NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Breman JG, Alilio MS, White NJ, editors. Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives: Supplement to Volume 77(6) of American Journal of Tropical Medicine and Hygiene. Northbrook (IL): American Society of Tropical Medicine and Hygiene; 2007 Dec.

Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives: Supplement to Volume 77(6) of American Journal of Tropical Medicine and Hygiene.
Show detailsDespite considerable efforts by multiple governmental and nongovernmental organizations to increase access to artemisinin-based combination therapies (ACTs), these life-saving antimalarial drugs remain largely unaffordable to the most vulnerable populations. The cost of artemisinin derivatives, ACTs’ crucial active ingredients, contributes significantly to the high price of these therapies. With a grant from the Bill and Melinda Gates Foundation, a partnership between Amyris Biotechnologies, the Institute for OneWorld Health, and the University of California, Berkeley is using synthetic biology to help reduce the cost of artemisinin. This article presents a description of the technological platform the partnership—called the Artemisinin Project—is developing to manufacture a low-cost, semi-synthetic artemisinin through a fermentation process. By making life-saving ACTs affordable to the people who most need them, the Artemisinin Project hopes to show that the power of biotechnology can be harnessed to provide solutions to global health problems.
Introduction
Over the last couple of decades, the rise of biotechnology has provided a cutting-edge technological platform driving innovation in the development of new therapies for life-threatening diseases such as cancer. These innovative approaches rely on years of research and development using technologies that are increasingly sophisticated and costly (the average total cost for the development of a new medicine is USD $800 million1). Thus, it has been assumed that these technologies would be too expensive to apply to the creation of new medicines that are desperately needed in the developing world.
The Artemisinin Project challenges this assumption. Armed with a grant from the Bill & Melinda Gates Foundation, a new partnership, comprised of Amyris Biotechnologies, the Institute for OneWorld Health, and the University of California, Berkeley, has been formed to inexpensively produce the antimalarial drug artemisinin through a new fermentation process. This San Francisco Bay Area–based public–private partnership is applying a combination of synthetic biology, industrial fermentation, chemical synthesis, and drug development expertise to a very specific need of the developing world. In doing so, the partnership is a paradigm of how groups with critical knowledge and skills can pool talents to address a major global health problem.
Malaria is a disease that overwhelmingly affects areas of poverty, producing 300–500 million new infections and 1–3 million deaths each year, with most of the disease burden falling on African children younger than 5 years of age.2 In the last few decades, Plasmodium falciparum—the parasite causing the most virulent form of malaria—has become increasingly resistant to first-line drug therapies. However, artemisinin-based combination therapies (ACTs) show nearly 100% effectiveness against these drug-resistant parasites. Unfortunately, because of their high cost, ACTs are still beyond the reach of the world’s poorest people. This unique partnership is using innovative technology to reduce the cost of ACTs, thereby making these life-saving therapies more accessible to people in the developing world.
ACTs: The Last Line of Defense against a Global Scourge
Because of widespread and unsupervised use of malaria drugs, chloroquine-resistant P. falciparum emerged in the early 1960s and rapidly spread around the world. Today there are reported cases of Plasmodium parasite resistance to most of the currently available antimalarial therapies, thus necessitating the development of new antimalarial treatments.3 A new class of antimalarials—artemisinin derivatives—were first isolated and developed in China in the 1980s. The world is greatly indebted to Chinese scientists and traditional healers for their discovery and open sharing of the antimalarial properties of the plant Artemisia annua, or sweet wormwood, also named qinghao in Chinese.4 Artemisinin extracted from the plant can be chemically converted into several active derivatives. Artemisinin derivatives such as artesunate, artemether, and dihydro-artemisinin (DHA) are extremely potent antimalarials that act rapidly against both the parasite’s asexual and sexual stages, which could potentially help to reduce the rate of malaria transmission.5 In addition, artemisinin-derived drugs have been shown to be highly efficacious against parasites resistant to other antimalarial drugs.6
As monotherapies, artemisinin derivatives are effective treatments for uncomplicated malaria, but because of their very rapid clearance in plasma, complete cure requires a longer treatment (up to 7 days), which is often not completed. This has raised concerns of a higher potential for this class of drugs to induce drug resistance in Plasmodium parasites. To prevent the development of drug resistance against artemisinin derivatives, ACTs were developed; this method of drug combination is also used for the treatment of HIV and tuberculosis. The treatment consists of the simultaneous administration of two or three antimalarial drugs, each with a distinct mechanism of action against the parasite.
