NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014.
19.1. HISTORICAL INTRODUCTION
The question arises as to whether humans possess pheromones. This issue is complex, since one has to first define what, in fact, a pheromone is. Once this is done, then some clear behavioral or endocrinological effect must be measured to demonstrate the effects of the putative agent.
The focus of this chapter is on whether humans possess pheromones, a question posed by Science magazine in 2005 as one of the top 100 outstanding issues of that era (Anonymous 2005). Its goal is to summarize a few concepts from The Great Pheromone Myth (Doty 2010) that address this issue.* While it is apparent that, like music and lighting, odors and fragrances can alter mood states and physiological arousal, is there evidence that unique agents exist, namely pheromones, which specifically alter such states? Are there special chemicals that can influence human endocrinology, such as altering the length of menstrual cycles?
Before addressing these questions, it is useful to put into perspective how the concept of pheromones arose in the first place. In the early 1930’s, the entomologist Bethe termed hormones secreted within the body of insects endohormones and those secreted outside the body of insects ectohormones. Ectohormones were further divided into chemicals with intraspecific effects, termed homoiohormones, and those with interspecific effects, termed alloiohormones (Bethe 1932). In 1959, Karlson and Luscher replaced the term homoiohormone with the term pheromone (Karlson & Lüscher 1959). These authors defined pheromones as “substances which are secreted to the outside by an individual of the same species, in which they release a specific reaction, for example, a definite behavior or a developmental process.” These authors distinguished between pheromones acting via olfaction and those acting via oral or ingestive routes. The former produced immediate releasing responses (e.g., initiating and guiding the flight of the male silk worm moth, Bombyx mori, to the female) and the latter delayed endocrine or reproductive effects, such as the caste-determining substances of many social insects.
Although numerous concerns and qualifications subsequently arose regarding the usefulness of this term in insects, such as the fact that insects have a remarkable capability to learn and respond to a wide range of odors, the pheromone concept and its associated term became codified by entomologists and other biologists. In retrospect, this codification has been questioned even in insects by some entomologists, who point out, among other things, that multiple chemicals, rather than single chemicals, which served as exemplars for insect pheromones (Karlson & Butenandt 1959) are the norm for eliciting most insect behavioral and reproductive processes (Holldobler 1999). Nevertheless, in the 1960s and 1970s, the pheromone concept was generalized to mammals. In an influential review appearing in Science, Parkes and Bruce (1961) reiterated the dichotomy employed by Bethe, noting that some “chemical messengers” act within an individual (e.g., hormones and “other excitatory substances” such as CO2), whereas others (i.e., “pheromones”) act between individuals via ingestion, absorption, or sensory receptors. These writers concluded that “Endocrinology has flowered magnificently in the last 40 years; exocrinology is now about to blossom.”
The generalization of the pheromone concept to mammals was popularized by the entomologist Wilson in a 1963 Scientific American article (Wilson 1963). This author explicitly set the tone for conceptualizing the nature of both priming and releasing mammalian pheromones. In the case of mammalian releasing pheromones, he focused on musks and noted:
Pheromones that produce a simple releaser effect–a single specific response mediated directly by the central nervous system–are widespread in the animal kingdom and serve a great many functions. The chemical structures of six attractants are shown <in the picture>. Although two of the six–the mammalian scents muskone and civetone–have been known for some 40 years and are generally assumed to serve a sexual function, their exact role has never been rigorously established by experiments with living animals. In fact, mammals seem to employ musk-like compounds, alone or in combination with other substances, to serve several functions: to mark territories, to assist in territorial defense and to identify the sexes.*
Subsequently, claims of the chemical isolation of mammalian pheromones appeared in the literature. The initial ones focused on releasing pheromones and included (a) chemicals within the vaginal secretions of rhesus monkeys that were said to elicit copulatory behaviors in males (Curtis et al. 1971; Michael & Keverne 1970; Michael et al. 1971), (b) agents within the tarsal scent glands of male black-tailed deer that elicited licking by females (Brownlee et al. 1969; Müller-Schwarze 1971; Müller-Schwarze et al. 1974), (c) a material from the midventral scent gland of Mongolian gerbils that received investigation from other gerbils (Thiessen et al. 1974), and (d) two steroids from the submaxillary salivary glands of boars that lowered the threshold for pressure-induced lordosis in female pigs (namely, 5α-androsten-16-en-3-one and its related alcohol) (Melrose et al. 1971). However, such isolated chemicals rarely mimicked completely the effectiveness of the virgin secretions and were largely dependent on learning for their efficacy. Moreover, a number of mammalogists pointed out that mammals are not insects and chemicals do not “release” behaviors in a simple manner in these organisms. Bronson suggested, for example, that the term signaling should replace the term releaser in describing one class of pheromones (Bronson 1968) and Whitten and Champlin suggested that “behavioral” should serve as the substitute (Whitten & Champlin 1973). Subsequent investigators suggested replacing the releasing pheromone term with such terms as social odors (Brown 1979), homeochemic substances (Martin 1980), or semiochemicals (Albone 1984). As Bronson (1976, p. 123) aptly pointed out,
The unfortunate side of such generalizations <from insects> is the tendency to think of mammalian communication in terms of simple stimulus-response systems. For example, it is now relatively common usage to refer to “aggression-promoting (or “eliciting”) and “aggression-inhibiting” pheromones in mice (e.g., Lee & Griffo 1974; Mugford & Nowell 1972). The obvious implication of this terminology is the existence of two simple urinary compounds which unequivocally either release or inhibit a stereotyped aggressive response. Mammalian social behavior simply does not work that way except at the purely reflexive level.
Bronson pointed out that the mouse’s nervous system not only contains many more neurons than that of an insect, but differs in terms of degree of encephalization, the numbers of associative neurons, and the flexibility afforded to the mediated behaviors. He continues,
Most insect pheromones are usually single compounds or simple mixtures, typically secreted by restricted glands, and normally evoking stereotyped responses even under totally inappropriate circumstances. Thus many of the standard tests for insect attractants have relied upon copulatory behavior in response to scented filter paper, repeated exposures in many cases providing little habituation of the response (Birch 1974). It is difficult to imagine a male mouse attempting copulation with a scented filter paper let alone doing so repeatedly, and, by extension, it is exceedingly difficult to apply the simple releaser concept to much of mammalian social behavior, whether elicited in part by odors or not. Additionally, experience is a profound modifier to mammalian social behavior. There have actually been relatively few attempts to examine the role of experience in odor-induced responses in mammals. Where investigated, however, the results usually have indicated a potent role for experience. Thus species identification apparently can be easily manipulated by odors early in the life of mammals (e.g., (Carter & Marr 1970; Mainardi, Marsan, & Pasquali 1965; Marr & Lilliston 1969) and adult sexual experience is a strong determinant of response to sex odors (e.g., (Caroom & Bronson 1971; Carr, Loeb, & Dissinger 1965; Carr, Loeb, & Wylie 1966). One wonders at this point whether the pheromone concept, so useful in insect behavior and physiology, should be bastardized to the point where it is used to cover situations in mammalian behavior where usually complex odors evoke highly variable responses which are easily modified by experience.
As described in great detail in my book (Doty 2010), attempts to identify mammalian pheromones have been generally unsuccessful, regardless as to whether such agents are viewed as releaser pheromones, priming pheromones, modulating pheromones, or any other type of putative pheromone. Such attempts fall prey to a number of conceptual, operational, and practical problems that go beyond issues of semantics, including assumptions related to innateness, chemical complexity, and the nature of perceptual systems in general.* Unfortunately, space does not permit a complete exposition of the involved issues, so the focus of this chapter is on issues related to the most popular claims of human pheromones.
19.2. QUEST FOR DISCOVERING HUMAN PHEROMONES
The search for human pheromones came to the fore soon after Alex Comfort published his influential 1971 Nature paper entitled Likelihood of Human Pheromones (Comfort 1971). However, nearly a half century has elapsed since this paper was published and a strong argument can be made that no chemical or simple set of chemicals has been identified that could be construed as a human pheromone. That being said, some investigators have assumed, a priori, that androgen-related steroids are pheromonal agents, as described later in this chapter. Some neurobiologists have even argued that the vomeronasal organ (VNO), a chemosensory structure at the base of the nasal septum in a number of mammals and other vertebrates, is the pheromone receptor, although most have stepped back from this dichotomous position in light of the discovery that this organ, at least in mice, also contains receptor proteins common to the main olfactory system. Although the weight of the evidence is that humans have a vestigial VNO, one group has claimed that this small pouch is functional and responds to chemicals in a sexually dimorphic manner, altering autonomic processes (Berliner et al. 1996; Monti-Bloch et al. 1994). Since there is no neural connection to this rudimentary organ and humans lack the brain structure to which such a connection would be made (i.e., the accessory olfactory bulb), such effects are more aptly explained on the basis of stimulation of the ethmoidal branch of the trigeminal nerve.
