NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee on Pain, Disability, and Chronic Illness Behavior; Osterweis M, Kleinman A, Mechanic D, editors. Pain and Disability: Clinical, Behavioral, and Public Policy Perspectives. Washington (DC): National Academies Press (US); 1987.

Pain and Disability: Clinical, Behavioral, and Public Policy Perspectives.
Show detailsPain is a subjective experience with two complementary aspects: one is a localized sensation in a particular body part; the other is an unpleasant quality of varying severity commonly associated with behaviors directed at relieving or terminating the experience.
Pain has much in common with other sensory modalities (National Academy of Sciences, 1985). First, there are specific pain receptors. These are nerve endings, present in most body tissues, that only respond to damaging or potentially damaging stimuli. Second, the messages initiated by these noxious stimuli are transmitted by specific, identified nerves to the spinal cord. The sensitive nerve ending in the tissue and the nerve attached to it together form a unit called the primary afferent nociceptor. The primary afferent nociceptor contacts second-order pain-transmission neurons in the spinal cord. The second-order cells relay the message through well-defined pathways to higher centers, including the brain stem reticular formation, thalamus, somatosensory cortex, and limbic system. It is thought that the processes underlying pain perception involve primarily the thalamus and cortex.
In this chapter we review the anatomy and physiology of pain pathways. We also discuss some of the physiological processes that modify the pain experience and that may contribute to the development of chronicity. For obvious reasons, most of this information comes from animal experiments. However, in recent years, experimental studies of human subjects using physiological, pharmacological, and psychophysical methods indicate that much of what has been learned in animals is applicable to humans (National Academy of Sciences, 1985). Research into basic mechanisms underlying pain is an increasingly exciting and promising area. However, most of what is known about the anatomy and physiology of pain is from studies of experimentally induced cutaneous (skin) pain, while most clinical pain arises from deep tissues. Thus, while experimental studies provide fairly good models for acute pain, they are poor models for clinical syndromes of chronic pain. Not only do they provide little information about the muscles, joints, and tendons that are most often affected by chronically painful conditions, but they do not address the vast array of psychosocial factors that influence the pain experience profoundly. To improve our understanding and treatment of pain we will need better animal models of human pain and better tools for studying clinical pain.
Pain Processes
Figure 7-1 illustrates the major components of the brain systems involved in processing pain-related information. There are four major processes: transduction, transmission, modulation, and perception. Transduction refers to the processes by which tissue-damaging stimuli activate nerve endings. Transmission refers to the relay functions by which the message is carried from the site of tissue injury to the brain regions underlying perception. Modulation is a recently discovered neural process that acts specifically to reduce activity in the transmission system. Perception is the subjective awareness produced by sensory signals; it involves the integration of many sensory messages into a coherent and meaningful whole. Perception is a complex function of several processes, including attention, expectation, and interpretation.
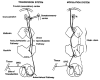
Figure 7-1
Diagrammatic outline of the major neural structures relevant to pain. The sequence of events leading to pain perception begins in the transmission system with transduction (lower left), in which a noxious stimulus produces nerve impulses in the primary (more...)
Transduction, transmission, and modulation are neural processes that can be studied objectively using methods that involve direct observation. In contrast, although there is unquestionably a neural basis for it, the awareness of pain is a perception and, therefore, subjective, so it cannot be directly and objectively measured. Even if we could measure the activity of pain-transmission neurons in another person, concluding that that person feels pain would require an inference based on indirect evidence.
Transduction
Three types of stimuli can activate pain receptors in peripheral tissues: mechanical (pressure, pinch), heat, and chemical. Mechanical and heat stimuli are usually brief, whereas chemical stimuli are usually long lasting. Nothing is known about how these stimuli activate nociceptors. The nociceptive nerve endings are so small and scattered that they are difficult to find, let alone study. Nonetheless, there have been some studies of the effects of chemicals on the firing frequency of identified primary afferent nociceptors.
A variety of pain-producing chemicals activate or sensitize primary afferent nociceptors (Bisgaard and Kristensen, 1985; Juan and Lembeck, 1974; Keele, 1966). Some of them, such as potassium, histamine, and serotonin, may be released by damaged tissue cells or by the circulating blood cells that migrate out of blood vessels into the area of tissue damage. Other chemicals, such as bradykinin, prostaglandins, and leukotrienes, are synthesized by enzymes activated by tissue damage (Armstrong, 1970; Ferreira, 1972; Moncada et al., 1985; Vane, 1971). All of these pain-producing chemicals are found in increased concentrations in regions of inflammation as well as pain. Obviously, the process of transduction involves a host of chemical processes that probably act together to activate the primary afferent nociceptor. In theory, any of these substances could be measured to give an estimate of the peripheral stimulus for pain. In practice, such assays are not available to clinicians.
It should be pointed out that most of our knowledge of primary afferent nociceptors is derived from studies of cutaneous nerves. Although this work is of general importance, the bulk of clinically significant pain is generated by processes in deep musculoskeletal or visceral tissues. Scientists are beginning to study the stimuli that activate nociceptors in these deep tissues (Cervero, 1982, 1985; Coggeshall et al., 1983; National Academy of Sciences, 1985). In muscle, there are primary afferent nociceptors that respond to pressure, muscle contraction, and irritating chemicals (Kumazawa and Mizumura, 1977; Mense and Meyer, 1985; Mense and Stahnke, 1983). Muscle contraction under conditions of ischemia is an especially potent stimulus for some of these nociceptors.
Despite progress in our understanding of the physiology of musculoskeletal nociceptors, we still know very little about the mechanisms underlying common clinical problems such as low back pain. Even when there is degeneration of the spine and compression of a nerve root—a condition generally acknowledged to be extremely painful—we do not know which nociceptors are activated or how they are activated. Neither do we know what it is about the process that leads to pain.