The World Health Organization (WHO) first endorsed ACTs for the treatment of malaria in 2004 and recommended a switch to ACTs as the first-line malaria treatment in 2005.6 Unfortunately, because of their high cost, these therapies are not widely accessible to the people who most need them, many of whom resort to using inexpensive but failing drugs such as chloroquine. Alternatively, some patients may use artemisinin monotherapies, which are generally cheaper than ACTs but could accelerate the development of parasite resistance, as has been recently suggested by the work of Jambou and others.7 At present, there have been no documented cases of ACT treatment failure because of resistance in Plasmodium parasites, but the study of Jambou and others strongly supports the need to protect artemisinin derivatives from the development of parasite resistance. In 2006, the WHO requested the discontinuation of manufacturing and marketing of all artemisinin monotherapies, except for the treatment of severe malaria, in an effort to prevent the development of resistance. Many drug manufacturers should be commended for their responsible response to this call from the WHO.
Challenges of Supplying Affordable ACTs
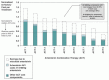
Despite notable efforts by pharmaceutical companies, governments, and nongovernmental organizations to make ACTs available to malaria patients, ACTs remain too expensive for the majority of people in endemic countries. Although there are many elements that influence the cost of these drugs, such as packaging and the non-artemisinin component of the combination drugs, the high cost of artemisinin itself (reported to be a range of $900–$1,600/kg in 2006; Boston Consulting Group, personal communication) is currently a key cost driver for ACTs (Figure 1).
Artemisinin is a natural product, and like many other natural products in the current pharmacopoeia, they have presented a supply chain challenge for the pharmaceutical industry that—in the case of a few active pharmaceutical ingredients (APIs) derived from natural products—had to make substantial financial investments to manage the API manufacturing and supply chain. To manufacture APIs, pharmaceutical companies often prefer to use a consistent and reliable source of starting material—ideally a chemical that can be completely synthesized from common and inexpensive chemicals rather than extracted from the natural source.
Artemisinin, the starting material for the derivatives used in ACTs, is currently extracted from dried leaves and influorescences from A. annua, an annual herb that is primarily cultivated throughout China and Southeast Asia. A. annua is a very labor-intensive crop with a lengthy growing cycle; the period from time of planting to artemisinin extraction is ∼12–18 months.8 In addition, the plant’s artemisinin content is quite sensitive to genetic backgrounds, cultivation conditions, and harvesting periods. The commonly accepted artemisinin recovery yield is ∼5 kg per 1,000 kg of dry leaves, produced from ∼1 ha of A. annua plants.8 Based on this yield, an estimated 17,000 ha are required to produce enough artemisinin to manufacture 100 million adult treatments per year. In 2004, there was only an estimated 4,700 ha of Artemisia grown in the world, mainly in China and Vietnam.8 Recent efforts to scale-up the cultivation of A. annua in Asia and East Africa are forecasted to increase the total acreage to ∼11,200 ha. However, an additional source for artemisinin production is clearly needed to meet the projections of a global demand of 400 million ACT treatments per year.8 In addition to the challenges inherent to the scale-up of A. annua cultivation, the artemisinin extraction and purification processes are difficult and costly. These processes currently rely on methods that use organic solvents such as hexane and petroleum ether, which are relatively inefficient, potentially unsafe, and environmentally damaging.
The plants—and the many farmers who grow them—are currently the sole source of artemisinin worldwide and are responsible for saving the lives of countless numbers of people who are treated with ACTs each year. A. annua will continue to play an important role in the ACT manufacturing process in the future and provide the additional benefit of supporting the development of local economies in the malaria endemic countries where A. annua is cultivated. Nonetheless, it is also clear that there is a need for an additional source of artemisinin that is consistent, reliable, pure, and inexpensive. By reducing the cost of this important API intermediate, the cost of ACTs can in turn be reduced, and these life-saving drugs will be more accessible to the people who need them most.
Synthetic Biology as a Cost-Effective Solution to the Problem of Artemisinin Supply
The genetic engineering and recombinant DNA technology revolutions have profoundly changed modern medicine. Today, diseases such as diabetes are effectively treated with recombinant proteins. These proteins are produced through genetic engineering of microorganisms, which can be industrially fermented to produce copious amounts of pure and potent biotherapeutics at a lower cost. Unfortunately, protein therapeutic approaches have not yet been applicable to the treatment of infectious diseases, which overwhelmingly affect populations in the developing world.
