All copyright for chapters belongs to the individual authors who created them. However, for non-commercial, academic purposes, images and content from the chapters portion of Webvision may be used with a non-exclusive rights under a Attribution, Noncommercial 4.0 International (CC BY-NC) Creative Commons license. Cite Webvision, http://webvision.med.utah.edu/ as the source. Commercial applications need to obtain license permission from the administrator of Webvision and are generally declined unless the copyright owner can/wants to donate or license material. Use online should be accompanied by a link back to the original source of the material. All imagery or content associated with blog posts belong to the authors of said posts, except where otherwise noted.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Kolb H, Fernandez E, Nelson R, editors. Webvision: The Organization of the Retina and Visual System [Internet]. Salt Lake City (UT): University of Utah Health Sciences Center; 1995-.

Size of the Retina
- 32 mm from ora to ora along the horizontal meridian (1) (Kolb, unpublished measurements).
Size of Optic Nerve Head or Disc
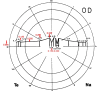
- 1.86 × 1.75 mm (Fig. 1)
Figure 1
Topography and dimensions of optic nerve and fovea.
Degrees and Distance in Micometers
- One degree of visual angle is equal to 288 μm on the retina without correction for shrinkage (4).
Foveal Position
- 11.8° or or 3.4 mm temporal to the optic disk edge
Cross Diameter of the Macula
- 3 mm of intense pigmentation, surrounded by a 1-mm-wide zone of less pigmentation (5).
Cross Diameter of the Central Fovea from Foveal Rim to Foveal Rim
- 1.5 mm (5)

Figure 2a
Vertical section of the human fovea. os, outer segments; is, inner segments; OLM, outer limiting membrane; ONL, outer nuclear layer; H, Henle fibers; INL, inner nuclear layer; ILM, inner limiting membrane; G, ganglion cells. From Yamada (7).

Figure 2b
Vertical section of the monkey fovea. From Hagerman and Johnson (19).
Vertical Thickness of the Fovea from Inner Limiting Membrane (ILM) to ELM
- in the foveal pit, 150 μm (7)
- foveal rim, 400 μm
Length of Foveal Axons (Henle Fibers)
- 150-300 μm (9)
Vertical Thickness of the Retina in Different Areas
The vertical extent of the retina across the horizontal meridian at different eccentricities is shown in Fig. 3. This is taken from data given by Sigelman and Ozanics (20). The small black numbers are the originals from Sigelman and Ozanics which were measured in typical histological preparations where there is a great deal of shrinkage. The figures in red are those recently measured by Ahnelt (personal communication) in well fixed EM quality material where there is little or no shrinkage. Hence the latter numbers are larger. The numbers are in mm.
Age When Fovea Is Fully Developed
- not before 4 years of age (6)
Highest Density of Cones at Center of the Fovea (50 × 50 μm)

Figure 4
Tangential section through the human fovea. Larger cones (arrows) are blue cones.
Total Number of Cones in Fovea
- approximately 200,000. 17,500 cones/degree2. Rod-free area is 1°; thus, there are 17,500 cones in the central rod-free fovea.
Total Number of Cones in the Retina
- 6,400,000 (10)
Total Number of Rods in the Retina
- 110,000,000 to 125,000,000 (10)
Rod Distribution
- Rods peak in density 18° or 5 mm out from the center of the fovea, in a ring around the fovea at 160,000 rods/mm2
- No rods in central 200 μm
- Average 80-100,000 rods/mm2.
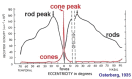
Figure 5
Graph to show rod and cone densities along the horizontal meridian.