Transmission
Peripheral Nervous System
The nociceptive message is transmitted from the periphery to the central nervous system by the axon of the primary afferent nociceptor. This neuron has its cell body in the dorsal root ganglion and a long process, the axon, that divides and sends one branch out to the periphery and one into the spinal cord (Figure 7-2). The axons of primary afferent nociceptors are relatively thin and conduct impulses slowly.
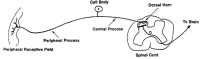
Figure 7-2
The primary afferent nociceptor. This is the route by which the central nervous system is informed of impending or actual tissue damage. Its peripheral process runs in peripheral nerves, and its peripheral terminals are present in most body structures (more...)
It is possible to place an electrode into a human peripheral nerve and record the activity of primary afferent nociceptors (Fitzgerald and Lynn, 1977; Torebjork and Hallin, 1973). The nociceptor is characterized by its response to noxious heat, pressure, or chemical stimuli. The ''pain'' message is coded in the pattern and frequency of impulses in the axons of the primary afferent nociceptors. There is a direct relation between the intensity of the stimulus and the frequency of nociceptor discharge (Figure 7-3). Furthermore, combined neurophysiological and psychophysical studies in humans have shown a direct relation between discharge frequency in a primary afferent nociceptor and the reported intensity of pain (Fitzgerald and Lynn, 1977; LaMotte et al., 1983). Blocking transmission in the small-diameter axons of the nociceptors blocks pain, whereas blocking activity of the larger-diameter axons in a peripheral nerve does not. These identified primary afferent nociceptors are thus necessary for detecting noxious stimuli.
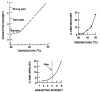
Figure 7-3
The relation of discharge frequency in primary afferent nociceptors to subjective pain intensity in human subjects. Top left: The skin of human subjects was subjected to brief, calibrated temperature increases. Subjects began to identify the temperature (more...)
Monitoring activity in identified primary afferent nociceptors is a potential tool for the evaluation of certain types of clinical pain. In fact, this method has been used clinically to demonstrate pain-producing neural activity arising from a damaged nerve (Nystrom and Hagbarth, 1981). At present, this method should be considered just a research tool; however, it is technically feasible and is of great potential value for evaluating pain patients. It raises the possibility of actually demonstrating nociceptor activity coming from a painful area. This method could be an advance over other correlative techniques for assessing pain because it measures the presumed noxious input, that is, the neural activity that ordinarily causes pain. Most of the other measures assess responses that could be, but are not necessarily, caused by noxious stimuli.
It is important to point out that (1) there can be pain without activity in primary afferent nociceptors, and (2) there can be activity in primary afferent nociceptors without pain. These phenomena occur when there has been damage to the central or peripheral nervous systems. In addition, the modulating system can suppress central transmission of activity elicited by nociceptor input. Thus, there is a variable relation between nociceptor input and perceived pain intensity. For this reason the method of recording primary afferent nociceptors could be used to confirm the presence of an input, but it could not be used to prove that pain was not present.
Besides these theoretical limitations of trying to assess subjective pain intensity by recording primary afferent nociceptors, there are important practical problems in measuring either pain-producing substances or primary afferent nociceptor activity. One is that the largest group of patients disabled by pain localize it to musculoskeletal structures in the lower back. Because the nerves innervating these structures are not near the skin, they are difficult to find. Another problem is that pain arising from deep structures is often felt at sites distant from where the tissue damage occurs. In contrast to the pain produced by skin damage, which is sharp or burning and well localized to the site of injury, the pain that arises from deep tissue injury is generally aching, dull, and poorly localized (Lewis, 1942). When the damage to deep tissues is severe or long lasting, the sensation it produces may be misperceived as arising from a site that is distant from the actual site of damage (Head, 1893; Kellgren, 1938; Lewis, 1942; Sinclair et al., 1948). This phenomenon, known as referred pain, helps to explain the frequent discrepancy between physical findings and patient complaints. The mechanism of referred pain is unknown for any particular case.
Referred pain can be a major source of confusion in the examination of patients complaining primarily of pain. The fact that pain is referred from visceral internal organs to somatic body structures is well known and commonly used by physicians. For example, the pain of a heart attack is not always localized to the heart but commonly is felt diffusely in the chest, the left arm, and sometimes in the upper abdomen. Less widely recognized is the fact that irritable spots, such as myofascial trigger points, in skeletal muscles also cause feelings of pain in locations distant from the irritable spot. This was demonstrated experimentally in muscle and fascia by Kellgren in the late 1930s (Kellgren, 1938). Specific patterns of pain referred from particular muscles have been described clinically (Travell and Rinzler, 1952; Travell and Simons, 1983). (See Chapter 10 and Appendix.)
At least four physiological mechanisms have been proposed to explain referred pain: (1) activity in sympathetic nerves, (2) peripheral branching of primary afferent nociceptors, (3) convergence projection, and (4) convergence facilitation. The latter two involve primarily central nervous system mechanisms.
- 1.
Sympathetic nerves may cause referred pain by releasing substances that sensitize primary afferent nerve endings in the region of referred pain (Procacci and Zoppi, 1981), or possibly by restricting the flow of blood in the vessels that nourish the sensory nerve fiber itself.
- 2.
Peripheral branching of a nerve to separate parts of the body causes the brain to misinterpret messages originating from nerve endings in one part of the body as coming from the nerve branch supplying the other part of the body.
- 3.
According to the convergence-projection hypothesis, a single nerve cell in the spinal cord receives nociceptive input both from the internal organs and from nociceptors coming from the skin and muscles. The brain has no way of distinguishing whether the excitation arose from the somatic structures or from the visceral organs. It is proposed that the brain interprets any such messages as coming from skin and muscle nerves rather than from an internal organ. The convergence of visceral and somatic sensory inputs onto pain projection neurons in the spinal cord has been demonstrated (Milne et al., 1981; Foreman et al., 1979).
- 4.
