often with concurrent coronary angiography) is warranted.
Control of chronic VT is pharmacological – typical drugs that are used include sotalol, flecainide, amiodarone, propafenone, and disopyramide – although radiofrequency ablation of the right ventricular (RV) outflow tract VT can be successful, and in some cases an automatic implantable cardioverter defibrillator (AICD) can save lives.
Ventricular ectopics
Ventricular ectopics, sometimes known as ventricular premature beats, are common in the general population. The shape of the complex is highly variable and depends on the ventricular source. Their significance is debated. They can be a marker of coronary disease and increased risk, but, without a precipitating cause, treatment generally does not lower risk. Electrolyte abnormalities should be excluded. If ventricular ectopics appear frequently during exercise (eg, an exercise tolerance test), the patient should be investigated further for coronary artery disease.
Torsades de pointes
This is a form of polymorphic VT that occurs when the SR shown on an ECG has a prolonged QT interval. The ECG exhibits a continuously changing axis (hence, "turning of points"; see Figure 17), which can look like ventricular fibrillation (VF). The prolonged QT interval can be caused by:
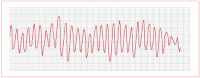
Figure 17
Electrocardiogram showing torsades de pointes.
- antiarrhythmic agents
- hypokalemia
- hypomagnesemia
- bradycardia
However, in very rare cases it may be congenital (Jervell and Lange–Nielsen syndrome or Romano–Ward syndrome).
Atrial fibrillation
AF is the single most common cardiac arrhythmia. It is a condition where there is disorganized electrical and mechanical activity of the atria with a mechanism of multiple re-entrant wavelets. It may be chronic or occur in a paroxysmal fashion (see Table 6). On the ECG, it is recognized as an irregular rhythm with absent P waves (see Figure 18). Low-amplitude wavelets are frequently seen, but in many cases the baseline is flat. Usually, the ventricular response is controlled by the physiological conduction delay of the AV node and the ventricular response is slower in patients with diseases of the conducting system, in the elderly, or in individuals receiving medications that impair AV nodal conduction (eg, β-blockers, digitalis, or calcium-channel blockers). With intense sympathetic stimulation, it may be as rapid as 160–180 bpm.
Table 6
Classification of atrial fibrillation.

Figure 18
Electrocardiogram of atrial fibrillation with irregular rhythm and absent P waves.
The clinical manifestations of AF range from a complete absence of symptoms (usually in the young and fit) to hemodynamic collapse (in the elderly or those with systolic dysfunction). In addition to symptoms of palpitations, patients with AF have an increased risk of stroke and may also develop decreased exercise tolerance and LV dysfunction. The incidence of AF increases with age and its development is concentrated in patients with hypertensive heart disease, congestive heart failure, and rheumatic heart disease; the association with coronary artery disease is not as strong as with these other conditions. Among the noncardiac causes of AF, the association is strongest with hyperthyroidism, electrolyte abnormalities, and alcohol excess.
The patient with newly discovered AF
You will often be faced with a patient presenting with or without symptoms of AF, and an ECG showing the classic irregular rhythm with a flat or irregular baseline (see Figure 18). The approach to management is guided, as before, by the clinical picture – is the patient compromised? If the answer is yes, you should refer immediately.
If not, you then have to decide whether the AF is persistent or paroxysmal (see Figure 19). A large proportion of patients experience spontaneous cardioversion within 24–48 hours of AF onset, but it is rarely clear whether this is their first episode. The best approach is to teach the patient to take his or her own pulse and monitor its regularity. This way, you can gain some idea of how long the patient spends in AF and in SR over the next few days.
If they spontaneously cardiovert, chronic treatment of paroxysmal AF takes three forms:
- anticoagulation
- rate control (if necessary)
- antiarrhythmics (the choice of which is guided by symptoms)
Most recent studies show that even patients with paroxysmal AF or successful cardioversion should remain on anticoagulation (warfarin, international normalized ratio [INR] 2–3) since these individuals have a greater risk of cerebrovascular events than of bleeding complications.
The use of rate control and antiarrhythmics is also individualized – some patients will require neither. However, a good choice is a beta-blocker (eg, carvedilol, metoprolol), which can impact both of these factors. Another choice for patients with heart failure or hypertension associated with AF is flecainide (in the absence of coronary artery disease) or amiodarone (if coronary artery disease is present).