19.2.1. Sources of Putative Human Pheromones
Like all vertebrates, humans excrete or secrete many different chemicals via their urine, anal excrement, breath, genitalia, saliva, and skin glands. Most proponents of the human pheromone concept assume that skin glands are the source of the active pheromonal agents. All three major skin glands—apocrine sweat glands, eccrine sweat glands, and sebaceous glands—can produce chemicals that become odorous. Conceptually, such chemicals could be sensed by the olfactory, gustatory, or trigeminal neural systems, or enter the general circulation by way of the vasculature of the nose, sinuses, oral cavity, and lungs (Doty 2008). In some body areas the three major skin glands are associated with hair follicles, as shown in Figure 19.1.
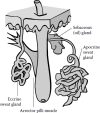
FIGURE 19.1
Schematic of a pilosebaceous unit with apocrine and eccrine sweat glands. (Copyright 2013, Richard L. Doty.)
Among the secretions of the apocrine sweat glands are lipids, including cholesterol, sterol esters, triglycerides, diglycerides, fatty acids, and wax esters. These secretions are usually considered odorless until acted upon by aerobic diphtheroid bacteria (Leyden et al. 1981). The highest density of apocrine glands occur in the axillae and in the perineum (Doty 1981). These glands become functional around the time of puberty and release their secretions in response to such emotions as anxiety, fear, pain, or sexual arousal (Wilke et al. 2007). In horses and some other ungulates, apocrine glands are involved in thermal regulation, but this not the case in humans and most other mammals (Scott et al. 2001). Conceivably, in our own species, they are a vestigial remnant of a defense system useful at one time in warding off predators or unwanted conspecifics.
The eccrine sweat glands, which are located on nearly all body surfaces, play a key role in regulating body temperature and, in humans, are capable of secreting as much as 3 liters of aqueous solution in 1 hour (Tobin 2006). Unlike apocrine glands, they can connect directly with the skin surface independently of hair cells. Their secretions are controlled largely by the sympathetic nervous system and are produced in response to stress, exercise, and other strenuous activity (Nicolaides 1974). While eccrine sweat is generally considered odorless, it can become odoriferous as a result of diet (e.g., garlic) and disease, such as fish odor syndrome (Simehoff et al. 1977).
Most of the lipids and antimicrobial products of the skin are produced by sebaceous glands (Nicolaides 1974). Their secretions are said to have a weak pleasant odor when not infected by bacteria. Like apocrine glands, they enlarge at the time of puberty. They are most dense on the forehead, face, and scalp, and like eccrine sweat glands are absent on the palms of the hand and the soles of the feet. The eyelids, the ear canals, the nares, the lips, the buccal mucosae, the breasts, the prepuce, and the anogenital region all contain specialized sebaceous glands. These glands are also involved in the regulation of local steroidogenesis, skin barrier function, and the production of both anti- and proinflammatory compounds (Tobin 2006).
19.2.2. Sensory Studies of Axillary Secretions
Can gender be determined from the odor of axillary secretions?
The question arises as to whether information critical for reproductive behavior can be sensed from axillary secretions, since most proponents of human pheromones have focused their attention on such secretions or compounds contained therein. To test this concept, Russell (1976) had 13 women and 16 men wear T-shirts for 24 hours without bathing or using deodorants. The armpit regions of the T-shirts were then sniffed in a test session where (a) the subject’s own T-shirt, (b) a male stranger’s T-shirt, and (c) a female stranger’s T-shirt were presented. The task was to identify their own odor and then to guess which of the two remaining odors came from a man. Nine of the 13 women and 13 of the 16 men performed both of these tasks correctly. This suggested to Russell that “at least the rudimentary communication of sexual discrimination and individual identification can be made on the basis of olfactory cues.”
The results of this study could, however, be based on the fact that male odors are generally stronger and less pleasant than female odors, reflecting (a) larger male apocrine glands and (b) the lack of shaved axillae. Axillary hair increases the surface area for bacterial activity and molecular diffusion. Thus, subjects may have used the strategy of assigning stronger and more unpleasant odors to the male category and weaker and less unpleasant ones to the female category, analogous to assigning, from a list of body heights, heavier weights to men and lighter weights to woman.
To test this possibility, we performed a series of studies in which axillary secretions were collected on gauze pads worn in the axillae of male and female donors for approximately 18 hours (Doty et al. 1978). These pads were presented for sampling in small sniff bottles to 10 men and 10 women, along with blank stimuli. The subjects were given the following instructions:
You will be presented with a series of sniff bottles containing human sweat. We wish you to tell us <by smell> which sex each of the odor samples comes from. The set of odors may include samples from both men and women, or from only men or from only women. Thus, some may be from females, some from males, or, alternatively, all may be from females or all from males. Therefore, don’t allow yourself to assume that some predetermined number of one or the other sex is represented.
The subjects were also required to estimate the relative intensity and pleasantness of the stimuli using a magnitude estimation procedure (Doty & Laing 2003). In one study, half of the stimuli were from men and half from women. In other studies, all of the stimuli were from women or from men.
The results of the study in which axillary odors from both men and women were smelled are presented in Figure 19.2. Four of the five male odors were correctly identified as male by at least half of the subjects, whereas three of the four female odors were correctly identified as female by at least half of the subjects. It is clear that stimulus intensity was strongly related to the sex assignments and that the blank (B) stimulus was nearly always assigned to the female category.
Figure 19.3 shows the results of the study in which only female odors were presented. In this case, the strongest odors were assigned to the male category and the weakest ones to the female category, even though all odors came from women. All subjects assigned the blank to the female category. These findings suggest that intensity and/or pleasantness was the primary basis for the sex classifications.
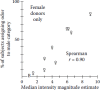
FIGURE 19.3
Relationship between perceived intensity and percent of subjects that assigned each axillary odor to the male gender category. All of the axillary odors came from women. B indicates a blank control. (Reprinted from Doty, R. L. (1981). Chem. Senses 6: (more...)
If human axillary odors serve as sex attractants without extensive conditioning, one would expect that pleasantness ratings of female axillary odors would be higher in men than in women, and that the reverse would be true for male axillary odors. However, we found that both men and women rated the male odors as more intense and less pleasant than female odors, with the relative magnitude of the responses of the two sexes being similar (rs > 0.90) (Doty et al. 1978). An inverse association was found between the overall intensity and pleasantness estimates of the odors (r = –0.94, p < 0.001), a nonculture-specific phenomenon observed by others (Schleidt et al. 1981).
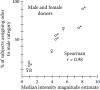
FIGURE 19.2
Relationship between perceived intensity, sex of odor donor, and percent of subjects assigning each axillary odor to the male gender category. Male and female symbols signify the sex of the odor donor. B indicates blank control. (From Doty, R. L. (1981). (more...)
It is telling that no differences in odor pleasantness or intensity are perceived when equivalent quantities of male and female apocrine sweat are incubated in vitro with aerobic coryneform bacteria, which produces characteristic body odor (Gower et al. 1994). Moreover, axillary hygiene markedly decreases the ability of subjects to correctly assign gender to axillary odors, in accord with the concept that relative intensity or pleasantness is the basis for the sex assignments (Schleidt 1980). When presented in equivalent volumes, axillary odors are not differentially pleasant to males and females, as would be expected if they contained sexually dimorphic pheromones associated with attraction or repulsion (Zeng et al. 1996). The extent to which learning influences hedonic responses to axillary odors is not known, although children who can identify their source as the armpits rate such odors as more intense and less pleasant than children who cannot identify their source, implying some role of concept learning. In accord with adult findings, adolescent girls rate axillary odors as more intense and less pleasant than do adolescent boys (Stevenson & Repacholi 2003).
A critical point is that no sex differences are present in the odorless precursor proteins within apocrine glands that are ultimately responsible for axillary odor, although males may be more prone than females to release their contents in relation to stressful stimuli (Spielman et al. 1998). Another critical point is that the intensity and pleasantness of axillary odors are significantly influenced by diet. In one study, 17 men were placed on a meat diet for 2 weeks and on a nonmeat diet for 2 weeks, with the order of the diets counterbalanced in time (Havlicek & Lenochova 2006). Axillary secretions were collected using gauze pads worn for 24 hours at the end of the dietary periods. The odors of donors on the meat diet were rated as less attractive, less pleasant, and more intense than the odors from the donors on the nonmeat diet. These findings suggest the possibility that some sex differences observed in axillary odor studies could be confounded by sex-specific dietary habits since, on average, men tend to eat much more meat than women (Shiferaw et al. 2012).
Do axillary secretions influence mood?
Like sights and sounds, there is no doubt that odors can influence human moods, emotions, and feelings. Is there any evidence that human secretions or excretions, such as those from the axillae, influence such emotions in ways different from other odors? Unfortunately, this topic has rarely been addressed scientifically and no study has provided control odorants to determine whether the purported effects are specific to the putative pheromonal stimuli.