Recently, the field of synthetic biology has expanded the techniques pioneered in the field of recombinant protein therapeutics to the realm of small molecule drugs. Synthetic biology combines science and engineering to design and build novel biologic systems or to redesign existing systems for useful purposes. In this case, the Artemisinin Project team is using synthetic biology to assemble a biosynthetic pathway of genes from the plant A. annua and other organisms into microbes. Professor Jay Keasling’s laboratory at the University of California, Berkeley, and scientists at Amyris Biotechnologies are completing the synthetic biologic process to produce artemisinic acid, a precursor to artemisinin. Berkeley scientists are elucidating the metabolic pathway in the wormwood plant and identifying the genes required to make artemisinic acid. They are inserting this pathway into microorganisms and optimizing the resulting microbial strains for commercial production of the precursor through fermentation.
The technology required for the microbial production underpinning this approach was developed in the Keasling laboratory.9,10 By constructing a new metabolic pathway comprised of bacterial, yeast, and plant genes into a bacterial “chassis,” they created a platform organism capable of synthesizing copious supply of isoprenoid precursors. These precursors can then be converted to any isoprenoid product of choice through the addition of a specific biosynthetic gene—amorphadiene synthase in the case of the artemisinin biosynthetic pathway (Figure 2). Through synthetic biology efforts by the Amyris/Keasling laboratory collaboration as well as fermentation process optimization by Amyris, titers of amorphadiene have increased nearly 100-fold in 1.5 years and > 10-million-fold since the initial inception of the project in 1999.
To complete the artemisinin biosynthetic pathway, amorphadiene undergoes three oxidation steps to form artemisinic acid. A very significant milestone of the project was met with the discovery of the CYP71AV1 gene, whose product belongs to a large class of enzymes known as cytochrome P450s.11 Using the yeast eukaryotic system as a screening platform, the UC Berkeley scientists identified a single gene, CYP71AV1, which catalyzes all three oxidation steps required for the conversion of amorphadiene into artemisinic acid. Taken together, these results show that an artemisinic acid– producing strain of baker’s yeast (Saccharomyces cerevisiae) can be created by engineering the endogenous farnesyl pyrophosphate (FPP) pathway and by expressing the A. annua genes, amorphadiene synthase, and CYP71AV1 and its redox partner (CPR). Efforts are currently underway to optimize the CYP71AV1 gene expression in microbial strains that have been optimized to sustain high-level production of amorphadiene.
In the A. annua plant, artemisinic acid is subsequently oxidized to yield artemisinin. However, a singlet oxygen-generating enzyme capable of peroxidizing artemisinic acid has not been identified. Furthermore, it is currently postulated that the transformation of artemisinic acid to artemisinin is performed in a light-driven reaction. Thus, these steps are difficult to complete through the microbial fermentation process, and the required chemical conversion has traditionally been considered costly and extremely difficult to perform at a commercial scale. We have overcome these challenges by developing— at Amyris Biotechnologies— a novel and inexpensive chemical process that can be scaled-up for commercial application. Using this proprietary chemistry process, we have successfully completed the synthesis of artemisinin, identical in all ways to natural material. Once the artemisinin is produced, it must be further chemically converted into derivatives such as artesunate or artemether, which are integrated into ACTs.
Although the discoveries to date constitute a good proof of concept, a substantial amount of work remains to be done for this process to be scalable and cost-effective. Efforts are underway to significantly increase the yield of artemisinic acid and to optimize the downstream chemistry process converting artemisinic acid into artemisinin. To effectively steer these efforts and to maximize the project probability of success, OneWorld Health is working with Amyris Biotechnologies and the Keasling laboratory, the Bill & Melinda Gates Foundation, and scientific and business advisors to determine the best overall product development strategy. This includes determining decision criteria to move the project forward and future partnerships needed to transition the research and laboratory-scale development work to pilot-scale and eventual manufacture at a commercial scale. In addition, One-World Health is developing a commercialization strategy based on a thorough understanding of the worldwide regulatory requirements and on an analysis of the current ACT manufacturing supply chain and distribution models. The outcome of this analysis will allow OneWorld Health to select scale-up and manufacturing partners that will maximize the impact of the semi-synthetic artemisinin on global ACT pricing.