If, several days following chronic treatment, patients remain in AF, there are two avenues of management: accept progression to permanent AF (and attend to rate control and anticoagulation) or attempt cardioversion.
Deciding between the two (the decision will often be in the hands of the cardiologist) is influenced by several factors:
- How long has AF been present?
- How likely is SR to be maintained?
- What is the risk of thromboembolism?
- How severe are the symptoms?
Patients with longstanding AF or AF caused by structural abnormalities are least likely to stay in SR. Most cardiologists would agree that every patient should have one attempt at restoration of SR. However, this is not always the best policy. For example, in an elderly patient with asymptomatic, rate-controlled AF, the toxicity of antiarrhythmics may outweigh the benefit of restoration of SR.
The following points regarding treatment are helpful when treating a patient with AF.
- Regardless of the treatment strategy, all patients should have an ECG, chest x-ray, echo, and testing of thyroid function.
- If the decision is made to accept permanent AF, then control the rate with a combination of digoxin, a β-blocker, or a rate-limiting calcium-channel antagonist, and anticoagulate with warfarin, aiming for an INR of 2–3. The INR should be determined weekly (at least) in the initial stages and monthly thereafter. Patients over the age of 75 years who are considered at high risk for bleeding complications can be targeted to a lower INR of 2. In patients with contraindications to full anticoagulation, a daily dose of 300 mg aspirin can be used as an alternative to warfarin.
- If elective cardioversion is to be attempted, patients should be anticoagulated for 4 weeks with warfarin (aim for an INR of 2–3.5) and potassium levels should be kept in the upper normal range (>4.2 mmol) as this increases the chance of success of the cardioversion. Cardioversion can be electrical or chemical. If DC cardioversion is used, the patient receives a general anesthetic with a short-acting induction agent (usually propofol) and then receives shocks (synchronized to the R waves) of increasing energy: 100 J, 200 J, 360 J, and 360 J using paddles on the anterior and posterior chest (the patient lies on their side).
Chemical cardioversion is also possible using flecainide. In the event of successful cardioversion, patients should continue warfarin for at least 4 weeks. This is because the thromboembolic risk relates to "stunned" atria that do not resume normal mechanical function during this time.
Famous Scots
The first insight into the mechanism of irregular pulse in atrial fibrillation was provided by James Mackenzie, a Scottish GP who used an ink polygraph to record and label jugular venous pulses. He noticed that the jugular "a" wave was lost when patients went from a normal to an irregular rhythm.
New directions in the management of atrial fibrillation
It has recently been established that triggers in pulmonary veins can initiate AF and that circumferential or segmental disconnection of these veins at the left atrial junction can provide effective therapy. In certain patients, success rates for catheter-based pulmonary vein isolation range from 70% to 90%, but more than one procedure is often necessary. Surgery for AF is now usually reserved for use as an adjunctive treatment in patients having mitral or coronary surgery. Nevertheless, the large experience of the Maze procedure provides an important source of information to guide those performing catheter ablation. The Maze procedure was designed to exclude any place in the atria where the macro re-entrant circuits that underlie AF can form. Until recently, this involved an extensive series of atrial incisions, but the more recent cryosurgical Maze is just as effective and technically less demanding.
Atrial flutter
Atrial flutter is a rapid, regular rhythm with atrial rates of 250–350 bpm. The ventricular response rate varies, but it is usually a 2:1 block (creating the classic 150 bpm regular ventricular rhythm). The ECG pattern is typical – classic flutter waves are positive in the inferior leads and negative in lead V1 (see Figure 20).

Figure 20
Electrocardiogram of atrial flutter.