In one of the few studies to have assessed the influences of human axillary odors on mood, Chen and Haviland-Jones (1999) concluded that “Exposure to underarm odors of older women, women, and older adults, led to a greater reduction in depressive mood than exposure to underarm odors of young men, men, and young adults.” In this study, gauze pads were worn in the axillae for several days by five prepubertal girls, five prepubertal boys, five college women, five college men, five older women, and five older men. Before and after smelling the odor-laden pads located in Petri dishes, subjects rated their current mood on the Differential Emotion Scale (Izard 1993). More than 300 volunteers took part, with each one smelling only one type of the aforementioned stimuli. However, a close and critical analysis of this study by Black (2001) found that the average positive mood actually declined in subjects exposed to the axillary odors from each of the six donor groups, contrary to the conclusions of the authors. Moreover, this critic pointed out that the Differential Emotion Scale was inappropriately employed as a measure of mood, since it is designed to determine how often different moods are experienced, not to assess short-term changes in mood. This suggested to Black that the subjects were likely confused as to how to respond on the post-odor test administration. Most importantly, Black noted that while Chen and Haviland-Jones collected data from a control condition (pads just left in homes but not placed in the armpit), these data were not properly included in their statistical analyses. When the mean data of each of the target groups was to with those of the controls, no statistically significant effects emerged. Black concluded (p. 216) that “it is clear that their work provides no evidence for their conclusion that certain human odors can decrease depressive mood.”
19.2.3. Androstenone, Androstenol, and Androstadienone as Human Pheromones
Dozens of studies have been performed on what have been purported, a priori, to be human pheromones, namely 5α-androst-16-en-3α-one (androstenone), 5α-androst-16-en-3α-ol (androstenol), or androsta-4,16-dien-3-one (androstadienone).
In this section I outline the basic logic of why it seems unlikely that these agents are human pheromones. The reader is referred elsewhere to reviews of the numerous studies that have employed these agents in attempts to show their pheromonal properties in humans (Havlicek et al. 2010; Doty 2010).
There are a number of reasons why such steroids have been assumed to be human pheromones by many workers (Doty 2010). These include the following:
- Being steroids, they fit into the pheromone concept of an “externally secreted hormone”
- They are among the few identified compounds that have been conceptually linked to mammalian reproductive behavior that some have deemed as pheromonal (i.e., lordosis in the female pig)
- They typically have urine- or musklike smells for those who can smell them, reinforcing the notion of their animal-like nature and the folklore that musks are social attractants in humans (Gower et al. 1985; Kloek 1961; Le Magnen 1952)
- They are commonly present, albeit at low levels, in human urine, axillary apocrine sweat, saliva, and semen (Brooksbank & Haslewood 1961; Brooksbank et al. 1974; Gustavson et al. 1987; Kwan et al. 1992; Nixon et al. 1988), making them potentially available to the external milieu for transfer from one person to another
- They occur in higher concentrations in men than in women, implying sexual dimorphism in their production (Gower & Ruparelia 1993; Lundström & Olsson 2005)
- Women are more sensitive, on average, to these agents than are men (Doty 1986), implying sexual dimorphism in their ability to be perceived
- In light of findings that humans can distinguish between the sexes to some degree on the basis of axillary and breath odors (Doty et al. 1978, 1982; Hold & Schleidt 1977), it is conceivable that these steroids serve to make this possible
- “Because androstenol has no known function in humans, these findings have suggested to several investigators that the steroid may function as a human pheromone” (Gustavson et al. 1987)
While such observations seem convincing on the surface, close scrutiny reveals the following issues:
- In reality, the levels of such steroids in the human axillae are low and highly variable. Using capillary gas chromatography–mass spectrometry with specific ion monitoring, Nixon et al. found that only 10 of 24 men had androstenone in their axillary hair (Nixon et al. 1988) and no relationship was evident between the age of the donors and presence of the steroid. Others, using different analytical methods, have reported finding no androstenone in samples of fresh apocrine sweat or secretions sampled by sterile gauze pads (Bird & Gower 1981; Labows et al. 1979). Before bacterial action, androstadienone levels are too low to be detected in the axillae by smell (Gower et al. 1994; Labows 1988).
- It does not follow that simply because these compounds are found in body fluids or axillae that they communicate meaningful social information or influence reproductive processes in humans. Indeed, androstenone and androstenol are common in the animal and plant kingdoms, being found even in the roots of vegetables such as parsnip and celery (Claus & Hoppen 1979). In one study, for example, androsterone was found in 60–80% of the plant species investigated (Janeczko & Skoczowski 2005).
- Under the assumption that olfaction is involved, none of these steroids contributes much to the generation of prototypical body odor, which arises largely from a mixture of C6-C11 normal, branched, and unsaturated acids (Hasegawa et al. 2004; Zeng et al. 1991, 1992).
- A significant number of persons cannot smell androstenone and related steroids (Amoore et al. 1977; Koelega 1980; Ohloff et al. 1983), although exposure to high concentrations can result in eventual detection in some individuals (Wysocki et al. 1989). This fact, along with evidence that most persons who can smell these agents find them repulsive or unpleasant (Gower et al. 1985; Jacob et al. 2006; Koelega 1980), would seem to limit their value in social interactions considered as reflecting influences from “sex pheromones.” In the case of androstadienone, repeated exposure results in an increase in its perceived unpleasantness (Boulkroune et al. 2007).
- It is questionable as to whether musky or urine-like smells, as such, reflect a logical criterion for defining odorants as pheromones.
- Sex differences and subtle menstrual cycle-related fluctuations are present for a wide range of odorants, including synthetic ones, so there seems to be nothing special about these agents in this regard (Doty & Cameron 2009).
- As noted earlier in this chapter, the ability of humans to determine the sex of another human on the basis of axillary odors as well as other odors common to the sexes such as breath odors, appears to be dependent on the intensity or pleasantness of the involved odors, not on the intrinsic chemical makeup of the secretions (Doty et al. 1978, 1982).
- If pheromones are species-specific, which is inherent in the original and most subsequent definitions of pheromones (Doty 2010), generalizing findings from pig studies to human studies is an oxymoron.
- Lack of knowledge of a known function of a secretion does not increase the likelihood that is serves as a human pheromone.
The concept that androstenone and androstenol are pheromones in pigs originally stemmed from observations that they increased the incidence of the standing response of sows when sprayed near the sows’ noses (Hafez & Signoret 1969; Melrose et al. 1971; Patterson 1966, 1968; Stefanczyk-Krzymowska et al. 2000). As noted by Claus and Hoppen (1979, p. 1674), “For female pigs in oestrus … <androstenone> is a very desirable ‘male perfume’ which is released by the boar’s saliva before mating and stimulates the female’s ‘standing reflex,’ thus acting as an aphrodisiac pheromone.”
It should be emphasized, however, that androstenone and androstenol do not have invariant influences even on the mating stance of sows. As shown in Table 19.1, such steroids fail to facilitate lordosis in all sows and are not the sole stimuli that facilitate pressure-induced standing. Sows do not lordose for all males, likely exhibiting mating preferences analogous to those that occur in dogs (Beach & LeBoeuf 1967). The most parsimonious explanation of the behavioral effects of androstenone and androstenol in the domestic pig is that the sow is conditioned through experience to exhibit lordosis in response to the smell of these agents. Albone (1984) summarizes the complexity as follows (p. 238):
TABLE 19.1
Influence of Various Steroids Found in Sexually Mature Boar Secretions on Pressure-Induced Lordosis in Estrous Sows
The situation is, however, a little more complicated than it seems. The oestrous sow will ‘stand’ in response not only to olfactory signals, but also, for example, to the sound of the boar’s grunting. In the natural situation the sow is exposed to a simultaneous combination of cues of many kinds, olfactory, visual, tactile and auditory, all of which play some part in stimulating the standing response, although it is clear that among these, olfactory signals are very important. Also, it is found experimentally that the oestrous sow will stand in response to the odour of boar urine or boar preputial fluid, substances in which these particular C19-Δ16 steroids <androstenone and androstenol> are either absent or present at very low levels. Further, the oestrous female will respond to varying degrees to the odours of some other closely related steroids.
If these steroids were truly effective as pheromones in humans, one might ask the following questions: Are women, in fact, attracted to the odors of male pigs or more willing to have sex in the presence of such odors? Are birth rates or other indices of sexual behavior higher in states or counties with pig farms?
19.2.4. Human Menstrual Synchrony Pheromones
A highly publicized 1971 Nature paper reported that the menstrual cycles of close friends or dormitory roommates synchronize over time (i.e., the onset of their periods of menstrual bleeding became closer over a 6-month period) (McClintock 1971). Many studies subsequently reported similar synchrony (for review, see Doty 2010). However, no chemical identification of the alleged pheromone has yet been made. Importantly, as described below, literature has since appeared that questions, largely on statistical grounds, whether menstrual synchrony itself is a true phenomenon with a viable evolutionary basis (Arden & Dye 1998; Schank 1997, 2000, 2001, 2006; Strassmann 1997, 1999; Wilson 1987, 1992; Yang & Schank 2006; Ziomkiewicz 2006).
Does menstrual synchrony exist?
On the basis of statistical issues, Wilson (1987) concluded that synchrony was not demonstrated in any of studies performed up to the time of his analysis (i.e., studies by Graham & McGrew 1980; McClintock 1971; Preti et al. 1986; Quadagno et al. 1981; Russell et al. 1980). He noted that the only apparent difference between studies reporting and not reporting synchrony was that the latter included persons with irregular menstrual cycles. When persons with such cycles were omitted from analysis, the results were biased towards synchrony. He described three sources of error that were inherent in the McClintock method of synchrony analysis as follows:
- Error I: The assumption that differences between menses onsets of randomly paired subjects vary randomly over consecutive onsets. This reflects the failure to account for the fact that ~50% of paired cycles of unequal length will show a tendency to synchronize by chance when relatively few cycles are evaluated.