According to the convergence-facilitation hypothesis, the background (resting) activity of pain projection neurons in the spinal cord that receive input from one somatic region is amplified (facilitated) in the spinal cord by activity arising in nociceptors originating in another region of the body. In this model, nociceptors producing the background activity originate in the region of perceived pain and tenderness; the nerve activity producing the facilitation originates elsewhere, for example, at a myofascial trigger point. This convergence-facilitation mechanism is of clinical interest because one would expect that blocking sensory input in the reference zone with cold or a local anesthetic should provide temporary pain relief. One would not expect such relief according to the convergence-projection theory. Clinical experiments have demonstrated both kinds of responses.
This phenomenon of referred pain can present a serious problem to both patients and physicians when it goes unrecognized. Because the source of the pain lies overlooked at a distant location, the lack of any demonstrable lesion at the site of pain and tenderness often leads to the suspicion that the pain has a strong psychological component. When health professionals insist that there is no reason for the pain, patients sometimes begin to wonder whether the pain is "all in their head." As is discussed in later chapters, this can exacerbate anxiety and other psychological reactions to the pain, is likely to frustrate both the doctor and the patient, and may lead to "doctor shopping" and inappropriate treatment.
Pain Pathways In the Central Nervous System
Primary afferent nociceptors transmit impulses into the spinal cord (or if they arise from the head, into the medulla oblongata of the brain stem). In the spinal cord, the primary afferent nociceptors terminate near second-order nerve cells in the dorsal horn of the gray matter (Willis, 1985). The primary afferent nociceptors release chemical transmitter substances from their spinal terminals. These transmitters activate the second-order pain-transmission cells. The identity of these transmitters has not been established, but candidates include small polypeptides such as substance P and somatostatin, as well as amino acids such as glutamic or aspartic acid.
The axons of some of these second-order cells cross over to the opposite side of the spinal cord and project for long distances to the brain stem and thalamus. The pathway for pain transmission lies in the anterolateral quadrant of the spinal cord. Most of our information about the anatomy and physiology of pain-transmission pathways in the central nervous system is derived from animal studies. However, it is known that in humans, lesions of this anterolateral pathway permanently impairs pain sensation and that electrical stimulation of it produces pain (Cassinari and Pagni, 1969; White et al., 1950; Willis, 1985).
There are two major targets for ascending nociceptive axons in the anterolateral quadrant of the spinal cord: the thalamus and the medial reticular formation of the brain stem. Our knowledge is most extensive for the spinal cells whose axons project directly to the thalamus, that is, the spinothalamic tract cells. The spinothalamic pathway is implicated in human pain perception because lesions of it, at any level, produce lasting impairments of pain sensation.
Studies of the properties of spinothalamic tract cells have been carried out in several species. In all these species, a major proportion of spinothalamic neurons respond maximally to noxious stimulation. Furthermore, there is a direct relationship in spinothalamic tract cells of firing frequency to stimulus intensities in the noxious range for human subjects (Kenshalo et al., 1980; Willis, 1985). These observations, coupled with decades of careful clinical studies, strongly implicate the spinothalamic tract as a major pathway for pain in humans.
The other major ascending nociceptive pathway in the anterolateral quadrant is the spinoreticular tract. The medullary reticular formation receives a major direct projection from the spinal cord as well as from branches of some of the spinal neurons that project to the thalamus (Kevetter and Willis, 1984; Mehler, 1962).
At the thalamic level, pain pathways have two major sites of termination: ventrocaudal and medial. The ventrocaudal thalamus receives nociceptive input directly from projecting spinal neurons. Neurons in the ventrocaudal thalamus project directly to the somatosensory cortex (Willis, 1985). The medial thalamus receives some indirect input from the spinal cord, but in addition, it receives a major input from the region of the brain stem reticular formation to which the nociceptive spinoreticular neurons project. The medial thalamus projects to widespread areas of the forebrain, including the somatosensory cortex (Jones and Leavitt, 1974). Thus there are two major ascending pathways for pain: a direct lateral spinothalamic pathway and an indirect medial spinoreticulothalamic pathway. It is thought that the lateral pathway from the spinal cord to the ventrocaudal thalamus and to the cortex is responsible primarily for sharp, well-localized pains that arise near the body surface. In contrast, the medial spinoreticulothalamic pathway responds more to stimuli of deep somatic and visceral structures.
There is some evidence for further functional differences between medial and lateral thalamic pathways. Lesions of the ventrocaudal thalamus and somatosensory cortex produce long-lasting deficits in the sensory aspects of pain that are very similar to those produced by lesions of the anterolateral spinal cord pathway. Lesions of the medial thalamus have very little effect on pain sensation per se; pain threshold is unaffected, as are the other sensory aspects of the pain experience. In contrast, the emotional or reactive aspects may be totally abolished (Barber, 1959).
Sensory Versus Affective Aspects of Pain
The processes set in motion by noxious stimuli can be divided into two broad categories. On one hand, there are the sensory processes that lead to the detection and identification of the stimulus. On the other hand, presumably because of the tissue-damaging potential of the noxious stimulus, aversive behavioral sequelae such as withdrawal and escape can terminate the stimulus and protect the organism. Correlated with these two categories of response are two subjective aspects of pain: sensory and affective.
The sensory aspects concern detecting, localizing, assessing the intensity of, and identifying the stimulus. Focusing on the sensory aspects, a person might describe his or her pain as a mild burning pain located on the back of the hand. In contrast, the affective or unpleasantness aspect of pain correlates with the aversive drive to terminate the noxious stimulus and is described by terms that are not specifically tied to a sensory experience, for example, nagging, uncomfortable, or excruciating. The affective aspects would also be accompanied by mood changes such as anxiety and depression, which are usually considered psychological rather than sensory.
The difference between the sensory and affective aspects of pain can be illustrated further by distinguishing between pain threshold and pain tolerance. For example, if one delivers calibrated thermal stimuli to the skin, most people will report that the sensation becomes painful over a narrow range of skin temperatures (43-46ºC) (LaMotte et al., 1983; Willis, 1985). The temperature that is called painful 50 percent of the time would be the pain detection or sensory threshold.