Overall, atrial flutter is managed very much like AF:
- if the patient is hemodynamically compromised, one treatment option is DC cardioversion with low energy (50–100 J)
- a relatively easy way to convert atrial flutter to SR in the hospital setting is to use overdrive pacing in the high right atrium (RA)
- radiofrequency catheter ablation is now considered a curative approach in patients with recurrent atrial flutter. It can be eliminated by creating a linear lesion in the isthmus between the tricuspid annulus and the inferior vena cava. Acute success rates of 85%–90% and recurrence rates of 10%–15% have been reported
- patients with atrial flutter require anticoagulation therapy and, although there is more effective atrial contraction in atrial flutter (which may explain the decreased incidence of thromboembolism), guidelines are similar to those for AF
Pacemakers
Implantation of a permanent electronic replacement for the heart's natural pacemaker began in the 1950s and is now a well-established treatment that increases patient longevity and improves their quality of life. Since its invention, advances in programmability, telemetry, and the ability to sense and pace two chambers have improved the level of care that can be provided. Miniaturization has made the process of implantation more straightforward and long-term complications less common. Advances in pacemaker technology mean that several new indications are likely to be added to the standard ones (see Table 7).
Table 7
Standard indications for pacemakers.
Pacemaker codes
Pacemaker codes are, to many, one of the most confusing aspects of electrocardiology (see Table 8). In fact, the labels, which are standardized, tell you all you need to know about the underlying programming and, in combination with the basic electrical wiring diagram of the heart (see Figure 1), can allow you to diagnose most problems.
Table 8
Pacemaker codes.
The first letter refers to the chamber or chambers paced (atrium, ventricle, both [dual]). The second letter refers to the chamber sensed (atrium, ventricle, both [dual]) and the third letter details the response to sensing (triggered, inhibited, both [dual]). Thus, the most common pacemaker codes are the following.
- DDD – this box senses both chambers and if it detects a missing or late atrial or ventricular contraction it will pace one or both. The most common scenarios are:
- sinus bradycardia – atrial pacing will "kick in" and, assuming AV conduction is normal, no ventricular pacing will occur
- AV block (Mobitz type II; third-degree) – the ventricle is paced either following a normal atrial contraction or a paced atrial contraction. The benefit of this – that ventricular contraction will "track" the increased atrial rate of exercise tachycardia – is also its biggest drawback; it will also increase the ventricular pacing rate in the presence of abnormally increased atrial contraction, eg, atrial tachycardia
- VVI – this type of box is considerably cheaper than those boxes capable of DDD programming. It senses and paces only the ventricle and, consequently, only requires one lead. VVI boxes are indicated for use in AF with bradycardia and AV block, and in sinus bradycardia with no AV block. They can cause pacemaker syndrome and retrograde atrial tachycardia. They can also trigger AF.
- AAI – this device senses and paces only the atrium. It does not pace if a normal P wave is sensed. Thus, it is indicated for use in sick sinus syndrome to prevent bradycardia.
- Rate-adaptive pacemakers, eg, VVI(R). Some patients do not elevate their heart rate normally in response to exercise. This is known as chronotropic incompetence, and is defined as a failure to elevate the heart rate to 70% of predicted heart rate or to >100 bpm. It is most common in sick sinus syndrome (40% of cases), but it can also occur in AF. The solution to this condition is to use a rate-adaptive pacemaker. This type of pacemaker uses one of three methods to detect the need for an increased heart rate and responds accordingly. Examples of detection methods are: mechanical accelerometers that detect movement; changes in transthoracic impedance that can be used to detect changes in ventilation or RV filling; and QT sensors that respond to the shortening of the paced QT interval by catecholamines.
Implantation
Pacemaker implantation is carried out under sedation. Leads are inserted via the subclavian or cephalic vein into the RA and/or RV. Atrial leads are J-shaped and are positioned in the right atrial appendage (anteriorly and superiorly in the RA). Ventricular leads are positioned in the RV apex. Most ventricular leads are placed in position and left against the myocardial wall. In certain circumstances, eg, when these leads become easily displaced or do not provide adequate threshold values, an active fixation lead can be used. This lead has a screw "thread" on its end –usually covered by a dissolvable tip, so that it is not exposed until it is in position – that allows the lead to be fixed into the myocardium.
After the leads are positioned, a series of tests are carried out to determine if the lead position is satisfactory from an electrical point of view. These would typically assess:
- threshold
- the voltage required to cause a contraction. This increases if the lead is not well-positioned and can be a sensitive method of detecting poor placement
- lead impedance
- this is a test of the integrity of the lead. It is essentially a measure of electrical resistance and is measured in Ohms
- abnormal stimulation of the phrenic nerve
- a high-voltage protocol tests for a diaphragmatic twitch
Once all the required tests are completed, the pacemaker pocket (in the fascia overlying the pectoral muscle) is created by blunt dissection and the wound sutured.