Number of Cones to Ganglion Cells in the Fovea
- 1 cone to 2 ganglion cells out to about 2.2° (16)
Number of Cones/Retinal Pigment Epithelial Cell (RPE)
- 30 cones/RPE in fovea (17)
Number of Rods/Retinal Epithelial Cell (RPE)
- in periphery, 22 rods/RPE cell
- in rod, peak (4-5 mm from foveal center), 28 rods/RPE cell (17)
Number of Neural and Glial Types in the Retina
The retina consists of many millions of cell types packed together in a tightly knit network spread over the surface of the back of the eye fundus as a thin film of tissue only 1/2 millimeter thick. The retina is like a three-layered cake with three layers containing cell bodies of neurons and two filling layers where synapses between the neurons occur. There are two basic kinds of photoreceptors—rods and cones. The cones are further subdivided into two types (long- and short-wavelength sensitive) in the majority of mammals, i.e., most mammals, which are dichromats and have divariant color vision. In primates, a third wavelength-sensitive cone has developed closely related to the long-wavelength cone type but a little more sensitive in the middle wavelength (i.e., green cone). Thus, primates including humans are trichromats and have trivariant color vision. Many reptiles, birds, and fish have four or even five types of cone, each sensitive to a slightly different peak wavelength.
The second-order neurons, postsynaptic to the photorecepors in the first synaptic (filling layer) (outer plexiform layer), are bipolar cells and horizontal cells. There are nine types of bipolar cell and two to four types of horizontal cell in species from mammals to fish. The third-order neurons are amacrine cells and ganglion cells that synapse in the inner synaptic filling layer (inner plexiform layer). There are two types of interplexiform cells stretching between both plexiform layers, in most vertebrate retinas. There are approximately 22 types of amacrine cell and 20 types of ganglion cell in the typical mammalian retina. There may be 30 or more amacrine cell types in fish and reptilian retinas and 22 or so ganglion cell types. The increased number of third-order neurons is attributable to the greater information processing taking place in the non-mammalian retinas than in mammalian retinas.
All vertebrate retinas also contain large numbers of glial cells. The radial Muller cells stretch from outer to inner limiting membranes and surround and isolate all neural cell types from each other except at synapses. Microglia arise in times of injury and are blood-borne cell types. Astrocytes surround ganglion cell axons and inner retinal blood vessels.
Fig. 6 shows a drawing of the human retina close to the fovea, where all of the cell types that have been studied in detail are depicted in their intricate and marvellous network.

Figure 6
Drawing of a vertical section through the human retina to show the organization of the different neurons and glial cells constituting it.
Useful Units in Vision Science
From Wandell (18):
- Radiometric units represent a physical measurement, e.g., radiance is measured in watts sr−1 m−2.
- Calorimetric units adjust radiometric units for visual wavelength sensitivity, e.g., luminance is measured in candela per square meter, cd/m2.
- Lux are units of illumination. Thus, a light intensity of 1 candela produces an illumination of 1 lux at 1 meter.
- Scotopic luminance units are proportional to the number of photons absorbed by rod photoreceptors to give a criterion psychophysical result.
- Photopic luminance units are proportional to a weighted sum of the photons absorbed by L- and M-cones to give a criterion psychophysical result.
- Typical ambient luminance levels (cd/m2):
- Starlight: 0.001
- Moonlight: 0.1
- Indoor lighting: 100
- Sunlight: 10.000
- Maximum intensity of common CRT monitors: 100
- One Troland (Td) of retinal illumination is produced when an eye with a pupil size of 1 mm2 looks at a surface whose luminance is 1 cd/m2.
- Lens focal length: f (meters); lens power= 1/f (diopters).
Image Formation
From Wandell (18):
- The eyes are 6 cm apart and halfway down the head.
- Visual angle of common objects (degrees, deg)
- The sun or moon = 0.5 deg
- Thumbnail (at arm's length) = 1.5 deg
- Fist (at arm's length) = 8-10 deg
- Visual field (measured from central fixation)
- Monocular: 160 deg (w) × 175 deg (h)
- Binocular: 200 deg (w) × 135 deg (h)
- Region of binocular overlap: 120 deg (w) × 135 deg (h)
- Range of pupil diameters: 1-8 mm
- Refractive indices
- Air: 1.000
- Glass: 1.520
- Water: 1.333
- Cornea: 1.376
- Optical power (diopters)
- Cornea: 43
- Lens (relaxed): 20
- Whole eye: 60
- Change in power due to accomodation: 8
- Axial chromatic aberration over the visible spectrum: 2 diopters
- Visible spectrum: 370-730 nanometers (nm)
- Peak wavelength sensitivity
- Scotopic: 507 nm
- Photopic: 555 nm
- Spectral equilibrium hues
- Blue: 475 nm
- Green: 500 nm
- Yellow: 575 nm
- No spectral equilibrium: red
References
- 1.