- Error II: The incorrect determination of the initial onset of absolute differences between subjects. Two issues are involved:
- 1. An incorrect onset difference (which only occurs for the initial onset calculations in McClintock’s method) is always greater than a correct onset difference (which occurs for subsequent onset calculations), thereby increasing the mean onset absolute difference and erroneously leading to what seems to be synchrony in subsequent onsets.
- 2. An incorrect onset difference reverses the direction of change between the consecutive onset differences of a pair. This occurs because the subject with the earliest recorded onset has the latest recorded onset after the correction.
- Error III: Exclusion of subject data on the basis of not having the number of onsets specified by the research design, which biases samples toward showing menstrual synchrony by reducing dispersion in final onset absolute differences, a common phenomenon in studies finding evidence of menstrual synchrony.
A simple explanation of Error II appeared in Cecil Adam’s column, the Straight Dope, in the Chicago Reader newspaper (Adams 2002). Assume the menstrual cycle study starts on October 1 (see Figure 19.4). The first study subject reports a 28-day-cycle with an onset of menses on September 27, another onset on October 25, and a third on November 22. The second study subject, with a 30-day-cycle, reports a menses onset on October 5 and another on November 4. Using McClintock’s calculation in which only cycle onsets are recorded within the study period, 20 days separated the two menses onset dates (October 5 vs. October 25) and 18 days separated the second pair of menses onset dates (November 4 vs. November 22). This calculation would suggest that the two cycles are synchronizing; that is, going from 20 to 18 days, when, in fact, they were eight days apart to begin with (September 27 vs. October 5). In fact, the two cycles are actually diverging from one another (November 4 – October 25 = 10 days relative to the original 8 days).

FIGURE 19.4
Demonstration of how calculating cycle lengths according the McClintock procedure leads to an erroneous conclusion of synchrony. See text for details.
In an attempt to overcome such problems, Weller and Weller employed a “last months only” (LMO) paradigm in establishing synchrony (e.g., Weller & Weller 1993a, b, 1997a, b, 1998; Weller et al. 1999a, b). In this procedure expected frequencies of onset differences are calculated from random onset occurrences or new random pairs of women from the sample.
Unfortunately, the LMO approach has its own set of limitations, some of which reflect issues related to volunteering, accurate record keeping, and provision of requested data (e.g., return of menstrual calendars; Arden & Dye 1998; Schank 2000, 2001). In a computer simulation of the LMO procedure, Schank (2000) found that cycle variability introduced a systematic bias toward synchrony; the greater variability in the simulated cycle distribution, the greater the bias. Even when cycle onsets are completely randomly related, he found that the LMO synchrony measurement leads to data distributions skewed toward synchrony “in a way that is qualitatively and quantitatively like the actual data distributions they <Weller and Weller> report.”
The assumption that menstrual synchrony, if indeed present, has biological meaning was questioned by Strassmann 1997, who pointed out that in most preindustrialized societies pregnancy and lactation, not menstrual cycling, takes up the majority of the female’s reproductive years. In a long-term prospective study of the Dogon of Mali, Strassmann examined 477 untruncated menstrual cycles from 58 women over a 2-year period (Strassmann 1997). In the Dogon society, menstruating women are segregated in special huts at night. Accurate information about the onset of menses was obtained from a nightly census of women present in the huts (736 days). This allowed data collection without interviews and errors in recall or reporting. Compared to American women who have, on average, more than 400 menstruations in their lifetimes, Dogon women have an average of only 128 menstruations. The proportion of women cycling on a given day was found to be ~25%. Sixteen percent were pregnant, 29% were in lactational amenorrhea, and 31% were postmenopausal. Subfecund women were most common among the cycling women, and conception usually occurred for the most fecund women on one of their first postpartum ovulations, resulting in their dropping out of the pool of regularly menstruating women. No evidence for synchrony was found for the cycling women who habitually ate and worked together or who lived with a particular lineage of related males. Similarly, no evidence for synchrony was found for any of the remaining cycling women. Strassmann concluded (p. 128), “Given the paucity of evidence, it is surprising that belief in menstrual synchrony is so widespread. I suggest that this belief arises, in part, from a popular misconception about how far apart one would expect the menstrual onsets of two women to be by chance alone.” Strassmann further elaborated on this point elsewhere (Strassmann 1999, p. 579):
Popular belief in menstrual synchrony stems from a misperception about how far apart menstrual onsets should be for two women whose onsets are independent. Given a cycle length of 28 days (not the rule – but an example), the maximum that two women can be out of phase is 14 days. On average, the onsets will be 7 days apart. Fully half the time they should be even closer (Wilson 1992; Strassmann 1997). Given that menstruation often lasts 5 days, it is not surprising that friends commonly experience overlapping menses, which is taken as personal confirmation of menstrual synchrony.
Such studies cast significant doubt on whether menstrual synchrony is a real phenomenon. If synchrony is, in fact, biologically meaningful, it would seem more important to focus on ovulation than on menses since menses is an imprecise index of synchrony, particularly when anovulatory cycles are included (Weller & Weller 1997b). In the unlikely event that menstrual synchrony is present in some groups of subjects under very specific circumstances, are “pheromones” involved in the synchronization process? As noted in the next section, evidence for such involvement seems weak, and like synchrony itself, is fraught with procedural issues (e.g., Doty 1981; Schank 2002, 2006; Whitten 1999; Wilson 1987, 1992).
If menstrual synchrony exists, what evidence is there that pheromones are involved?
The first claim of a demonstration of pheromone-induced synchronization of menses was that of Russell et al. 1980). These investigators collected axillary secretions on gauze pads taped under the arm of a woman who had a history of regular 28-day menstrual cycles and a “previous experience of ‘driving’ another woman’s menstrual cycle on three separate occasions, over three consecutive years; i.e., a friend had become synchronous with her when they roomed together in the summer and desynchronized when they moved apart in the fall.” The pads were cut into four squares, combined with four drops of 70% alcohol, and frozen in dry ice. Following thawing, the material from appropriate phases of the cycle was rubbed on the upper lips of five women, three times a week, for four months. Six control women had their lips similarly rubbed with pads that had received only the alcohol treatment. A mean pretreatment difference of 9.3 days between the day of the onset of the donor’s menses and those of the subjects was observed. After 4 months of treatments, this difference decreased to 3.4 days. The authors concluded, “The data indicate that odors from one woman may influence the menstrual cycle of another and that these odors can be collected from the underarm area, stored as frozen samples, for at least short periods, and placed on another woman. Further, the experiment supports the theory that odor is a communicative element in human menstrual synchrony, and that at least a rudimentary form of olfactory control of the hormonal system is occurring in humans in a similar fashion to that found in other mammals.”
Unfortunately, this study has several problems. First, it was not performed either single- or double-blind. Second, the woman who donated the samples (the second author of the paper) also acted as one of the two female experimenters who rubbed the stimuli on the subjects (Doty 1981). Aside from potentially providing subtle social cues that might affect the experiment’s outcome, under the assumption that pheromones are actually involved, this would confound the experiment with a second source of pheromones (i.e., those on her person as she interacted with the subjects). Third, the purpose of the study was explained to each subject, potentially introducing another possible factor that might influence cycle lengths.
Wilson (1992) examined the data of this study in light of the three errors outlined on page 541 indicating that the study
“… shows evidence of all three errors: The number of synchronous cases is too few to be statistically significant (Error I), one of the four synchronous cases has an incorrect initial onset difference which, when corrected, causes the initial mean onset difference to be greater than the final mean onset difference (Error II), and one or more subjects may have withdrawn from the experiment because their cycle behavior was not meeting the expectations of the investigators (Error III). I conclude that Russell et al. (1980) did not demonstrate menstrual synchrony in subjects treated with axillary extract from a female donor.”
A subsequent study by Preti et al. (1986) sought to correct some of the methodological problems of the Russell et al. study. Double-blinding was employed and the purpose of the study was explained to the subjects only after study completion. The 19 subjects were selected from a larger number on the basis of self-reports of regular cycles (29.5 ± 3 days) in an effort to minimize the potential adverse influences of highly irregular cycles. In a procedure similar to that of the Russell study, axillary secretions from cotton pads previously worn in the axillae during “a convenient 6- to 9-hr period” of four female donors was applied in an alcohol base to the upper lips of 10 subjects three times a week for three complete menstrual cycles. The stimuli employed reflected 3-day segments of the cycles of all four donors from which they were collected. This produced a set of “donor cycle” stimuli whose midpoints consisted of cycle days 2, 5, 8, 11, 14, 17, 20, 23, 26, and 29. The extracts were applied at 22- to 25-day intervals. After two complete cycles, 8 of the 10 subjects in the experimental group reportedly synchronized with the extract treatment schedules, whereas only 3 of 9 of the control women did so. The authors conclude (pp. 480–481) that “This study represents the first systematically designed, prospectively conducted, double-blind research in humans to attempt to manipulate the menstrual cycle with female-derived secretions. In this experiment naturally occurring 29.5 ± 3 day cycles could be modulated with repeated applications of extract at a 22 to 25 day interval. This study establishes phenomena in humans which are analogous to previously demonstrated olfactory/reproductive relationships in nonhuman mammals.”