In contrast to this relatively reproducible pain-detection threshold, tolerance for pain differs widely among individuals. For example, subjects immersing their hands in ice water fall into distinct groups those who keep their hands in for over 5 minutes and those who pull them out after less than 90 seconds (Turk and Kerns, 1983-1984). The tolerance for pain is a complex function that may be modified by personality traits, attitudes, previous experience, economic factors, gender, and the particular circumstance under which the pain is experienced. Tolerance may be thought of as a response threshold. Pain of a certain intensity and duration may be ignored, whereas a somewhat more intense pain might induce some people to take painkillers, stay home from work, or consult a physician. The particular behavior elicited by pain of a given intensity is highly individual and greatly influenced by what the patient believes will be helpful and how serious he or she thinks the situation is. For example, most people with headaches do not seek medical attention because headaches are not considered indicative of serious disease (and usually are not). In contrast, a person whose father died recently from a brain tumor might be very frightened by even a mild headache and seek medical attention (see Chapter 8).
Tolerance is also tied to the cognitive and affective aspects of pain. For patients with cancer, pain may be a sign that the tumor has recurred or spread and that death is near. For such patients, the suffering is due not only to the pain's intensity but also to its meaning. Anguish, suffering, and anxiety commonly accompany pain.
In the 1950s many patients with severe pain due to malignancy were given frontal lobotomies (Barber, 1959). These operations disrupt the projections to the frontal lobe from the medial spinoreticulothalamic pathway. In such patients, pain intensity and threshold were unaffected, but the emotional aspects (suffering and anguish) were abolished. Unfortunately, the severe personality changes that accompanied the elimination of suffering made this an unacceptable approach to the treatment of pain. However, these clinical observations show that the affective component of pain has a separate anatomical substrate from the sensory component.
Modulation
The abovementioned processes were discussed in terms of a highly reliable pain-transmission system, the assumption being that pain intensity is a direct function of nociceptor activity. In fact, the excellent correlation among stimulus intensity, impulses in primary afferent nociceptors, and reported pain intensity demonstrated in human subjects under experimental conditions often does not apply to the clinical situation. The most remarkable observations are those in which patients subjected to injuries that ought to be very painful report no significant pain (Beecher, 1959).
An hypothesis for spontaneous analgesia emerged when it was discovered that electrical stimulation of certain brain regions blocks responses to noxious stimulation in laboratory animals (Basbaum and Fields, 1978). This phenomenon, stimulation-produced analgesia (SPA), became more than a laboratory curiosity when it was shown that stimulating homologous brain regions provided relief for patients suffering from chronic pain (Hosobuchi et al., 1977; Richardson and Akil, 1977). SPA has been demonstrated in a variety of animal species and in hundreds of patients.
SPA can be elicited from well-defined brain stem sites. A body of evidence now indicates that SPA is mediated by a discrete neuronal network running from the midbrain to the medulla and then to the spinal cord (Figure 7-1) (Basbaum and Fields, 1978, 1984). This descending, pain-modulating pathway projects to regions of the spinal cord that contain pain-transmission neurons. Stimulation at brain stem sites that produce behavioral analgesia also selectively inhibits identified nociceptive spinothalamic tract neurons. This inhibition may underly the behavioral and clinical analgesia produced by brain stem stimulation.
In addition to electrical stimulation, the analgesia network can be activated by morphine and other opiate analgesic drugs (Yaksh, 1978). The brain stem sites for SPA and the spinal cord are both sensitive to directly applied opiates. The weight of evidence indicates that opiates produce analgesia in part by activating these pain-modulating networks.
One of the most important discoveries in pain research was that the brain contains substances that have the same pharmacological properties as plant-derived opiates and synthetic opioid drugs. These substances, called endogenous opioid peptides, axe present within nerve cells of the peripheral and central nervous systems (Palkovits, 1984). Of particular importance for our discussion is the presence in high concentrations of these peptides in those brain stem sites implicated in pain suppression (Basbaum and Fields, 1984). As discussed in Chapter 9, these findings have led to some promising new psychopharmacological applications.
Studies of this endorphin-mediated analgesia system in laboratory animals have shown that it can be activated by a variety of stressful manipulations, including painful stimuli (Basbaum and Fields, 1984). Clinical studies indicate that it is activated after surgery and can have a significant analgesic effect (Fields and Levine, 1984; Levine et al., 1979). The important point is that there is a well-defined network for controlling pain transmission. Current evidence indicates that this network accounts for some of the striking variability of reported pain intensity in different patients who have had apparently similar noxious stimuli.
It has been suggested that failure of the pain-suppression system accounts for certain types of chronic pain states (Sicuteri et al., 1984; Terenius, 1985), but most pain experts consider this conclusion premature. Much more work is needed to determine the extent to which this pain-modulating network operates on chronic pain.
Physiological Processes That Enhance Pain and May Lead to Chronicity
One of the most troublesome issues for patients, clinicians, and disability examiners is how to account for pain experiences that seem disproportionate to physical findings or objectively verifiable disease or injury. Although it is well known and well accepted that various psychosocial factors may enhance pain, the role of several physiological processes in amplifying and maintaining pain is perhaps not adequately taken into account when assessing patients' complaints.
Sensitization
Tissue damage initiates a variety of processes that sustain and amplify pain. With repeated stimuli, the thresholds of primary afferent nociceptors progressively decrease, so that normally innocuous stimuli become painful (Campbell et al., 1979; Gybels et al., 1979; LaMotte et al., 1983). For some primary afferent nociceptors, repeated noxious stimuli may induce continuous activity lasting for hours (National Academy of Sciences, 1985). The most familiar example of this is sunburn, in which the skin becomes a source of pain; hot water applied to the skin is perceived as unbearably painful and a friendly slap on the back is excruciating. Other examples are the tenderness of a sprained ankle or an arthritic joint. In these situations it is painful to bear weight or even move the affected joint. Sensitization is a major feature of many and perhaps most clinically significant pains, but its cellular mechanism is unknown.