- Van Buren JM. The retinal ganglion cell layer. Springfield (IL): Charles C. Thomas; 1963.
- 2.
- Michels RG, Wilkinson CP, Rice TA. Retinal detachment. The C.V. Mosby Company; 1990. p. 17.
- 3.
- Penkhus J. The ora serrata and its anatomical variations. M.S. Thesis. University of California, Los Angeles; 1965.
- 4.
- Drasdo N, Fowler CW. Non-linear projection of the retinal image in a wide-angle schematic eye. Br J Ophthalmol. 1974;58:709–714. [PMC free article: PMC1215006] [PubMed: 4433482]
- 5.
- Polyak SL. The retina. Chicago: University of Chicago Press; 1941.
- 6.
- Hendrickson AE, Youdelis C. The morphological development of the human fovea. Ophthalmology. 1984;91:603–612. [PubMed: 6462623]
- 7.
- Yamada E. Some structural features of the fovea central is in the human retina. Arch Ophthalmol. 1969;82:151–159. [PubMed: 4183671]
- 8.
- Ahnelt PK, Kolb H, Pflug R. Identification of a subtype of cone photoreceptor, likely to be blue sensitive, in the human retina. J Comp Neurol. 1987;255:18–34. [PubMed: 2434534]
- 9.
- Ahnelt PK, Pflug R. Telodendrial contacts between foveolar cone pedicles in the human retina. Experientia. 1986;42:298–300. [PubMed: 3956685]
- 10.
- Osterberg G. Topography of the layer of rods and cones in the human retina. Acta Ophthalmol Suppl. 1935;6:1–103.
- 11.
- Curcio CA, Sloan KR, Packer O, Hendrickson AE, Kalina RE. Distribution of cones in human and monkey retina: individual variability and radial asymmetry. Science. 1987;236:579–582. [PubMed: 3576186]
- 12.
- Mariani AP, Kolb H, Nelson R. Dopamine-containing amacrine cells of rhesus monkey retina parallel rods in spatial distribution. Brain Res. 1984;322:1–7. [PubMed: 6518360]
- 13.
- Bruesch SR, Arey LB. The number of myelinated and unmyelinated fibres in the optic nerve of vertebrates. J Comp Neurol. 1942;77:631.
- 14.
- Balazsi AG, Rootman J, Drance SM, Schulzer M, Douglas GR. The effect of age on the nerve fiber population of the human optic nerve. Am J Ophthalmol. 1984;97:760–766. [PubMed: 6731540]
- 15.
- Quigley HA, Addicks EM, Green WR. Optic nerve damage inhuman glaucoma. III. Quantitative correlation of nerve fibre loss and visual defect in glaucoma ischemic neuropathy and toxic neuropathy. Arch Ophthalmol. 1982;100:135. [PubMed: 7055464]
- 16.
- Schein SJ. Anatomy of macaque fovea and spatial densities of neurons in foveal representation. J Comp Neurol. 1988;269:479–505. [PubMed: 3372725]
- 17.
- Rapaport DH, Rakic P, Yasamura D, LaVail MM. Genesis of the retinal pigment epithelium in the macaque monkey. J Comp Neurol. 1995;363:359–376. [PubMed: 8847405]
- 18.
- Wandell BA. Foundations of vision. Sunderland (MA): Sinauer Associates, Inc; 1965.
- 19.
- Hagerman GS, Johnson LV. The photoreceptor-retinal pigmented epithelial interface. In: Heckenlively JR, Arden GB, editors. Principles and practice of clinical electrophysiology of vision. St. Louis: Mosby Year Book; 1991.
