All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: tni.ohw@sredrokoob). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: tni.ohw@snoissimrep).
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. Geneva: World Health Organization; 2009.

WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care.
Show detailsTransmission of health care-associated pathogens from one patient to another via HCWs’ hands requires five sequential steps (Figures I.7.1–6): (i) organisms are present on the patient’s skin, or have been shed onto inanimate objects immediately surrounding the patient; (ii) organisms must be transferred to the hands of HCWs; (iii) organisms must be capable of surviving for at least several minutes on HCWs’ hands; (iv) handwashing or hand antisepsis by the HCW must be inadequate or entirely omitted, or the agent used for hand hygiene inappropriate; and (v) the contaminated hand or hands of the caregiver must come into direct contact with another patient or with an inanimate object that will come into direct contact with the patient. Evidence supporting each of these elements is given below.

Figure I.7.1
Organisms present on patient skin or the immediate environment. A bedridden patient colonized with Gram-positive cocci, in particular at nasal, perineal, and inguinal areas (not shown), as well as axillae and upper extremities. Some environmental surfaces (more...)

Figure I.7.2
Organism transfer from patient to HCWs’ hands. Contact between the HCW and the patient results in cross-transmission of microorganisms. In this case, Gram-positive cocci from the patient’s own flora transfer to HCW’s hands. Reprinted (more...)

Figure I.7.3
Organism survival on HCWs’ hands. (A) Microorganisms (in this case Gram-positive cocci) survive on hands. Reprinted from Pittet, 2006 with permission from Elsevier. (B) When growing conditions are optimal (temperature, humidity, absence of hand (more...)
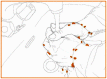
Figure I.7.4
Incorrect hand cleansing. Inappropriate handwashing can result in hands remaining contaminated; in this case, with Gram-positive cocci. Reprinted from Pittet, 2006 with permission from Elsevier.

Figure I.7.5a
Failure to cleanse hands results in between-patient cross-transmission. (A) The doctor had a prolonged contact with patient A colonized with Gram-positive cocci and contaminated his hands. Reprinted from Pittet, 2006 with permission from Elsevier.
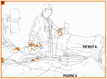
Figure I.7.5b
Failure to cleanse hands results in between-patient cross-transmission. (B) The doctor is now going to have direct contact with patient B without cleansing his hands in between. Cross-transmission of Gram-positive cocci from patient A to patient B through (more...)
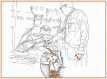
Figure I.7.6
Failure to cleanse hands during patient care results in within-patient cross-transmission. The doctor is in close contact with the patient. He touched the urinary catheter bag previously and his hands are contaminated with Gram-negative rods from touching (more...)
7.1. Organisms present on patient skin or in the inanimate environment
Health care-associated pathogens can be recovered not only from infected or draining wounds, but also from frequently colonized areas of normal, intact patient skin.82–96 The perineal or inguinal areas tend to be most heavily colonized, but the axillae, trunk, and upper extremities (including the hands) are also frequently colonized.85,86,88,89,91,93,97 The number of organisms such as S. aureus, Proteus mirabilis, Klebsiella spp. and Acinetobacter spp. present on intact areas of the skin of some patients can vary from 100 to 106 CFU/cm2.86,88,92,98 Diabetics, patients undergoing dialysis for chronic renal failure, and those with chronic dermatitis are particularly likely to have skin areas colonized with S. aureus.99–106. Because nearly 106 skin squames containing viable microorganisms are shed daily from normal skin,107 it is not surprising that patient gowns, bed linen, bedside furniture and other objects in the immediate environment of the patient become contaminated with patient flora.93–96,108–114 Such contamination is most likely to be due to staphylococci, enterococci or Clostridium difficile which are more resistant to desiccation. Contamination of the inanimate environment has also been detected on ward handwash station surfaces and many of the organisms isolated were staphylococci.115 Tap/faucet handles were more likely to be contaminated and to be in excess of benchmark values than other parts of the station. This study emphasizes the potential importance of environmental contamination on microbial cross contamination and pathogen spread.115 Certain Gram-negative rods, such as Acinetobacter baumannii, can also play an important role in environmental contamination due to their long-time survival capacities.116–119
7.2. Organism transfer to health-care workers’ hands