Public Health Windfalls of Cheaper Artemisinin
The implementation of adequate vector control approaches, (such as insecticide indoor residual spraying), prevention methods (such as insecticide-treated nets), and the development of malaria vaccines are crucial in the campaign to decrease the incidence of malaria. Nonetheless, access to effective and safe antimalarial drugs has always been— and remains today— a key component of any malaria control program.
In sub-Saharan Africa, the region with the greatest burden of malaria, high cost is the primary factor limiting access to life-saving ACTs. The ultimate goal of the Artemisinin Project is to lower the cost of ACTs by reducing the cost of artemisinin derivatives. Lowering the cost of ACTs is necessary to enable the adoption of these drugs as first-line antimalarials in impoverished countries. Currently, ACTs are not economically competitive with chloroquine and sulfadoxine-pyrimethamine (SP), and the economic incentive for countries to implement a policy switch to ACTs is low. To some extent the situation is comparable— and maybe even worse, considering the currently high cost of ACTs— to the situation in the 1990s, when a policy switch from chloroquine to the more expensive SP was recommended but was quite costly to African countries. For example, it was estimated that the budget for the planned change in the United Republic of Tanzania was equivalent to 1% of the total annual Ministry of Health budget over an 18-month period.12 The creation of the Global Fund to Fight AIDS, Tuberculosis, and Malaria has provided a financial mechanism to allow for governments to implement the WHO recommendations and switch to ACTs as first-line therapies. Nonetheless, additional approaches aiming to reduce the ACT cost such as the Artemisinin Project are much needed to further expand the use of these life-saving drugs.
Fermentation has been used for decades to produce important chemicals and medicines, such as vitamins, antibiotics, and protein-based pharmaceuticals. The Artemisinin Project aims to develop and validate inexpensive processes to begin the scale-up process of fermentation combined with synthetic chemistry to produce artemisinin. It is anticipated that bulk production of this important compound will significantly reduce the price of these lives-saving medicines (Figure 1), making them accessible to the hundreds of millions of impoverished people who contract malaria each year. In addition to reducing the production cost, industrial fermentation processes offer advantages in scalability, reliability, and flexibility, which allow for a greater and faster ability to adjust to market changes.
If successful, the Artemisinin Project could impact public health significantly. First and foremost, by increasing access to ACTs, this project is expected to have an impact on malaria mortality. Rapid access to effective treatments at the first onset of symptoms has been clearly shown to prevent the progression of the disease to more life-threatening stages such as severe and cerebral malaria.13 Another expected project outcome is a dramatic reduction of the use of artemisinin monotherapies. Artemisinin monotherapies are a real threat to the longevity of this class of drugs in the field. As mentioned previously, recent studies support the hypothesis that the use of artemisinin monotherapies could potentially increase the risk of drug resistance development.7 The Institute for OneWorld Health is leading the manufacturing and commercialization effort for the Artemisinin Project in the developing world, and their policy will be to supply artemisinin exclusively to pharmaceutical manufacturers who only provide ACTs; artemisinin will not be supplied to companies that produce monotherapies. By providing low-cost artemisinin only to ACT manufacturers, it is expected that ACTs will be able to outcompete the artemisinin monotherapies.
It is also anticipated that the Artemisinin Project will have an impact on the pervasiveness of counterfeited drugs. The problem of counterfeited drugs in the developing world has steadily grown in magnitude, and some reports state that up to 50% of the drugs sold on the private market in certain parts of Africa and Asia are counterfeited.14 By providing the market with low-cost ACTs, thereby diminishing the potential profits generated by criminal counterfeiting activities, the incentive to manufacture and sell counterfeited drugs could be substantially lowered.
This project is a unique opportunity to provide the world with an additional consistent, reliable, and inexpensive source of artemisinin. By using state-of-the art technologies to solve a global health problem, the Artemisinin Project hopes to bring affordable antimalarials to the people who most need them.
Acknowledgments: The authors thank Janice Culpepper, Nina Grove, Mireille Mather, and Kristina Rudd for critical review and editorial contributions to the manuscript
Financial support: The Artemisinin project is financed by a grant from The Bill & Melinda Gates Foundation.
References
- 1.
- DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22:151–185. [PubMed: 12606142]
- 2.
- Eline Korenromp JM, Nahlen B, Wardlaw T, Young M. World Malaria Report: World Health Organization (WHO), Roll Back Malaria (RBM) and United Nations Children’s Fund (UNICEF). Geneva: World Health Organization; 2005.
- 3.
- Bloland P. Geneva: World Health Organization; Drug Resistance in Malaria. A Background Document for the WHO Global Strategy for Containment of Antimicrobial Resistance. 2001
- 4.
- Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228:1049–1055. [PubMed: 3887571]
- 5.
- Sutherland CJ, Ord R, Dunyo S, Jawara M, Drakeley CJ, Alexander N, Coleman R, Pinder M, Walraven G, Targett GA. Reduction of malaria transmission to Anopheles mosquitoes with a six-dose regimen of co-artemether. PLoS Med. 2005;2:e92. [PMC free article: PMC1087200] [PubMed: 15839740]
- 6.
- Olumese P. Guidelines for the Treatment of Malaria. Geneva: World Health Organization; 2006.
- 7.
- Jambou R, Legrand E, Niang M, Khim N, Lim P, Volney B, Ekala MT, Bouchier C, Esterre P, Fandeur T, Mercereau-Puijalon O. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet. 2005;366:1960–1963. [PubMed: 16325698]
- 8.
- Grupper M. Meeting on the Production of Artemisinin and ACTs. Arusha, Tanzania: DFID Health Resource Centre, Roll Back Malaria, World Health Organization; 2005.
- 9.
- Martin VJ, Yoshikuni Y, Keasling JD. The in vivo synthesis of plant sesquiterpenes by Escherichia coli. Biotechnol Bioeng. 2001;75:497–503. [PubMed: 11745124]
- 10.
- Martin VJ, Pitera DJ, Withers ST, Newman JD, Keasling JD. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotechnol. 2003;21:796–802. [PubMed: 12778056]
- 11.
- Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MC, Withers ST, Shiba Y, Sarpong R, Keasling JD. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. [PubMed: 16612385]
- 12.
- Abdulla S. The Costs, Effects and Cost-Effectiveness of Changing the First Line Drug for the Treatment of Malaria in Tanzania. Ministry of Health, United Republic of Tanzania, and World Health Organization; 2000.
- 13.
- Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. [PubMed: 11832955]
- 14.
- Cockburn R, Newton PN, Agyarko EK, Akunyili D, White NJ. The global threat of counterfeit drugs: why industry and governments must communicate the dangers. PLoS Med. 2005;2:e100. [PMC free article: PMC1062889] [PubMed: 15755195]
Authors’ addresses: Victoria Hale and Thierry T. Diagana, The Institute for OneWorld Health, 50 California Street, Suite 500, San Francisco, CA 94111, Telephone: 415–421–4700, Fax: 415–421–4747, E-mail: gro.htlaehdlroweno@anagaidt. Jay D. Keasling, Berkeley Center for Synthetic Biology, University of California, Berkeley, 717 Potter Street, Building 977, MC 3224, Berkeley, CA 94720–3224, Telephone: 510–495–2620, E-mail: ude.yelekreb@gnilsaek. Neil Renninger, Amyris Biotechnologies, 5980 Horton Street, Ste. 450, Emeryville, CA 94608, Telephone: 510–450–0761, E-mail: moc.hcetoibsiryma@regninneR.
- Microbially Derived Artemisinin: A Biotechnology Solution to the Global Problem ...Microbially Derived Artemisinin: A Biotechnology Solution to the Global Problem of Access to Affordable Antimalarial Drugs - Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives
- PVX_094660 [Plasmodium vivax]PVX_094660 [Plasmodium vivax]Gene ID:5473688Gene
- FAM78A family with sequence similarity 78 member A [Homo sapiens]FAM78A family with sequence similarity 78 member A [Homo sapiens]Gene ID:286336Gene
- Mtbp Mdm2, transformed 3T3 cell double minute p53 binding protein [Mus musculus]Mtbp Mdm2, transformed 3T3 cell double minute p53 binding protein [Mus musculus]Gene ID:105837Gene
- 1700110C19Rik RIKEN cDNA 1700110C19 gene [Mus musculus]1700110C19Rik RIKEN cDNA 1700110C19 gene [Mus musculus]Gene ID:76634Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...