Hyperactivity of the Sympathetic Nervous System: Reflex Sympathetic Dystrophy
Patients with relatively minor injuries occasionally develop pain disproportionate to their injuries. Such pain often becomes progressively worse rather than following the usual course of lessening with time. It is important to stress that the pain persists well beyond the time when the original tissue-damaging process has ended. Furthermore, the location of the pain may be quite different from the site of the precipitating pathology.
In some of these patients hyperactivity of the sympathetic nervous system clearly plays a major role in sustaining the pain because selective blockade of the sympathetic outflow produces immediate and dramatic relief. The pain is usually accompanied by signs of sympathetic hyperactivity, such as a cold (vasoconstricted), sweaty limb. In addition, the skin may be hypersensitive to touch, as if the nociceptors were sensitized. With time, osteoporosis, arthritis, and muscle atrophy may set in and a permanent impairment of function may ensue. This condition, called reflex sympathetic dystrophy, usually responds to sympathetic blocks and physical therapy (De Takats, 1937; Livingston, 1943; Procacci et al., 1975). Physiological studies in animals indicate that the sympathetic outflow can induce discharge of primary afferent nociceptors. This is most prominent in damaged and regenerating afferents (Devor, 1984) but also occurs in undamaged, sensitized afferents (Roberts, 1986) (Figure 7-4).
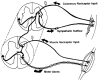
Figure 7-4
Reflex activation of nociceptors in self-sustaining pain. There are two important reflex pathways for pain. The top loop illustrates the sympathetic component. Nociceptor input activates sympathetic reflexes, which activate or sensitize nociceptor terminals. (more...)
The reflex sympathetic dystrophy syndrome is relatively uncommon in its full-blown form, but sympathetic activity could be a common factor in sustaining or amplifying pain that would ordinarily fade as the injured tissues heal. If this were the ease, local signs of increased sympathetic activity could help provide objective evidence that a pain-producing pathological process is present.
Muscle Contraction
Nociceptor activity results in sustained contraction in muscles. In limbs, this muscle contraction produces flexion, a form of primitive withdrawal that is presumably a protective movement. Disease in the abdominal viscera (e.g., gut, liver) produces tension in the muscles of the abdominal wall. Pain arising from musculoskeletal structures also produces contraction and tenderness in other muscles innervated by the same spinal segment (Head, 1893; Kellgren, 1938).
There is some evidence that this spreading muscle contraction plays an important role in clinically significant pains. In patients with persistent pain it is common to find small areas in muscles that are quite tender. Pressure over these myofascial trigger points can reproduce the patient's pain, and locally anesthetizing the points (or other manipulations of them) can give relief lasting days to months (Simons and Travell, 1983). The physiological basis of these trigger points is unknown, but the clinical evidence suggests that they are often involved in sustaining pain in the absence of ongoing tissue damage.
Self-Sustaining Painful Processes: Livingston's ''Vicious Circle''
From the material just discussed, clinical observations clearly indicate that several processes are set in motion by tissue-damaging stimuli that activate nociceptors. In the peripheral tissues, pain-producing substances are released that sensitize the nociceptors so that normally innocuous stimuli can activate them. In addition, nociceptors themselves release factors such as substance P that in turn cause vasodilation, edema, and the release of sensitizing substances from nonneural cells (Lembeck, 1983). Presumably, these processes play a role in the activation of host defenses against infection or toxins. However, they do prolong and amplify pain.
For example, a noxious stimulus to the skin would activate nociceptors. These nociceptors then activate spinal reflexes that produce sustained muscle contraction with consequent activation of muscle nociceptors (Figure 7-4). In this case, the production of a second site of noxious input in muscle is due to a spinal reflex. In some cases (e.g., reflex sympathetic dystrophy), the nociceptive input also activates the sympathetic nervous system, which can feed back to the periphery to sensitize or even activate nociceptive primary afferents. Livingston (1943) was the first to emphasize the clinical importance of these positive feedback loops; that is, the pain produces muscle contraction and sympathetic outflow that in turn activate nociceptors, which produce more sympathetic outflow and muscle contraction, and so on (Figure 7-4). The point is that painful injuries set in motion secondary processes, not associated with tissue damage, that cause a prolongation and spread of nociceptive input and may contribute to chronicity. These secondary processes set up foci of nociceptive input that are independent of the original site of injury. The pain acquires, so to speak, a life of its own.
Although there is no question that these factors contribute to the pain in some cases, it is not clear what proportion of patients with chronic pain have it because of these factors. This would obviously be an important area for future research on chronic pain.
Neuropathic Pain
Damage to the peripheral or central nervous systems can produce chronic pain. For example, in some diseases that affect peripheral nerves, such as diabetes mellitus or alcohol toxicity, pain is very common. Traumatic injury to a peripheral nerve is rarely painful, but when it is, it may be dramatically so. Causalgia (heat pain) is an example of pain induced by traumatic injury to a peripheral nerve. Causalgia is a syndrome characterized by severe burning pain and signs of sympathetic nervous system hyperactivity (Mitchell, 1965; Roberts, 1986). Similarly, lesions of the central nervous system are rarely painful, but when they are, the pain is severe and resistant to treatment (Cassinari and Pagni, 1969; Riddoch, 1938).
There are certain characteristics of neuropathic pain. It frequently begins several days to weeks after the injury that produces it and tends to worsen before stabilizing. It is usually accompanied by sensory abnormalities, including, paradoxically, deficits in pain sensation and painful hyperreactivity to ordinarily innocuous stimuli (Noordenbos, 1959; Ochoa, 1982).
The mechanisms of neuropathic pain are not completely understood, but there are several factors that could contribute to them (Ochoa, 1982). Damaged primary afferents, presumably including nociceptors, acquire certain properties when they begin to regenerate. These include spontaneous activity, mechanical sensitivity, and sensitivity to sympathetic nervous system activity (Ochoa, 1982; Scadding, 1981).
Note that under these circumstances there can be pain either without any stimulus or with a very gentle, non-tissue-damaging stimulus.