- 20.
- Sigelman J, Ozanics V. In: Jokobiec FA, editor. Ocular anatomy, embryology and teratology. Philadelphia: Harper and Rowe; 1982.
- Size of the Retina
- Size of Optic Nerve Head or Disc
- Degrees and Distance in Micometers
- Foveal Position
- Cross Diameter of the Macula
- Cross Diameter of the Central Fovea from Foveal Rim to Foveal Rim
- Cross Diameter of Central Rod-free Area
- Vertical Thickness of the Fovea from Inner Limiting Membrane (ILM) to ELM
- Central Region of Fovea Where There Are No Cone Pedicles
- Length of Foveal Axons (Henle Fibers)
- Vertical Thickness of the Retina in Different Areas
- Age When Fovea Is Fully Developed
- Highest Density of Cones at Center of the Fovea (50 × 50 μm)
- Total Number of Cones in Fovea
- Total Number of Cones in the Retina
- Total Number of Rods in the Retina
- Rod Distribution
- Number of Axons in the Optic Nerve
- Number of Cones to Ganglion Cells in the Fovea
- Number of Cones/Retinal Pigment Epithelial Cell (RPE)
- Number of Rods/Retinal Epithelial Cell (RPE)
- Number of Neural and Glial Types in the Retina
- Useful Units in Vision Science
- Image Formation
- References
- Facts and figures concerning injurious substances of exhaust gases from automobiles and in general from motors with internal combustion.[Rev Pathol Gen Physiol Clin. 1...]Facts and figures concerning injurious substances of exhaust gases from automobiles and in general from motors with internal combustion.SERRUYS M. Rev Pathol Gen Physiol Clin. 1958 Feb; 58(695):239-47.
- Facts and figures concerning the relationship of tonsillectomy and dental extraction to poliomyelitis.[Eye Ear Nose Throat Mon. 1958]Facts and figures concerning the relationship of tonsillectomy and dental extraction to poliomyelitis.UNGER M. Eye Ear Nose Throat Mon. 1958 Apr; 37(4):254-6.
- Preview of Hawaii Cancer Facts and Figures 2010.[Hawaii Med J. 2010]Preview of Hawaii Cancer Facts and Figures 2010.Hernandez BY, Green MD, Cassel KD, Pobutsky AM, Vu V, Wilkens LR. Hawaii Med J. 2010 Sep; 69(9):223-4.
- Review Comparing Two Programs for Helping Patients Make Informed Decisions about Rheumatoid Arthritis Treatment[ 2020]Review Comparing Two Programs for Helping Patients Make Informed Decisions about Rheumatoid Arthritis TreatmentBlalock SJ, Hunt C, Hickey G, Carpenter D, Chapman SB, Keebler M, Solow E, Reyna V, Curtis J, Edmonds CC, et al. 2020 Dec
- Review Leprosy: A Review of Some Facts and Figures.[Trans Epidemiol Soc Lond. 1889]Review Leprosy: A Review of Some Facts and Figures.Abraham PS. Trans Epidemiol Soc Lond. 1889; 8:118-151.
- Facts and Figures Concerning the Human Retina - WebvisionFacts and Figures Concerning the Human Retina - Webvision
- argB [Escherichia coli str. K-12 substr. MG1655]argB [Escherichia coli str. K-12 substr. MG1655]Gene ID:948464Gene
- SPATA31A1 SPATA31 subfamily A member 1 [Homo sapiens]SPATA31A1 SPATA31 subfamily A member 1 [Homo sapiens]Gene ID:647060Gene
- ST20 suppressor of tumorigenicity 20 [Homo sapiens]ST20 suppressor of tumorigenicity 20 [Homo sapiens]Gene ID:400410Gene
- Scml4 Scm polycomb group protein like 4 [Mus musculus]Scml4 Scm polycomb group protein like 4 [Mus musculus]Gene ID:268297Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...