Relatively few data are available regarding the types of patient-care activities that result in transmission of patient flora to HCWs’ hands.72,89,110,111,120–123 In the past, attempts have been made to stratify patient-care activities into those most likely to cause hand contamination,124 but such stratification schemes were never validated by quantifying the level of bacterial contamination that occurred. Casewell & Phillips121 demonstrated that nurses could contaminate their hands with 100–1000 CFU of Klebsiella spp. during “clean” activities such as lifting patients; taking the patient’s pulse, blood pressure or oral temperature; or touching the patient’s hand, shoulder or groin. Similarly, Ehrenkranz and colleagues88 cultured the hands of nurses who touched the groin of patients heavily colonized with P. mirabilis and found 10–600 CFU/ml in glove juice samples. Pittet and colleagues72 studied contamination of HCWs’ hands before and after direct patient contact, wound care, intravascular catheter care, respiratory tract care or handling patient secretions. Using agar fingertip impression plates, they found that the number of bacteria recovered from fingertips ranged from 0 to 300 CFU. Direct patient contact and respiratory tract care were most likely to contaminate the fingers of caregivers. Gram-negative bacilli accounted for 15% of isolates and S. aureus for 11%. Importantly, duration of patient-care activity was strongly associated with the intensity of bacterial contamination of HCWs’ hands in this study. A similar study of hand contamination during routine neonatal care defined skin contact, nappy/diaper change, and respiratory care as independent predictors of hand contamination.73 In the latter study, the use of gloves did not fully protect HCWs’ hands from bacterial contamination, and glove contamination was almost as high as ungloved hand contamination following patient contact. In contrast, the use of gloves during procedures such as nappy/diaper change and respiratory care almost halved the average increase of bacteria CFU/min on HCWs’ hands.73
Several other studies have documented that HCWs can contaminate their hands or gloves with Gram-negative bacilli, S. aureus, enterococci or C. difficile by performing “clean procedures” or touching intact areas of skin of hospitalized patients.89,95,110,111,125,126 A recent study that involved culturing HCWs’ hands after various activities showed that hands were contaminated following patient contact and after contact with body fluids or waste.127 McBryde and colleagues128 estimated the frequency of HCWs’ glove contamination with methicillin-resistant S. aureus (MRSA) after contact with a colonized patient. HCWs were intercepted after a patient-care episode and cultures were taken from their gloved hands before handwashing had occurred; 17% (confidence interval (CI) 95% 9–25%) of contacts with patients, a patient’s clothing or a patient’s bed resulted in transmission of MRSA from a patient to the HCWs’ gloves. In another study involving HCWs caring for patients with vancomycin-resistant enterococci (VRE), 70% of HCWs contaminated their hands or gloves by touching the patient and the patient’s environment.114 Furthermore, HCWs caring for infants with respiratory syncytial virus (RSV) infections have acquired infection by performing activities such as feeding infants, nappy/diaper change, and playing with the infant.122 Caregivers who had contact only with surfaces contaminated with the infants’ secretions also acquired RSV. In the above studies, HCWs contaminated their hands with RSV and inoculated their oral or conjunctival mucosa. Other studies have also documented that the hands (or gloves) of HCWs may be contaminated after touching inanimate objects in patients’ rooms.73,111,112,125–130 Furthermore, a recent two-part study conducted in a non-health-care setting found in the initial phase that patients with natural rhinovirus infections often contaminated multiple environmental sites in their rooms. In the second part of the study, contaminated nasal secretions from the same individuals were used to contaminate surfaces in rooms, and touching contaminated sites 1–178 hours later frequently resulted in the transfer of the virus to the fingertips of the individuals.131
Bhalla and colleagues studied patients with skin colonization by S. aureus (including MRSA) and found that the organism was frequently transferred to the hands of HCWs who touched both the skin of patients and surrounding environmental surfaces.96 Hayden and colleagues found that HCWs seldom enter patient rooms without touching the environment, and that 52% of HCWs whose hands were free of VRE upon entering rooms contaminated their hands or gloves with VRE after touching the environment without touching the patient.114 Laboratory-based studies have shown that touching contaminated surfaces can transfer S. aureus or Gram-negative bacilli to the fingers.132 Unfortunately, none of the studies dealing with HCW hand contamination was designed to determine if the contamination resulted in the transmission of pathogens to susceptible patients.