In addition to the peripheral sources of pain, damage to primary afferents produces changes in the pain-transmission neurons to which they project in the central nervous system. These cells become spontaneously active and could be a source of pain, again in the absence of any noxious stimuli (Lombard and Larabi, 1983; Roberts, 1986).
Trigeminal neuralgia and post-herpetic neuralgia are among the most common types of neuropathic pains. These conditions tend to strike older individuals, many of whom are retired. This may be why patients with pains that are obviously neuropathic account for only a small proportion of those who seek disability benefits. On the other hand, some patients with low back pain might have an element of nerve damage that adds to the painfulness of their problem as well as to its chronicity and resistance to conventional treatment. Further research on this issue is clearly needed, as are better methods for detecting injuries to nerves that innervate deep structures.
Acute Versus Chronic Pain
Is there any physiological basis for differentiating between acute and chronic pain? Little is known about the effects of prolonged pain on the central nervous system. There is some evidence that the transition from acute pain to chronic pain alters patients' neurophysiology in a way that makes them somewhat different from people with acute pain. In arthritic rats, for example, there are changes in the peripheral nerves that alter their range of response to applied stimuli, and there may be changes in the central pathways for pain transmission as well (Guilbaud et al., 1985; Kayser and Guilbaud, 1984). Experiments with rats in which nerves have been injured and observed over time have shown changes in the central nervous system, but it is not known how these changes relate to pain (Markus et al., 1984).
People with recurrent headaches, arthritis, low back pain, angina, or low-grade malignancies may have had pain for years. The complaints, treatment, and patients' reactions may be different for each of these conditions. In some cases, psychological factors loom large. These factors are particularly prominent in patients with low back pain, facial pain, and headaches and seem to be more prominent the longer the pain persists.
Psychological and somatic factors are not completely separate in maintaining pain. For example, stress and anxiety increase both muscle contraction and sympathetic outflow and would be expected to exacerbate any ongoing pain problem to which they contribute. Conversely, any treatment that induces relaxation will reduce these factors and lessen pain. This may be one important connection between the psychosocial and the somatic factors that influence pain tolerance.
Potential Methods of Physiological Monitoring
In this chapter we have briefly surveyed the anatomy, physiology, and pharmacology of nociceptive transduction, transmission, and modulation. These are objective and potentially observable phenomena initiated by stimuli that damage or threaten tissue.
As we learn more about the transduction process, it may be feasible to measure the concentration of substances in regions of ongoing tissue damage that activate or sensitize primary afferent nociceptors. This could give an estimate of the level of stimulation of chemically sensitive nociceptors. The most promising technique at present is direct recording of the electrical activity in primary afferents. This is technically feasible and has been used in research, but it is not presently available for general clinical use.
The monitoring of central pain transmission pathways is not practical with the technology available. Although it is theoretically possible, recording single units within the human nervous system requires a potentially dangerous surgical procedure. Multiunit, or evoked-potential, studies do not have the required specificity or spatial resolution to permit collecting meaningful data about clinical pain. It is technically possible to measure the chemicals released at spinal synapses by primary afferent nociceptors. If the concentration of such chemicals in the cerebrospinal fluid could be shown to correlate with either the activity of the primary afferent nociceptors or with the severity of clinical pain, this could provide evidence similar to that derived from recording the activity of the primary afferents. However, at the present time, the transmitter or transmitters for the primary afferent nociceptors are unknown.
Another approach is to use positron emission tomography (PET) to monitor metabolic activity in central nervous system pain pathways. PET is a noninvasive scanning technique that can provide evidence of focal brain activity and of the concentration of certain chemicals. This technique requires that enough neurons be active in a large enough region for a long enough period of time to be detected. Because of the topographical organization of the cortex, this technique might be used to monitor the somatosensory cortex. A precise map of the body surface spreads over many millimeters of the cortex. Representation of the face and hand on this map is very large, so it might be possible to detect ongoing activity produced by nociceptive input from these regions. At present, there is no evidence that such measurements show anything in patients with chronic pain.
Indirect measures, such as those of sympathetic nervous system activity (skin temperature or skin resistance) or of muscle contraction in painful areas might be helpful in providing objective evidence of sustained nociceptive input. The measurement of skin temperature over extensive areas of the body surface, thermography, is being used clinically but is still not widely accepted as a reliable indicator of pain. Although they are simple, painless, and safe indicators of sympathetic function, indirect measures of painful input like thermography could be misleading. Sympathetic changes could be produced by nonspecific factors such as surprise or anxiety that do not involve pain. On the other hand, if the changes in sympathetic activity are highly localized, persistent, and consistent with the reported location of the patients' pain, routine evaluation of sympathetic function with techniques like thermography in patients with chronic pain might provide clues about the mechanisms sustaining the pain.
Ultimately, the presence of pain in another individual is always inferred. Even if we could measure pain directly, such a measure would not be adequate to describe the experience of pain, and it is the experience that affects functioning, including the ability to work.
References
- Armstrong, D. Bradykinin, Kallidin and Kallikrein. Vol. 25 of Handbook of Experimental Pharmacology (Erdos, E.G., editor. , ed.). Berlin: Springer-Verlag, 1970.
- Barber, T.X. Toward a theory of pain: relief of chronic pain by prefrontal leucotomy, opiates, placebos, and hypnosis. Psychological Bulletin 56:430-460, 1959. [PubMed: 13796585]
- Basbaum, A.I., and Fields, H.L. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annual Review of Neuroscience 7:309-338, 1984. [PubMed: 6143527]
- Basbaum, A.I., and Fields, H.L. Endogenous pain control mechanisms: review and hypothesis. Annals of Neurology 4:451-462, 1978. [PubMed: 216303]
- Beechef, H.K. The Measurement of Subjective Responses. New York: Oxford University Press, 1959.