The Pacemaker's Maker
Canadian John Hopps invented the first cardiac pacemaker. Hopps was trained as an electrical engineer at the University of Manitoba and joined the National Research Council in 1941, where he conducted research on hypothermia. While experimenting with radiofrequency heating to restore body temperature, Hopps made an unexpected discovery: if a heart stopped beating due to cooling, it could be started again by artificial stimulation using mechanical or electrical means. In 1950, this led to Hopps' invention of the world's first cardiac pacemaker. His device was far too large to be implanted inside the human body – it was an external pacemaker.
Complications
Acute complications will generally occur in hospital. These may include pneumothorax, RV perforation and cardiac tamponade, and hematoma.
Chronic complications are more likely to present to the generalist. Lead infection is fortunately rare, but can present a very difficult management problem. Skin commensals are the most common culprits in right-sided endocarditis. If you suspect this, you should immediately refer the patient for an echo and blood cultures. Treatment is initially with antibiotics, but the lead system should be quickly replaced if this is unsuccessful.
Lead displacement can occur as a result of concurrent right-sided pathology, eg, RV dilatation or valve abnormalities, and can be detected by changes in threshold. Changes in impedance can point to deterioration of old wires or loss of insulation properties. Subclavian vein or superior vena cava occlusion is more common with multiple lead systems. Classic signs include unilateral superficial vein engorgement around the upper thorax, neck, and face.
Erosion occasionally occurs if the pacemaker box becomes gradually more superficial. Unless it causes chronic pain, or erodes completely, it does not demand referral. Some patients move their own box back and forward under the skin (Twiddler's syndrome). This can cause hemorrhage or lead breaks.
Infection of the implantation site can be problematic and should be taken seriously. In the early stage, superficial redness of the skin or swelling around the box will be noticed. For this, standard treatment is indicated, eg, a skin swab, cloxacillin (flucloxacillin) (oral dosage, 500 mg four times daily). If the infection does not respond, or if you suspect deep infection for another reason, eg, marked constitutional symptoms, refer immediately. Infections resistant to antibiotic therapy demand box and lead extraction – a procedure not without difficulty or complications.
Pacemaker syndrome occurs in some patients with a VVI pacemaker who are in SR. It is thought to relate to the fact that although sometimes the atria contract "in time" and cardiac output is normal, at other times they contract against closed AV valves, which causes elevated venous pressure and a fall in cardiac output. The patient will experience dizziness, and the solution is to upgrade the patient to a DDD pacemaker.
Finally, atrial-sensing pacemakers, eg, DDD, respond to atrial arrhythmia with tachycardic pacing. If this happens, it is possible to alter the program (to DDI or set an upper rate limit) or use antiarrhythmic drugs. This problem can be avoided by using a mode-switching pacemaker that detects atrial arrhythmia and switches to VVI.
Pacemaker ECGs
ECGs are harder to interpret in a patient with a pacemaker as there are more variables; although the pacing spikes are usually straightforward to recognize. It is important to remember that the paced QRS complex will be in an LBBB pattern because the wave of depolarization begins where the lead is placed in the RV.
Doctor, I have a pacemaker, what should I avoid?
This is a common question that patients ask. In general, patients should live life as normal. They should avoid magnetic resonance imaging studies, and care should be taken with the following:
- electrocautery during surgery – this can cause sensing problems
- therapeutic radiation
- cardioversion/defibrillation – this should be carried out using the lowest effective energy with the paddles in the anterior–posterior positions on the body of the patient
- mobile phones – these should not be placed in a shirt pocket next to the pacemaker
- car batteries – batteries can produce large magnetic fields. Again, this is only a problem if the person is leaning over and the pacemaker comes close to the battery
- high-voltage cables
Most manufacturers supply each pacemaker recipient with a wallet-sized emergency card for identification as the bearer of an implanted device. This card should include important information about current pacing parameters, names and numbers of the pulse generator (including leads), indication for pacing, and underlying structural heart disease.
Patient follow-up
Application of a magnet to many pacemaker generators reveals the current battery status by pacing with a fixed pacing rate or "magnet rate". The pacemaker rate decreases in most models with declining battery charge. When a decrease indicates exhaustion of one battery capacity, the pulse generator should be replaced.