Preti et al.’s data were reanalyzed by Wilson (1987) who concluded that “the apparent synchrony in menses onsets in the axillary extract sample is explained on the bases of (a) chance variations, (b) mathematical properties of cocycling menses onsets, (c) features of the experimental design, and (d) failure to follow the experimental protocol, or calculation errors, or both.” In his reanalysis, Wilson found 20 instances, equally divided between the experimental and control group data, where the cycle length of the treatment application fell outside of the 22- to 25-day range stipulated in the protocol. In the extract sample, the donor’s cycle was found to be greater than 25 days in 9 instances, and less than 22 days in one instance, a point later acknowledged by Preti (1987). Wilson summarized his findings as follows:
In summary, the equal distribution of five preovulatory and five postovulatory cases in the extract sample is due to chance. Eight of these cases are shown <in Table 1> as having decreased absolute onset differences between the first and third onsets of the subjects and donor. The decreases in the four preovulatory cases, including two cases in which the subject had constant cycle lengths, are interpreted as a product of the experimental design, the mathematical properties of cocycling menses onsets, and chance variations. The decreases in the four postovulatory cases, including one case with constant cycle lengths, are interpreted as the result of “errors” in the cycle lengths of the treatment applications. If all of the treatment cycles were in the 22- to 25-day range specified by the experimental protocol, the extract sample would have the characteristics of a sample of randomly paired subjects. No evidence in this experiment suggests that the 29.5 ± 3 day cycles of the subjects in the extract sample were modulated by the applications of the female axillary extract or that humans have phenomena analogous to olfactory/reproductive relationships demonstrated in nonhuman mammals.
In another McClintock study published in Nature, Stern and McClintock (1998) reported (pp. 177–178) finding that “that odourless compounds from the armpits of women in the late follicular phase of their menstrual cycles accelerated the preovulatory surge of luteinizing hormone of recipient women and shortened their menstrual cycles. Axillary compounds from the same donors which were collected later in the menstrual cycle (at ovulation) had the opposite effect: they delayed the luteinizing-hormone surge of the recipients and lengthened their menstrual cycles. By showing in a fully controlled experiment that the timing of ovulation can be manipulated, this study provides definitive evidence of human pheromones.”
Unfortunately, this study did not take into account the statistical issues previously pointed out by Wilson and others. Nine donor women wore cotton pads in their axillae for 8 or more hours after bathing. The pads were collected daily, along with urinary LH and other information (e.g., menses, basal body temperature). This allowed them to “classify each pad as containing compounds produced during the follicular phase (2 to 4 days before the onset of the LH surge) or the ovulatory phase (the day of the LH surge onset and the 2 subsequent days).” The pads were prepared in a manner similar to those of Preti et al. and stored –80°C until use. Data from one initial cycle, when exposure to the axillary stimuli was made, was first obtained. During the next four cycles, the axilary secretions were then applied daily to the subjects’ upper lips. Ten subjects received rubs from pads, collected from donors during the follicular phase, each day for two menstrual cycles and then from pads collected from ovulatory phase donors for the next two cycles. The reverse was the case for the other 10 subjects. The donors served as a control group, receiving only the 70% alcohol carrier each day.
According to these investigators, the stimuli from the follicular phase produced shorter cycles than those from the ovulatory phase (–1.7 ± 0.9 days vs. +1.4 ± 0.4 days). Surprisingly, this effect occurred within the first cycle, unlike the synchrony in earlier work that took more than one cycle. The carrier had no effect on cycle lengths of the controls. The authors noted that “In five of the cycles, women had mid-cycle nasal congestion, which could have prevented their exposure to pheromones; including these cycles in the analysis made the results slightly less robust (follicular compounds: –1.4 ± 0.9 days; ovulatory compounds: +1.4 ± 0.5 days; ANOVA: follicular versus ovulatory compounds F (1,18) = 4.32, P ≤ 0.05; cycle 1 versus cycle 2 of exposure (not significant; NS); order of presentation (NS); alternations between factors were not significant).”
In a second component of the study, Stern and McClintock sought to “determine the specific mechanism of pheromone action.” To do so, they utilized the LH and progesterone data to establish the follicular and luteal cycle phases. They then “traced all the changes caused by the pheromones presented in our study to the follicular phase. For the menses and luteal phases, the distributions during the pheromone and control conditions were the same (indicated by overlapping log-survivor curves). Only the follicular phase was regulated, shortened by follicular compounds and lengthened by ovulatory compounds, suggesting that these ovarian-dependent pheromones have opposite effects on the recipient’s ovulation by differentially altering the rate of follicular maturation or hormonal threshold for triggering the LH surge.” They concluded that “This experiment confirms the coupled oscillator model of menstrual synchrony and refocuses attention on the ovarian-dependent pheromones that regulate ovulation, producing either synchrony, asynchrony or cycle stabilization within a social group, namely two distinct pheromones, produced at different times of the cycle, which phase-advance or phase-delay the preovulatory LH surge.”
The Stern and McClintock study, which in fact identified no putative pheromone or pheromones, has come under considerable criticism. For example, Schank (2006) points out that in their analysis of the five cycles, the investigators subtracted the onset dates of cycle 1 from those of cycles 2 and 3, and the onset dates of cycle 3 from those of cycles 4 and 5, rather than subtracting the onset dates of the first cycle from that of the following four cycles. Thus, cycle 3, in which axillary odor was being applied, was treated as a baseline period when, in fact, it was a treatment period. In his critique, Schank provided examples of why such an analysis is flawed. Moreover, he demonstrated how random data sets drawn from a truncated normal distribution with the means and standard deviations reported by Stern and McClintock become statistically significant only after being transformed using the flawed McClintock analysis procedure.
Strassmann (1999) has pointed out that Stern and McClintock disregarded all of the methodological problems with the McClintock procedure for establishing synchrony and questioned the statistical robustness of their findings (p. 580):
The conclusion that a change in cycle lengths of the subjects was caused by a pheromone, rather than by the well-documented variation in cycle length in women (Treloar, Boynton, Behn, & Brown 1967; Harlow & Zeger 1991), requires inordinate confidence in the biological importance of a P value of borderline statistical significance (P ≤ 0.055). From the data presented it is unclear whether the assumption of a normal distribution was justified. Moreover, in view of the small sample size, the entire effect might have been due to just one or two subjects who had undue leverage. Additional questions are raised by the following statement (Stern and McClintock, 1998): ‘Any condition preventing exposure to the compounds, such as nasal congestion anytime during the mid-cycle period from 3 days before to 2 days after the preovulatory LH, could weaken the effect. We analyzed the data taking this into account.’ It would be useful to know what a priori criteria were employed in making such adjustments, and whether the data analysis part of the project was done blind. In the absence of a theoretical reason for expecting menstrual synchrony to be a feature of human reproductive biology, and until a cycle-altering pheromone has been chemically isolated, it would appear that skepticism is warranted.
Similarly, Whitten (1999) questioned the validity of the Stern and McClintock study. Like Strassmann, he pointed out that “Each group has an apparent outlier favourable to the model: one of –14 comprises 25% of the total shortening, whereas that of +12 makes up 22% of the increase. Excluding these two outliers would abolish the claim of significance.” However, his major point of concern was as follows:
My main criticism of the study is the use of the value of single first cycles, receiving carrier-only treatment, to derive the data analyzed. Such single observations have no within-subject variance and the irregular statistical manoeuvre of converting all 20 observations to zero masks any between-subject variance and provides an illusory zero baseline with indeterminate confidence limits. Carrier-only treatments should have been distributed throughout this long experiment to give a balanced crossover design with three treatments (carrier, follicular and ovulatory) and two or more complete replications to confer confidence limits to the baseline observations, thus making comparisons valid.
This pioneer of mammalian pheromonology goes on to state, “I am not convinced of the validity of the coupled-oscillator model derived from rat studies. I also question the ‘definitive evidence’ that pheromones regulate human ovarian function because, if these exist, their characterization will require large, carefully designed experiments, a controlled social and physical environment, and a clearly defined endpoint measured in hours.”
Space does not permit in this chapter a review of critiques of the problems associated with the other element of the Stern and McClintock study, namely the changing of the timing of the LH surge. The reader is referred to Doty (2010) for such a review.
19.3. CONCLUSIONS
In this chapter I have provided a snapshot of the history of the pheromone concept and examples of its common applications to humans. As described in detail elsewhere (Doty 2010), there is a lack of consensus as to what defines a pheromone, making it difficult to tie down as an objective scientific entity. Most definitions imply that a pheromone (a) is comprised of one or only a few chemicals, (b) is species-specific, (c) has well-defined behavioral or endocrine effects, and (d) is little influenced by learning. To date, no chemicals have been isolated in humans that meet such criteria.