- Bisgaard, H., and Kristensen, J.K. Leukotriene B4 produces hyperalgesia in humans. Prostaglandins 30:791-797, 1985. [PubMed: 3001832]
- Campbell, J.N., Meyer, R.A., and LaMotte, R.H. Sensitization of myelinated nociceptive afferents that innervate monkey hand. Journal of Neurophysiology 42:1669-1679, 1979. [PubMed: 115969]
- Cassinari, V., and Pagni, C.A. Central Pain, A Neurosurgical Survey. Cambridge, MA: Harvard University Press, 1969.
- Cervero, F. Visceral nociception: peripheral and central aspects of visceral nociceptive systems. Philosophical Transactions of the Royal Society of London, Series B 308:325-337, 1985. [PubMed: 2858886]
- Cervero, F. Afferent activity evoked by natural stimulation of the biliary system in the ferret. Pain 13:137-151, 1982. [PubMed: 7122106]
- Coggeshall, R.E., Hong, K.A.P., Langford, L.A., Shaible, H.G., and Schmidt, R.F. Discharge characteristics of fine medial articular afferents at rest and during passive movements of inflamed knee joints. Brain Research 272:185-188, 1983. [PubMed: 6311338]
- De Takats, G. Reflex dystrophy of the extremities. Archives of Surgery 34:939-956, 1937.
- Devor, M. The pathophysiology and anatomy of damaged nerve. In: Textbook of Pain (Wall, P.D., editor; , and Melzack, R., editor. , eds.). Edinburgh: Churchill-Livingston, 1984.
- Ferreira, S.H. Prostaglandins, aspirin-like drugs and analgesia. Nature 240:200-203, 1972. [PubMed: 4629913]
- Fields, H.L., and Levine, J.D. Placebo analgesia—a role for endorphins? Trends in Neurosciences 7:271-173, 1984.
- Fitzgerald, M., and Lynn, B. The sensitization of high threshold mechanoreceptors with myelinated axons by repeated heating. Journal of Physiology 365:549-563, 1977. [PMC free article: PMC1307834] [PubMed: 850207]
- Foreman, R.D., Schmidt, R.F., and Willis, W.D. Effects of mechanical and chemical stimulation of fine muscle afferents upon primate spinothalamic tract cells. Journal of Physiology 286:215-231, 1979. [PMC free article: PMC1281567] [PubMed: 108391]
- Gullbaud, D., Iggo, A., and Tegner, R. Sensory receptors in ankle joint capsules of normal and arthritic rats. Experimental Brain Research 58:29-40, 1985. [PubMed: 3987851]
- Gybels, J., Handwerker, H.O., and Van Hees, J. A comparison between the discharges of human nociceptive nerve fibers and the subject's ratings of his sensations. Journal of Physiology 292:193-206, 1979. [PMC free article: PMC1280853] [PubMed: 490345]
- Head, H. On disturbances of sensation with especial reference to the pain of visceral disease. Brain 16:1-132, 1893.
- Hosobuchi, Y., Adams, J.E., and Lichitz, R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science 197:183-186, 1977. [PubMed: 301658]
- Jones, E.G., and Leavitt, R.Y. Retrograde axonal transport and the demonstration of non-specific projections to the cerebral cortex and striatum from thalamic intralaminar nuclei in the rat, cat and monkey. Journal of Comparative Neurology 154:349-378, 1974. [PubMed: 4132969]
- Juan, H., and Lembeck, F. Action of peptides and other algesic agents on paravascular pain receptors of the isolated perfused rabbit ear. Naunyn-Schmiedeberg's Archives of Pharmacology 283:151-164, 1974. [PubMed: 4369745]
- Kayser, V., and Guilbaud, G. Further evidence for changes in the responsiveness of somatosensory neurons in arthritic rats: a study of the posterior intralaminar region of the thalamus. Brain Research 323:144-147, 1984. [PubMed: 6525504]
- Keele, C.A. Measurement of responses to chemically induced pain. In: Touch Heat and Pain (De Reuck, A.V.S., editor; , and Knight, J., editor. , eds.). Boston: Little, Brown, 1966.
- Kellgren, J.H. Observations on referred pain arising from muscle. Clinical Science 3:175-190, 1938.
- Kenshalo, D.R., Jr., Giesler, G.J., Jr., Leonard, R.B., and Willis, W.D. Responses of neurons in primate ventral posterior lateral nucleus to noxious stimula. Journal of Neurophysiology 43:1594-1614, 1980. [PubMed: 7411178]
- Kevetter, G.A., and Willis, W.D. Collateralization in the spinothalamic tract: new methodology to support or deny phylogenetic theories. Brain Research Reviews 7:1-14, 1984. [PubMed: 6370375]
- Kumazawa, T., and Mizumura, K. Thin fibre receptors responding to mechanical, chemical and thermal stimulation in the skeletal muscle of the dog. Journal of Physiology 273:179-194, 1977. [PMC free article: PMC1353733] [PubMed: 599419]
- LaMotte, R.H., and Campbell, J.N. Comparison of responses of warm and nociceptor C-fiber afferents in monkey with human judgments of thermal pain. Journal of Neurophysiology 41:509-528, 1978. [PubMed: 418156]
- LaMotte, R.H., Thaihammer, J.G., and Robinson, C.J. Peripheral neural correlates of magnitude of cutaneous pain and hyperalgesia: a comparison of neural events in monkey with sensory judgments in human. Journal of Neurophysiology 50:1-26, 1983. [PubMed: 6875640]
- Letobeck, F. Sir Thomas Lewis's nocifensor system, histamine and substance-p-containing primary afferent nerves. Trends in Neuroscience 6:106-108, 1983.
- Levine, J.D., Gordon, N.C., and Fields, H.L. Naloxone dose-dependently produces analgesia and hyperalgesia in postoperative pain. Nature 278:740-741, 1979. [PubMed: 219371]
- Lewis, T. Pain. New York: Macmillan, 1942.
- Livingston, W.K. Pain Mechanisms. New York: Macmillan, 1943.