Implantable cardioverter defibrillators
The natural evolution of pacemaker technology led, in the late 1960s, to the development of the AICD. Early versions were implanted abdominally under general anesthesia. These boxes, now barely bigger than a VVI pacemaker, are implanted under heavy sedation/light general anesthesia, and have revolutionized the treatment of ventricular arrhythmias. Indications for use of the device are expanding as the evidence base grows, but at present these include VF or VT cardiac arrest without a reversible cause; spontaneous sustained VT; syncope of undetermined origin with hemodynamically significant sustained VT; and nonsustained VT with prior MI or LV dysfunction. A recent study suggested that all post-MI patients with an EF <30% should receive an ACD.
An AICD is expensive – approximately the same cost as bypass grafting – but the device is multifunctional. It is capable of bradycardia and tachyarrhythmia detection, overdrive pacing or defibrillation, and event memory (ie, it can "play back" the intracardiac trace from a few minutes before the event, which is extremely useful diagnostically).
The AICD contains a device program for termination of VT and this highlights its versatility. It can initiate:
- burst pacing – a short burst of paced beats delivered at approximately 90% of the rate of the VT
- ramp pacing – a short burst of paced beats at a rate increasing up to 90% of the rate of the VT (to try to achieve capture)
- low-energy shock
- high-energy shock
The programming of AICDs is sophisticated and is carried out by telemetry (a magnet detector is placed over the unit). The energy of shock delivered by the AICD is a magnitude less than that delivered across the chest wall by an external defibrillator (internal 5–35 J, external 100–360 J). If an AICD delivers a shock, it is important to refer the patient to the nearest center where the unit can be interrogated.
Electrophysiological studies
Electrophysiology is a rapidly advancing field in which the potential indications currently outweigh the availability of facilities. The indications for electrophysiology are detailed in Table 9.
Table 9
Potential indications for electrophysiology.
The essence of the electrophysiological test is a measurement of the intracardiac ECG: (1) during normal SR; (2) during an induced arrhythmia; and (3) following premature extrastimuli.
Measurements are usually taken from several intracardiac sites, allowing identification of sinus node function and recovery, sinoatrial conduction, AV nodal conduction, and triggered ventricular arrhythmias. Treatment is with radiofrequency stimulation, which can ablate accessory pathways or interrupt re-entry circuits.
Drugs in arrhythmia
Table 10 describes the Vaughan-Williams classification of antiarrhythmic drugs, and Figure 21 outlines the drugs used in the treatment of arrhythmia.
Table 10
The Vaughan-Williams classification of antiarrhythmic drugs.
Further reading
- Fuster V, Ryden LE, Asinger RW. et al. ACC/AHA/ESC Guidelines for the Management of Patients With Atrial Fibrillation: Executive Summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation) Developed in collaboration with the North American Society of Pacing and Electrophysiology. Eur Heart J. 2001;22:1852–923. [PubMed: 11601835]
- Guidelines for Clinical Intracardiac Electrophysiological and Catheter Ablation Procedures. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. (Committee on Clinical Intracardiac Electrophysiologic and Catheter Ablation Procedures). Developed in collaboration with the North American Society of Pacing and Electrophysiology. Circulation. 1995;92:673–91. [PubMed: 7634483]
- Silverman ME. From rebellious palpitations to the discovery of auricular fibrillation: contributions of Mackenzie, Lewis and Einthoven. Am J Cardiol. 1994;73:384–9. [PubMed: 8109554]
- Wolff L, Parkinson J, White PD. Bundle-branch block with short P-R interval in healthy young people prone to paroxysmal tachyardia. Am Heart J. 1930;5:685. [PMC free article: PMC6932258] [PubMed: 17040283]
- Arrhythmia - Cardiology ExplainedArrhythmia - Cardiology Explained
- HAGLR HOXD antisense growth-associated long non-coding RNA [Homo sapiens]HAGLR HOXD antisense growth-associated long non-coding RNA [Homo sapiens]Gene ID:401022Gene
- KEG1 [Saccharomyces cerevisiae S288C]KEG1 [Saccharomyces cerevisiae S288C]Gene ID:850603Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...