REFERENCES
- Adams C. Does menstrual synchrony really exist? The Straight Dope, The Chicago Reader. 2002 December 20.
- Albone E. S. Mammalian Semiochemistry. New York: John Wiley & Sons; 1984.
- Amoore J. E, Pelosi P, Forrester L. J. Specific anosmia to 5a-androst-16en-3-one and gamma-pentadecalactone: The urinous and musky primary odors. Chem. Senses Flav. 1977;2:401–425.
- So much more to know. Science. 2005;309:78–102. Anonymous. [PubMed: 15994524]
- Arden M. A, Dye L. The assessment of menstrual synchrony: Comment on Weller and Weller. J. Comp. Psychol. 1998;1997;112:323–324. [PubMed: 9770318]
- Beach F. A, LeBoeuf B. J. Coital behavior in dogs. 1. Preferential mating in the bitch. Anim. Behav. 1967;15:546–558. [PubMed: 5633411]
- Berliner D. L, Monti-Bloch L, Jennings-White C, Diaz-Sanchez V. The functionality of the human vomeronasal organ (VNO): Evidence for steroid receptors. J. Steroid Biochem. Mol. Biol. 1996;58:259–265. [PubMed: 8836161]
- Bethe A. Vernachlässigte Hormone. Naturwissenschaften. 1932;11:177–181.
- Birch M. C. Pheromones. New York: American Elsevier; 1974.
- Bird S, Gower D. B. The validation and use of a radioimmunoassay for 5 alpha-androst-16-en-3-one in human axillary collections. J. Steroid Biochem. 1981;14:213–219. [PubMed: 7193782]
- Black S. L. Does smelling granny relieve depressive mood? Commentary on “Rapid mood change and human odors.” Biol. Psychol. 2001;55:215–225. [PubMed: 11240215]
- Booth W. D. Endocrine and exocrine factors in the reproductive behaviour of the pig. Sym. Zool. S. London. 1980;45:289–311.
- Boulkroune N, Wang L. W, March A, Walker N, Jacob T. J. C. Repetitive olfactory exposure to the biologically significant steroid androstadienone causes a hedonic shift and gender dimorphic changes in olfactory-evoked potentials. Neuropsychopharmacology. 2007;32:1822–1829. [PubMed: 17251914]
- Bronson F. H. Pheromonal influences on mammalian reproduction. In: Diamond M, editor. Pheromonal Influences on Mammalian Reproduction. Bloomington, IN: Indiana University Press; 1968. pp. 341–361. In.
- Bronson F. H. Urine marking in mice: causes and effects. In: Doty R. L, editor. Mammalian Olfaction, Reproductive Processes and Behavior. New York: Academic Press; 1976. pp. 119–143. In.
- Brooksbank B. W. L, Brown R, Gustafsson J.-A. The detection of 5α-Androst-16-en-3α-ol in human male axillary sweat. Experientia. 1974;30:864–865. [PubMed: 4416149]
- Brooksbank B. W. L, Haslewood G. A. D. The estimation of androsten-16-en-3α-ol in human urine. Biochem. J. 1961;80:488–496. [PMC free article: PMC1243258] [PubMed: 16748922]
- Brown R. E. Mammalian social odors: A critical review. In: Rosenblatt J.S, editor. Advances in the Study of Behavior. Vol. 10. New York: Academic Press; 1979. pp. 103–162. In.
- Brownlee R. G, Silverstein R. M, Müller-Schwarze D, Singer A. G. Isolation, identification, and function of the chief component of the male tarsal scent in black-tailed deer. Nature. 1969;221:284–285.
- Caroom D, Bronson F. H. Responsiveness of female mice to preputial attractant: Effects of sexual experience and ovarian hormones. Physiol. Behav. 1971;7:659–662. [PubMed: 5164357]
- Carr W. J, Loeb L. S, Dissinger M. E. Responses of rats to sex odors. J. Comp. Physiol. Psychol. 1965;59:370–377. [PubMed: 14313776]
- Carr W. J, Loeb L. S, Wylie N. R. Responses to feminine odors in normal and castrated male rats. J. Comp. Physiol. Psychol. 1966;62:336–338. [PubMed: 6008017]
- Carter C. S, Marr J. N. Olfactory imprinting and age variables in the guinea-pig. Cavia porcellus. Anim. Behav. 1970;18:238–244. [PubMed: 5466043]
- Chen D, Haviland-Jones J. Rapid mood change and human odors. Physiol. Behav. 1999;68:241–250. [PubMed: 10627087]
- Claus R, Hoppen H. O. The boar-pheromone steroid identified in vegetables. Experientia. 1979;35:1674–1675. [PubMed: 520500]
- Comfort A. Likelihood of human pheromones. Nature. 1971;230:432–433. [PubMed: 4932036]
- Curtis R. F, Ballantine J. A, Keveren E. B, Bonsall R. W, Michael R. P. Identification of primate sexual pheromones and the properties of synthetic attractants. Nature. 1971;232:396–398. [PubMed: 4999879]
- Doty R. L. Olfactory communication in humans. Chem. Senses. 1981;6:351–376.
- Doty R. L. Gender and endocrine-related influences upon olfactory sensitivity. In: Meiselman H. L, Rivlin R. S, editors. Clinical Measurement of Taste and Smell. New York: Macmillan; 1986. pp. 377–413. In.
- Doty R. L. The olfactory vector hypothesis of neurodegenerative disease: Is it viable? Ann. Neurol. 2008;63:7–15. [PubMed: 18232016]
- Doty R. L. The Great Pheromone Myth. Baltimore MD: Johns Hopkins University Press; 2010.
- Doty R. L, Cameron E. L. Sex differences and reproductive hormone influences on human odor perception. Physiol. Behav. 2009;97:213–228. [PMC free article: PMC2693767] [PubMed: 19272398]
- Doty R. L, Green P. A, Ram C, Yankell S. L. Communication of gender from human breath odors: Relationship to perceived intensity and pleasantness. Horm. Behav. 1982;16:13–22. [PubMed: 7068124]
- Doty R. L, Laing D. G. Psychophysical measurement of olfactory function, including odorant mixture assessment. In: Doty R. L, editor. Handbook of Olfaction and Gustation. Second. New York: Marcel Dekker; 2003. pp. 203–228. In.
- Doty R. L, Orndorff M. M, Leyden J, Kligman A. Communication of gender from human axillary odors: Relationship to perceived intensity and hedonicity. Behav. Neural Biol. 1978;23:373–380. [PubMed: 697690]
- Gower D. B, Bird S, Sharma P, House F. R. Axillary 5 alpha-androst-16-en-3-one in men and women: Relationships with olfactory acuity to odorous 16-androstenes. Experientia. 1985;41:1134–1136. [PubMed: 4043321]
- Gower D. B, Holland K. T, Mallet A. I, Rennie P. J, Watkins W. J. Comparison of 16-androstene steroid concentrations in sterile apocrine sweat and axillary secretions: interconversions of 16-androstenes by the axillary microflora—A mechanism for axillary odour production in man? J. Steroid Biochem. Mol. Biol. 1994;48:409–418. [PubMed: 8142319]
- Gower D. B, Ruparelia B. A. Olfaction in humans with special reference to odorous 16-androstenes: Their occurrence, perception and possible social, psychological and sexual impact. J. Endocrinol. 1993;137:167–187. [PubMed: 8326246]
- Graham C. A, McGrew W. C. Menstrual synchrony in female undergraduates living on a coeducational campus. Psychoneuroendocrinology. 1980;5:245–252. [PubMed: 7191131]
- Gustavson A. R, Dawson M. E, Bonett D. G. Androstenol, a putative human pheromone, affects human (Homo sapiens) male choice performance. J. Comp. Psychol. 1987;101:210–212. [PubMed: 3608426]
- Hafez E. S. E, Signoret J. P. The behaviour of swine. In: Hafez E. S. E, editor. The Behavior of Domestic Animals. Baltimore, MD: Williams and Wilkins; 1969. pp. 349–390. In.
- Harlow S. D, Zeger S. L. An application of longitudinal methods to the analysis of menstrual diary data. J. Clin. Epidemiol. 1991;44:1015–1025. [PubMed: 1940994]
- Hasegawa Y, Yabuki M, Matsukane M. Identification of new odoriferous compounds in human axillary sweat. Chem. Biodivers. 2004;1:2042–2050. [PubMed: 17191839]
- Havlicek J, Lenochova P. The effect of meat consumption on body odor attractiveness. Chem. Senses. 2006;31:747–752. [PubMed: 16891352]
- Havlicek J, Murray A. K, Saxton T. K, Roberts S. C. Current issues in the study of androstenes in human chemosignaling. Vitam. Horm. 2010;83:47–81. [PubMed: 20831942]
- Hold B, Schleidt M. The importance of human odour in non-verbal communication. Zeitschrift für Tierpsychologie. 1977;43:225–238. [PubMed: 868320]
- Holldobler B. Multimodal signals in ant communication. J. Comp. Physiol. A. 1999;184:129–141.