Many other studies have reported contamination of HCWs’ hands with potential pathogens, but did not relate their findings to the specific type of preceding patient contact.78,79,94,132–142 For example, in studies conducted before glove use was common among HCWs, Ayliffe and colleagues137 found that 15% of nurses working in an isolation unit carried a median of 1× 104 CFU of S. aureus on their hands; 29% of nurses working in a general hospital had S. aureus on their hands (median count, 3.8 × 103 CFU), while 78% of those working in a hospital for dermatology patients had the organism on their hands (median count, 14.3 × 106 CFU). The same survey revealed that 17–30% of nurses carried Gram-negative bacilli on their hands (median counts ranged from 3.4 × 103 CFU to 38 × 103 CFU). Daschner135 found that S. aureus could be recovered from the hands of 21% of ICU caregivers and that 21% of doctors and 5% of nurse carriers had >103 CFU of the organism on their hands. Maki80 found lower levels of colonization on the hands of HCWs working in a neurosurgery unit, with an average of 3 CFU of S. aureus and 11 CFU of Gram-negative bacilli. Serial cultures revealed that 100% of HCWs carried Gram-negative bacilli at least once, and 64% carried S. aureus at least once. A study conducted in two neonatal ICUs revealed that Gram-negative bacilli were recovered from the hands of 38% of nurses.138
7.3. Organism survival on hands
Several studies have shown the ability of microorganisms to survive on hands for differing times. Musa and colleagues demonstrated in a laboratory study that Acinetobacter calcoaceticus survived better than strains of A. lwoffi at 60 minutes after an inoculum of 104 CFU/finger.143 A similar study by Fryklund and colleagues using epidemic and non-epidemic strains of Escherichia coli and Klebsiella spp. showed a 50% killing to be achieved at 6 minutes and 2 minutes, respectively.144 Noskin and colleagues studied the survival of VRE on hands and the environment: both Enterococcus faecalis and E. faecium survived for at least 60 minutes on gloved and ungloved fingertips.145 Furthermore, Doring and colleagues showed that Pseudomonas aeruginosa and Burkholderia cepacia were transmissible by handshaking for up to 30 minutes when the organisms were suspended in saline, and up to 180 minutes when they were suspended in sputum.146 The study by Islam and colleagues with Shigella dysenteriae type 1 showed its capacity to survive on hands for up to 1 hour.147 HCWs who have hand dermatitis may remain colonized for prolonged time periods. For example, the hands of a HCW with psoriatic dermatitis remained colonized with Serratia marcescens for more than three months.148 Ansari and colleagues149,150 studied rotavirus, human parainfluenza virus 3, and rhinovirus 14 survival on hands and potential for cross-transfer. Survival percentages for rotavirus at 20 minutes and 60 minutes after inoculation were 16.1% and 1.8%, respectively. Viability at 1 hour for human parainfluenza virus 3 and rhinovirus 14 was <1% and 37.8%, respectively.
The above-mentioned studies clearly demonstrate that contaminated hands could be vehicles for the spread of certain viruses and bacteria. HCWs’ hands become progressively colonized with commensal flora as well as with potential pathogens during patient care.72,73 Bacterial contamination increases linearly over time.72 In the absence of hand hygiene action, the longer the duration of care, the higher the degree of hand contamination. Whether care is provided to adults or neonates, both the duration and the type of patient care affect HCWs’ hand contamination.72,73 The dynamics of hand contamination are similar on gloved versus ungloved hands; gloves reduce hand contamination, but do not fully protect from acquisition of bacteria during patient care. Therefore, the glove surface is contaminated, making cross-transmission through contaminated gloved hands likely.
7.4. Defective hand cleansing, resulting in hands remaining contaminated
Studies showing the adequacy or inadequacy of hand cleansing by microbiological proof are few. From these few studies, it can be assumed that hands remain contaminated with the risk of transmitting organisms via hands. In a laboratory-based study, Larson and colleagues151 found that using only 1 ml of liquid soap or alcohol-based handrub yielded lower log reductions (greater number of bacteria remaining on hands) than using 3 ml of product to clean hands. The findings have clinical relevance since some HCWs use as little as 0.4 ml of soap to clean their hands. Kac and colleagues152 conducted a comparative, crossover study of microbiological efficacy of handrubbing with an alcohol-based solution and handwashing with an unmedicated soap. The study results were: 15% of HCWs’ hands were contaminated with transient pathogens before hand hygiene; no transient pathogens were recovered after handrubbing, while two cases were found after handwashing. Trick and colleagues153 did a comparative study of three hand hygiene agents (62% ethyl alcohol handrub, medicated handwipe, and handwashing with plain soap and water) in a group of surgical ICUs. They also studied the impact of ring wearing on hand contamination. Their results showed that hand contamination with transient organisms was significantly less likely after the use of an alcohol-based handrub compared with the medicated wipe or soap and water. Ring wearing increased the frequency of hand contamination with potential health care-associated pathogens. Wearing artificial acrylic fingernails can also result in hands remaining contaminated with pathogens after use of either soap or alcohol-based hand gel154 and has been associated with outbreaks of infection155 (see also Part I, Section 23.4).
