By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Purves D, Augustine GJ, Fitzpatrick D, et al., editors. Neuroscience. 2nd edition. Sunderland (MA): Sinauer Associates; 2001.

Neuroscience. 2nd edition.
Show detailsThere are five established biogenic amine neurotransmitters: the three catecholamines—dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline)—and histamine and serotonin (see Figure 6.3). In terms of synthesis, packaging, release, and degradation, the amine neurotransmitters fall somewhere between the properties of the other small-molecule neurotransmitters and those of the neuropeptides.
All the catecholamines (so named because they share the catechol moiety) are derived from a common precursor, the amino acid tyrosine (Figure 6.11). The first step in catecholamine synthesis is catalyzed by tyrosine hydroxylase in a reaction requiring oxygen as a co-substrate and tetrahydrobiopterin as a cofactor to synthesize dihydroxyphenylalanine (DOPA). Because tyrosine hydroxylase is rate-limiting for the synthesis of all three transmitters, its presence is a valuable criterion for identifying catecholaminergic neurons.

Figure 6.11
The biosynthetic pathway for the catecholamine neurotransmitters. The amino acid tyrosine is the precursor for all three catecholamines. The first step in this reaction pathway, catalyzed by tyrosine hydroxylase, is rate-limiting.
- Dopamine is produced by the action of DOPA decarboxylase on DOPA (see Figure 6.11). Although present in several brain regions (Figure 6.12A), the major dopamine-containing area of the brain is the corpus striatum, which receives major input from the substantia nigra and plays an essential role in the coordination of body movements. In Parkinson's disease, for instance, the dopaminergic neurons of the substantia nigra degenerate, leading to a characteristic motor dysfunction (see Box B in Chapter 18). Although dopamine does not readily cross the blood-brain barrier, its precursor, levodopa, does. Levodopa is absorbed in the small bowel but is rapidly catabolized in the GI tract and in peripheral tissues. Hence, the disease can be treated by administering levodopa together with carbidopa, a dopamine decarboxylase inhibitor, and selegiline, a monoamine oxidase inhibitor. Dopamine is also believed to be involved in motivation, reward, and reinforcement. For example, cocaine and other addictive drugs act by stimulating the release of dopamine from specific brain areas (see Box D). Once released, dopamine binds to specific dopamine receptors, as well as to some β-adrenergic receptors. It not only acts as a neurotransmitter in the central nervous system but also plays a poorly understood role in some sympathetic ganglia. Dopamine is also used clinically to treat shock because it dilates renal arteries by activating dopamine receptors and increases cardiac output by activating β-adrenergic receptors in the heart.
- Norepinephrine (also called noradrenaline) synthesis requires dopamine β-hydroxylase, which catalyzes the production of norepinephrine from dopamine (see Figure 6.11). Dopamine is transported by vesicles into adrenergic terminals, where it is converted to norepinephrine. The most prominent class of neurons that synthesize norepinephrine is sympathetic ganglion cells, since norepinephrine is the major peripheral transmitter in this division of the visceral motor system (see Chapter 21). Norepinephrine is also the transmitter used by the locus coeruleus, a brainstem nucleus that projects diffusely to a variety of forebrain targets (Figure 6.12B), where it influences sleep and wakefulness, attention, and feeding behavior.
- Epinephrine (also called adrenaline) is present in the brain at lower levels than the other catecholamines. The enzyme that synthesizes epinephrine, phenylethanolamine-N-methyltransferase (see Figure 6.11), is present only in epinephrine-secreting neurons. Epinephrine-containing neurons in the central nervous system are found in two groups in the rostral medulla, the function of which is not known.All three catecholamines are removed by reuptake into nerve terminals or surrounding glial cells by a Na+-dependent transporter. The two major enzymes involved in the catabolism of catecholamines are monoamine oxidase (MAO) and catechol O-methyltransferase (COMT). Both neurons and glia contain mitochondrial MAO and cytoplasmic COMT. Inhibitors of these enzymes, such as phenelzine and tranylcypromine, are used clinically as antidepressants (see Box C).
- Histamine is produced from the amino acid histidine by a histidine decarboxylase and is metabolized by the combined actions of histamine methyltransferase and MAO. (Figure 6.13A). High concentrations of histamine and histamine decarboxylase are found in neurons in the hypothalamus that send sparse but widespread projections to almost all regions of the brain and spinal cord (see Figure 6.12C). The central histamine projections mediate arousal and attention, similar to central ACh and norepinephrine projections. This partly explains why antihistamines that cross the blood-brain barrier, such as diphenhydramine (Benadryl®), act as sedatives. Histamine also is released from mast cells in response to allergic reactions or tissue damage. The close proximity of mast cells to blood vessels, together with the potent actions of histamine on blood vessels, raises the possibility that histamine may influence brain blood flow.
- Serotonin, or 5-hydroxytryptamine (5-HT), was initially thought to increase vascular tone by virtue of its presence in serum (hence the name serotonin). 5-HT is synthesized from the amino acid tryptophan, which is an essential dietary requirement. Tryptophan is taken up into neurons by a plasma membrane transporter and hydroxylated in a reaction catalyzed by the enzyme tryptophan-5-hydroxylase (Figure 6.13B), the rate-limiting step for 5-HT synthesis. As in the case of other biogenic amines, the synaptic effects of serotonin are terminated by transport back into serotonergic nerve terminals. The primary catabolic pathway is mediated by MAO. Serotonin is located in groups of neurons in the raphe region of the pons and upper brainstem, which have widespread projections to the forebrain (see Figure 6.12D) and have been implicated in the regulation of sleep and wakefulness (see Chapter 28). A number of antipsychotic drugs used in the treatment of depression and anxiety are thought to act specifically on serotonergic neurons.Because biogenic amines are implicated in such a wide range of behaviors (ranging from central homeostatic functions to cognitive phenomena such as attention), it is not surprising that drugs affecting the synthesis, receptor binding, or catabolism of these neurotransmitters are among the most important in the armamentarium of modern pharmacology (Box C).
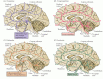
Figure 6.12
The distribution in the human brain of neurons and their projections (arrows) containing biogenic amine neurotransmitters. Curved arrows along the perimeter of the cortex indicate the innervation of lateral cortical regions not shown in this midsagittal (more...)
Box D
Addiction.
Box C
Biogenic Amine Neurotransmitters and Psychiatric Disorders.

Figure 6.13
Synthesis of histamine and serotonin. (A) Histamine is synthesized from the amino acid histidine. (B) Serotonin is derived from the amino acid tryptophan by a two-step process that requires the enzymes tryptophan-5-hydroxylase and a decarboxylase.
- PubMedLinks to PubMed
- The Biogenic Amines - NeuroscienceThe Biogenic Amines - Neuroscience
- Cell Membranes - The CellCell Membranes - The Cell
- Glossary - The CellGlossary - The Cell
- Transport of Small Molecules - The CellTransport of Small Molecules - The Cell
- Introduction - Beyond the HIPAA Privacy RuleIntroduction - Beyond the HIPAA Privacy Rule
Your browsing activity is empty.
Activity recording is turned off.
See more...