- Izard C. E, Libero D. Z, Putnam P, Haynes O. M. Stability of emotion experiences and their relations to traits of personality. J. Pers. Soc. Psychol. 1993;64:847–860. [PubMed: 8505713]
- Jacob T. J. C, Wang L. W, Jaffer S, McPhee S. Changes in the odor quality of androstadienone during exposure-induced sensitization. Chem. Senses. 2006;31:3–8. [PubMed: 16280418]
- Janeczko A, Skoczowski A. Mammalian sex hormones in plants. Fol. Histochem. Cytobiol. 2005;43:71–79. [PubMed: 16044944]
- Karlson P, Butenandt A. Pheromones (ectohormones) in insects. Ann. Rev. Entomol. 1959;4:39–58.
- Karlson P, Lüscher M. “Pheromones”: A new term for a class of biologically active substances. Nature. 1959;183:55–56. [PubMed: 13622694]
- Kloek J. The smell of some steroid sex-hormones and their metabolites: Reflections and experiments concerning the significance of smell for the mutual relation of the sexes. Psychiat. Neurol. Neurochir. 1961;64:309–344. [PubMed: 14036856]
- Koelega H. S. Preference for and sensitivity to the odours of androstenone and musk. In: van der Starre H, editor. Proceedings of the 7th International Symposium on Olfaction & Taste and the 4th Congress of the ECRO. London: IRL Press; 1980. In.
- Kwan T. K, Kraevskaya M. A, Makin H. L, Trafford D. J, Gower D. B. Use of gas chromatographic-mass spectrometric techniques in studies of androst-16-ene and androgen biosynthesis in human testis; cytosolic specific binding of 5alpha-androst-16-en-3-one. J. Steroid Biochem. Mol. Biol. 1997;60:137–146. [PubMed: 9182868]
- Kwan T. K, Trafford D. J, Makin H. L, Mallet A. I, Gower D. B. GC-MS studies of 16-androstenes and other C19 steroids in human semen. J. Steroid Biochem. Mol. Biol. 1992;43:549–556. [PubMed: 1419890]
- Labows J. Odor detection, generation and etiology in the axilla. In: Felger C, Laden K, editors. Antiperspirants and Deodorants. New York: Marcel Dekker; 1988. pp. 321–343. In.
- Labows J. N, Preti G, Hoelzle E, Leyden J, Kligman A. Steroid analysis of human apocrine secretion. Steroids. 1979;34:249–258. [PubMed: 158859]
- Le Magnen J. Les phénoménes olfacto-sexuels chez l’homme. C. R. Acad. Sci. Biol. 1952;6:125–160. [PubMed: 12987088]
- Lee C. T, Griffo W. Progesterone antagonism of androgen-dependent aggression-promoting pheromone in inbred mice (Mus musculus). J. Comp. Physiol. Psychol. 1974;87:150–155. [PubMed: 4472259]
- Leyden J. J, McGinley K. J, Holzle E, Labows J. N, Kligman A. M. The microbiology of the human axilla and its relationship to axillary odor. J. Invest. Dermatol. 1981;77:413–416. [PubMed: 7288207]
- Lundström J. N, Olsson M. J. Subthreshold amounts of social odorant affect mood, but not behavior, in heterosexual women when tested by a male, but not a female, experimenter. Biol. Psychol. 2005;70:197–204. [PubMed: 16242537]
- Mainardi D, Marsan M, Pasquali A. Causation of sexual preferences of the house mouse. The behaviour of mice reared by parents whose odour was artificially altered. Atti. Soc. Ital. Sci. Nat. 1965;104:325–338.
- Marr J. N, Lilliston L. G. Social attachment in rats by odor and age. Behaviour. 1969;XXXIII:277–282.
- Martin I. G. Letter to the editor. J. Chem. Ecol. 1980;6:517–519.
- McClintock M. K. Menstrual synchorony and suppression. Nature. 1971;229:244–245. [PubMed: 4994256]
- Melrose D. R, Reed H. C, Patterson R. L. Androgen steroids associated with boar odour as an aid to the detection of oestrus in pig artificial insemination. Br. Vet. J. 1971;127:497–502. [PubMed: 5167342]
- Michael R. P, Keverne E. B. Primate sex pheromones of vaginal origin. Nature. 1970;225:84–85. [PubMed: 4983029]
- Michael R. P, Keverne E. B, Bonsall R. W. Pheromones: Isolation of male sex attractants from a female primate. Science. 1971;172:964–966. [PubMed: 4995585]
- Monti-Bloch L, Jennings-White C, Dolberg D. S, Berliner D. L. The human vomeronasal system. Psychoneuroendocrinology. 1994;19:673–686. [PubMed: 7938363]
- Mugford R. A, Nowell N. W. The dose-response to testosterone propionate of preputial glands, pheromones and aggression in mice. Horm. Behav. 1972;3:39–46. [PubMed: 4681735]
- Müller-Schwarze D. Pheromones in black-tailed deer (Odocoileus heminonus columbianus). Anim. Behav. 1971;19:141–152. [PubMed: 5149786]
- Müller-Schwarze R, Müller-Schwarze D, Singer A. G, Silverstein R. M. Mammalian pheromone: Identification of active component in the subauricular scent of the male pronghorn. Science. 1974;183:860–862. [PubMed: 17780773]
- Nicolaides N. Skin lipids: Their biochemical uniqueness. Science. 1974;186:19–26. [PubMed: 4607408]
- Nixon A, Mallet A. I, Gower D. B. Simultaneous quantification of five odorous steroids (16-androstenes) in the axillary hair of men. J. Steroid Biochem. 1988;29:505–510. [PubMed: 3379959]
- Ohloff G, Maurer B, Winter B, Wolfgang G. Structural and configurational dependence of the sensory process in steroids. Helv. Chir. Acta. 1983;66:192–201.
- Parkes A. S, Bruce H. M. Olfactory stimuli in mammalian reproduction. Science. 1961;134:1049–1054. [PubMed: 14483939]
- Patterson R. L. S. Possible contribution of phenolic components to boar odour. Nature. 1966;212:744–745.
- Patterson R. L. S. Acidic components of boar preputial fluid. J. Sci. Food Agric. 1968;19:38–40.
- Pinker S. The Stuff of Thought: Language as a Window into Human Nature. New York: Penguin Group; 2007.
- Preti G. Reply to Wilson. Horm. Behav. 1987;21:547–550.
- Preti G, Cutler W. B, Garcia C. R, Huggins G. R, Lawley H. J. Human axillary secretions influence women’s menstrual cycles: The role of donor extract of females. Horm. Behav. 1986;20:474–482. [PubMed: 3793028]
- Quadagno D. M, Shubeita H. E, Deck J, Francoer D. The effects of males, athletic activities and all female living conditions on the menstrual cycle. Psychoneuroendocrinology. 1981;6:239–244. [PubMed: 7291434]
- Reed H. C, Melrose D. R, Patterson R. L. Androgen steroids as an aid to the detection of oestrus in pig artificial insemination. Br. Vet. J. 1974;130:61–67. [PubMed: 4856526]
- Russell M. J. Human olfactory communication. Nature. 1976;260:520–522. [PubMed: 1264204]
- Russell M. J, Switz G. M, Thompson K. Olfactory influences on the human menstrual cycle. Pharm. Biochem. Behav. 1980;13:737–738. [PubMed: 7443744]
- Schank J. C. Problems with dimensionless measurement models of synchrony in biological systems. Am. J. Primatol. 1997;41:65–85. [PubMed: 9050366]
- Schank J. C. Menstrual-cycle variability and measurement: Further cause for doubt. Psychoneuroendocrinology. 2000;25:837–847. [PubMed: 10996477]
- Schank J. C. Menstrual-cycle synchrony: Problems and new directions for research. J. Comp. Psychol. 2001;115:3–15. [PubMed: 11334217]
- Schank J. C. A multitude of errors in menstrual-synchrony research: Replies to Weller and Weller (2002) and Graham (2002). J. Comp. Psychol. 2002;116:319–322. [PubMed: 12234084]
- Schank J. C. Do human menstrual-cycle pheromones exist? Hum. Nat. 2006;17:448–470. [PubMed: 26181613]
- Schleidt M. Personal odor and nonverbal communication. Ethol. Sociobiol. 1980;1:225–231.