- Lombard, M.C., and Larabi, Y. Electrophysiological study of cervical dorsal horn cells in partially deafferented rats. Advances in Pain Research and Therapy 5:147-154, 1983.
- Markus, H., Pomeranz, B., and Krushelnycky, D. Spread of saphenous somatotropic projection map in spinal cord and hypersensitivity of the foot after chronic sciatic denervation in adult rat. Brain Research 296:27-39, 1984. [PubMed: 6713208]
- Mehler, W.R. The anatomy of the so-called "Pain Tract" in man: an analysis of the course and distribution of the ascending fibers of the fasciculus anterolateralis. In: Basic Research in Paraplegia (French, J.D., editor; , and Portor, T.W., editor. , eds). Springfield, IL: Charles C Thomas, 1962.
- Mense, S., and Meyer, H. Different types of slowly conducting afferent units in cat skeletal muscle and tendon. Journal of Physiology 363:403-417, 1985. [PMC free article: PMC1192937] [PubMed: 4020704]
- Mense, S., and Stahnke, M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. Journal of Physiology 342:383-397, 1983. [PMC free article: PMC1193965] [PubMed: 6631740]
- Milne, R.J., Forman, R.D., Giesler, G.J., Jr., and Willis, W.D. Convergence of cutaneous and pelvic visceral nociceptive inputs onto primate spinothalamic neurons. Pain 11:163-183, 1981. [PubMed: 7322601]
- Mitchell, S.W. Injuries of Nerves and Their Consequences. New York: Dover, 1965.
- Moncada, S., Flower, R.J., and Vane, J.R. Prostaglandins, prostacyclin, thromboxane A2 and leukotrienes. In: The Pharmacological Basis of Therapeutics, 7th Ed. (Goodman, L.S., editor; , and Gilman, A.G., editor. , eds.) New York: Macmillan, 1985.
- National Academy of Sciences. Research Briefing Panel on Pain and Pain Management. Washington, DC: National Academy Press, 1985.
- Noordenbos, W. Pain. Amsterdam: Elsevier, 1959.
- Nystrom, B., and Hagbarth, K.E. Microelectrode recordings from transected nerves in amputees with phantom limb pain. Neuroscience Letter 27:211-216, 1981. [PubMed: 7322453]
- Ochoa, J. Pain in local nerve lesions. In: Abnormal Nerves and Muscles as Impulse Generators (Culp, W.J., editor; , and Ochoa, J., editor. , eds.). New York: Oxford University Press, 1982.
- Palkovits, M. Distribution of neuropeptides in the central nervous system: a review of biochemical mapping studies. Progress in Neurobiology 23:151-189, 1984. [PubMed: 6395185]
- Procacci, P., Francini, F., Maresca, M., and Zoppi, M. Cutaneous pain threshold changes after sympathetic block in reflex dystrophies. Pain 1:167-175, 1975. [PubMed: 1235980]
- Procacci, P., and Zoppi, M. Pathophysiology and clinical aspects of visceral and referred pain. Pain, Supplement 1:S7, 1981.
- Richardson, D.E., and Akil, H. Pain reduction by electrical brain stimulation in man. Journal of Neurosurgery 47:178-183, 1977. [PubMed: 327030]
- Riddoch, G. The clinical features of central pain. The Lancet 234:1093-1209, 1938.
- Roberts, W.J. An hypothesis on the physiological basis for causalgia and related pains. Pain 24:297-311, 1986. [PubMed: 3515292]
- Scadding, J.W. Development of ongoing activity, mechanosensitivity and adrenaline sensitivity in severed peripheral nerve axons. Experimental Neurology 73:345-364, 1981. [PubMed: 7262242]
- Sicuteri, F., Anselmi, B., and Fanciullacci, M. The serotonin (5-HT) theory of migraine. Advances in Neurology 4:383-394, 1984.
- Simons, D.G., and Travell, J.G. Myofascial origins of low back pain. Parts 1,2,3. Postgraduate Medicine 73:66-108, 1983. [PubMed: 6218489]
- Sinclair, D.C., Weddell, G., and Feindel, W.H. Referred pain and associated phenomena. Brain 71:184-211, 1948. [PubMed: 18890914]
- Terenius, L. Families of opioid peptides and classes of opioid receptors. In: Advances in Pain Research and Therapy, Vol. 9 (Fields, H.L., editor; , Dubner, R., editor; , and Cervero, F., editor. , eds.). New York: Raven Press, 1985.
- Torebjork, H.E., and Hallin, R.G. Perceptual changes accompanying controlled preferential blocking of a and c fibre responses in intact human skin nerves. Experimental Brain Research 16:321-332, 1973. [PubMed: 4686614]
- Travell, J., and Rinzler, S.H. The myofascial genesis of pain. Postgraduate Medicine 11:425-434, 1952. [PubMed: 14920327]
- Travell, J.G., and Simons, D.G. Myofascial Pain and Dysfunction: The Trigger Point Manual. Baltimore, MD: Williams & Wilkins, 1983.
- Turk, D.C., and Kerns, R.D. Conceptual issues in the assessment of clinical pain. International Journal of Psychiatry in Medicine 13:57-68, 1983-1984. [PubMed: 6350203]
- Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirinlike drugs. Nature (London) New Biology 231:232-235, 1971. [PubMed: 5284360]
- White, J.C., Sweet, W.H., Hawkins, R., and Nilges, R.G. Anterolateral cordotomy: results, complications and causes of failure. Brain 73:346-367, 1950. [PubMed: 14812051]
- Willis, W.D. The Pain System. Basel: S. Karger, 1985.
- Yaksh, T.L. Narcotic analgesics: CNS sites and mechanisms of action as revealed by intracerebral injection techniques. Pain 4:299-359, 1978. [PubMed: 25403]
- The Anatomy and Physiology of Pain - Pain and DisabilityThe Anatomy and Physiology of Pain - Pain and Disability
- Variegate Porphyria - GeneReviews®Variegate Porphyria - GeneReviews®
Your browsing activity is empty.
Activity recording is turned off.
See more...