Sala and colleagues156 investigated an outbreak of food poisoning attributed to norovirus genogroup 1 and traced the index case to a food handler in the hospital cafeteria. Most of the foodstuffs consumed in the outbreak were handmade, thus suggesting inadequate hand hygiene. Noskin and colleagues145 showed that a 5-second handwash with water alone produced no change in contamination with VRE, and 20% of the initial inoculum was recovered on unwashed hands. In the same study, a 5-second wash with two soaps did not remove the organisms completely with approximately a 1% recovery; a 30-second wash with either soap was necessary to remove the organisms completely from the hands.
Obviously, when HCWs fail to clean their hands between patient contact or during the sequence of patient care – in particular when hands move from a microbiologically contaminated body site to a cleaner site in the same patient – microbial transfer is likely to occur. To avoid prolonged hand contamination, it is not only important to perform hand hygiene when indicated, but also to use the appropriate technique and an adequate quantity of the product to cover all skin surfaces for the recommended length of time.
7.5. Cross-transmission of organisms by contaminated hands
Cross-transmission of organisms occurs through contaminated hands. Factors that influence the transfer of microorganisms from surface to surface and affect cross-contamination rates are type of organism, source and destination surfaces, moisture level, and size of inoculum. Harrison and colleagues157 showed that contaminated hands could contaminate a clean paper towel dispenser and vice versa. The transfer rates ranged from 0.01% to 0.64% and 12.4% to 13.1%, respectively.
A study by Barker and colleagues158 showed that fingers contaminated with norovirus could sequentially transfer virus to up to seven clean surfaces, and from contaminated cleaning cloths to clean hands and surfaces. Contaminated HCWs’ hands have been associated with endemic HCAIs.159,160 Sartor and colleagues160 provided evidence that endemic S. marcescens was transmitted from contaminated soap to patients via the hands of HCWs. During an outbreak investigation of S. liquefaciens, BSI, and pyrogenic reactions in a haemodialysis centre, pathogens were isolated from extrinsically contaminated vials of medication resulting from multiple dose usage, antibacterial soap, and hand lotion.161 Duckro and colleagues126 showed that VRE could be transferred from a contaminated environment or patients’ intact skin to clean sites via the hands of HCWs in 10.6% of contacts.
Several HCAI outbreaks have been associated with contaminated HCWs’ hands.162–164 El Shafie and colleagues164 investigated an outbreak of multidrug-resistant A. baumannii and documented identical strains from patients, hands of staff, and the environment. The outbreak was terminated when remedial measures were taken. Contaminated HCWs’ hands were clearly related to outbreaks among surgical148,162 and neonatal163,165,166 patients.
Finally, several studies have shown that pathogens can be transmitted from out-of-hospital sources to patients via the hands of HCWs. For example, an outbreak of postoperative S. marcescens wound infections was traced to a contaminated jar of exfoliant cream in a nurse’s home.167 An investigation suggested that the organism was transmitted to patients via the hands of the nurse, who wore artificial fingernails. In another outbreak, Malassezia pachydermatis was probably transmitted from a nurse’s pet dogs to infants in an intensive care nursery via the hands of the nurse.168
- Transmission of pathogens by hands - WHO Guidelines on Hand Hygiene in Health Ca...Transmission of pathogens by hands - WHO Guidelines on Hand Hygiene in Health Care
- Bet v 1 e [Betula pendula]Bet v 1 e [Betula pendula]gi|452734|emb|CAA54483.1|Protein
- ABHD14A-ACY1 ABHD14A-ACY1 readthrough [Homo sapiens]ABHD14A-ACY1 ABHD14A-ACY1 readthrough [Homo sapiens]Gene ID:100526760Gene
- MTDHP3 metadherin pseudogene 3 [Homo sapiens]MTDHP3 metadherin pseudogene 3 [Homo sapiens]Gene ID:100418832Gene
- fabF 3-oxoacyl-[acyl carrier protein] synthase 2 [Escherichia coli str. K-12 sub...fabF 3-oxoacyl-[acyl carrier protein] synthase 2 [Escherichia coli str. K-12 substr. MG1655]Gene ID:946665Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...