- Schleidt M, Hold B, Attili G. A cross-cultural study on the attitude towards personal odors. J. Chem. Ecol. 1981;7:19–31. [PubMed: 24420424]
- Scott C. M, Marlin D. J, Schroter R. C. Quantification of the response of equine apocrine sweat glands to beta2-adrenergic stimulation. Equine Vet. J. 2001;33:605–612. [PubMed: 11720033]
- Shiferaw B, Verrill L, Booth H, Zansky S. M, Norton D. M, Crim S. et al. Sex-based differences in food consumption: Foodborne Diseases Active Surveillance Network (FoodNet) Population Survey, 2006–2007. Clin. Infect. Dis. 2012;54 Suppl. 5:S453–S457. [PubMed: 22572669]
- Simehoff M. L, Burke J. F, Saukkonen J. J, Ordinario A. T, Doty R. L. Biochemical profile of uremic breath New England. Journal of Medicine. 1977;297:132–135. [PubMed: 865584]
- Spielman A. I, Sunavala G, Harmony J. A. K, Stuart W. D, Leyden J. J, Turner G. et al. Identification and immunohistochemical localization of protein precursors to human axillary odors in apocrine glands and secretions. Arch. Derm. 1998;134:813–818. [PubMed: 9681344]
- Stefanczyk-Krzymowska S, Krzymowski T, Grzegorzewski W, Sowska W, Skipor J. Humoral pathway for local transfer of the priming pheromone androstenol from the nasal cavity to the brain and hypophysis in anaesthetized gilts. Exp. Physiol. 2000;85:801–809. [PubMed: 11187974]
- Stern K, McClintock M. K. Regulation of ovulation by human pheromones. Nature. 1998;392:177–179. [PubMed: 9515961]
- Stevenson R. J, Repacholi B. M. Age-related changes in children’s hedonic response to male body odor. Dev. Psychol. 2003;39:670–679. [PubMed: 12859121]
- Strassmann B. I. The biology of menstruation in Homo Sapiens: Total lifetime menses, fecundity, and nonsychrony in a natural-fertility population. Curr. Anthropol. 1997;38:123–129.
- Strassmann B. I. Menstrual synchrony pheromones: Cause for doubt. Hum. Reprod. 1999;14:579–580. [PubMed: 10221677]
- Thiessen D. D, Regnier F. E, Rice M, Goodwin M, Isaacks N, Lawson N. Identification of a ventral scent marking pheromone in the male Mongolian gerbil (Meriones unguiculatus). Science. 1974;184:83–85. [PubMed: 4815289]
- Tobin D. J. Biochemistry of human skin—Our brain on the outside. Chem. Soc. Rev. 2006;35:52–67. [PubMed: 16365642]
- Treloar A. E, Boynton R. E, Behn B. G, Brown B. W. Variation of the human menstrual cycle through reproductive life. Int. J. Fertil. 1967;13:77–126. [PubMed: 5419031]
- Weller A, Weller L. Menstrual synchrony between mothers and daughters and between roommates. Physiol. Behav. 1993a;53:943–949. [PubMed: 8511211]
- Weller A, Weller L. Menstrual synchrony under optimal conditions: Bedouin families. J. Comp. Psychol. 1997a;111:143–151. [PubMed: 9170279]
- Weller A, Weller L. Prolonged and very intensive contact may not be conductive to menstrual synchrony. Psychoneuroendocrinology. 1998;23:19–32. [PubMed: 9618749]
- Weller L, Weller A. Multiple influences of menstrual synchrony: Kibbutz roommates, their best friends, and their mothers. Am. J. Hum. Biol. 1993b;5:173–179. [PubMed: 28524333]
- Weller L, Weller A. Menstrual variability and the measurement of menstrual synchrony. Psychoneuroendocrinology. 1997b;22:115–128. [PubMed: 9149333]
- Weller L, Weller A, Roizman S. Human menstrual synchrony in families and among close friends: Examining the importance of mutual exposure. J. Comp. Psychol. 1999a;113:261–268. [PubMed: 10497793]
- Weller L, Weller A, Koresh-Kamin H, Ben Shoshan R. Menstrual synchrony in a sample of working women. Psychoneuroendocrinology. 1999b;24:449–459. [PubMed: 10341370]
- Whitten W. Reproductive biology: Pheromones and regulation of ovulation. Nature. 1999;401:232–233. [PubMed: 10499577]
- Whitten W. K, Champlin A. K. In Handbook of Physiology. Section 7: Endocrinology. Washington, DC: American Physiological Society; 1973. The role of olfaction in mammalian reproduction; pp. 109–123.
- Wilke K, Martin A, Terstegen L, Biel S. S. A short history of sweat gland biology. Int. J. Cosmet. Sci. 2007;29:169–179. [PubMed: 18489347]
- Wilson E. O. Pheromones. Sci. Am. 1963;208:100–114.
- Wilson H. C. Female axillary secretions influence women’s menstrual cycles: A critique. Horm. Behav. 1987;21:536–546. [PubMed: 3428891]
- Wilson H. C. A critical review of menstrual synchrony research. Psychoneuroendocrinology. 1992;17:565–591. [PubMed: 1287678]
- Wysocki C. J, Dorries K. M, Beauchamp G. K. Ability to perceive androstenone can be acquired by ostensibly anosmic people. Proc. Natl. Acad. Sci. U. S. A. 1989;86:7976–7978. [PMC free article: PMC298195] [PubMed: 2813372]
- Yang Z. W, Schank J. C. Women do not synchronize their menstrual cycles. Hum. Nat. 2006;17:433–447. [PubMed: 26181612]
- Zeng X. N, Leyden J. J, Brand J. G, Speilman A. I, McGinley K. J, Preti G. An investigation of human apocrine gland secretion for axillary odor precursors. J. Chem. Ecol. 1992;18:1039–1055. [PubMed: 24254146]
- Zeng X. N, Leyden J. J, Lawley H. J, Sawano K, Nohara I, Preti G. Analysis of characteristic odors from human male axillae. J. Chem. Ecol. 1991;17:1469–1491. [PubMed: 24257805]
- Zeng X. N, Leyden J. J, Spielman A. I, Preti G. Analysis of characteristic human female axillary odors: Qualitative comparison to males. J. Chem. Ecol. 1996;22:237–257. [PubMed: 24227407]
- Ziomkiewicz A. Menstrual synchrony: Fact or artifact? Hum. Nat. 2006;17:419–432. [PubMed: 26181611]
Footnotes
- *
Because of space limitations, all of the arguments presented in this book cannot be made in this chapter, and the reader is referred to the book for a complete exposé of the issues.
- *
The caption of the table in which the chemical structures of civetone and maskone were provided read as follows: “Six sex pheromones including the identified sex attractants of four insect species as well as two mammalian musks generally believed to be sex attractants. The molecular weight of most sex pheromones accounts for their narrow specificity and high potency.” Note that Wilson was classifying agents as pheromones without even knowing what effects, if any, they have on behavior or endocrine function.
- *
In my view it is erroneous to infer that a wide range of mammalian behaviors and endocrine responses are uniquely determined in an invariant way by single or small sets of chemical stimuli and to apply a generic and misleading name to the presumptive agents to support such an inference. Nonetheless, at some point semantics are involved. As noted by Pinker (2007, p. 3), “Semantics is about the relation of words to reality–the way that speakers <or writers> commit themselves to a shared understanding of the truth, and the way their thoughts are anchored to things and situations in the world.” Clearly, the belief that simple and presumptive hormone-like chemical agents, signified by the term pheromone, are responsible for an extensive array of behaviors and endocrine states assumes a reality that is questionable in humans and other mammals.
- Causes and consequences of variability in peptide mating pheromones of ascomycete fungi.[Mol Biol Evol. 2011]Causes and consequences of variability in peptide mating pheromones of ascomycete fungi.Martin SH, Wingfield BD, Wingfield MJ, Steenkamp ET. Mol Biol Evol. 2011 Jul; 28(7):1987-2003. Epub 2011 Jan 20.
- Review Introduction to Chemical Signaling in Vertebrates and Invertebrates.[Neurobiology of Chemical Commu...]Review Introduction to Chemical Signaling in Vertebrates and Invertebrates.Wyatt TD. Neurobiology of Chemical Communication. 2014
- Visualisation of the vomeronasal pheromone response system.[Bioessays. 2008]Visualisation of the vomeronasal pheromone response system.Keverne EB. Bioessays. 2008 Sep; 30(9):802-5.
- Pheromone attraction and cross-attraction of Nezara, Acrosternum, and Euschistus spp. stink bugs (Heteroptera: Pentatomidae) in the field.[Environ Entomol. 2010]Pheromone attraction and cross-attraction of Nezara, Acrosternum, and Euschistus spp. stink bugs (Heteroptera: Pentatomidae) in the field.Tillman PG, Aldrich JR, Khrimian A, Cottrell TE. Environ Entomol. 2010 Apr; 39(2):610-7.
- Review Odor and pheromone sensing via chemoreceptors.[Adv Exp Med Biol. 2012]Review Odor and pheromone sensing via chemoreceptors.Ma M. Adv Exp Med Biol. 2012; 739:93-106.
- Human Pheromones - Neurobiology of Chemical CommunicationHuman Pheromones - Neurobiology of Chemical Communication
- Gm24044 predicted gene, 24044 [Mus musculus]Gm24044 predicted gene, 24044 [Mus musculus]Gene ID:115485682Gene
- ptsH [Yersinia pestis CO92]ptsH [Yersinia pestis CO92]Gene ID:1175816Gene
- OR2AT4 olfactory receptor family 2 subfamily AT member 4 [Homo sapiens]OR2AT4 olfactory receptor family 2 subfamily AT member 4 [Homo sapiens]Gene ID:341152Gene
- GATC glutamyl-tRNA amidotransferase subunit C [Homo sapiens]GATC glutamyl-tRNA amidotransferase subunit C [Homo sapiens]Gene ID:283459Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...
