By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Purves D, Augustine GJ, Fitzpatrick D, et al., editors. Neuroscience. 2nd edition. Sunderland (MA): Sinauer Associates; 2001.

Neuroscience. 2nd edition.
Show detailsHuman sleep occurs with circadian (circa = about, and dia = day) periodicity, and biologists interested in circadian rhythms have explored a number of questions about this daily cycle. What happens, for example, when individuals are prevented from sensing the cues they normally have about night and day? This question has been answered by placing volunteers in an environment (caves or bunkers have sometimes been used) without external cues about time (Figure 28.3). During a five-day period of acclimation that included social interactions, meals at normal times, and temporal cues (radio, TV), the subjects arose and went to sleep at the usual times and maintained a 24-hour sleep-wake rhythm. After removing these cues, however, the subjects awakened later each day, and the cycle of sleep and wakefulness gradually lengthened to about 28 hours instead of the normal 24. When the volunteers were returned to a normal environment, the 24-hour cycle was rapidly restored. Thus, humans (and many other animals; see Box B) have an internal “clock” that continues to operate in the absence of any external information about the time of day; under these conditions, the clock is said to be “free running.”
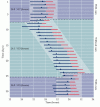
Figure 28.3
Rhythm of waking (blue lines) and sleeping (red lines) of a volunteer in an isolation chamber with and without cues about the day-night cycle. Numbers represent the mean ± standard deviation of a complete waking/sleeping cycle during each period (more...)
Box B
Molecular Mechanisms of Biological Clocks.
Presumably, circadian clocks evolved to maintain appropriate periods of sleep and wakefulness in spite of the variable amount of daylight and darkness in different seasons and at different places on the planet. To synchronize physiological processes with the day-night cycle (called photoentrainment), the biological clock must detect decreases in light levels as night approaches. The receptors that sense these light changes are, not surprisingly, in the outer nuclear layer of the retina; although removing the eye abolishes photoentrainment. The detectors are not, however, the rods or cones. Rather, these poorly understood cells lie within the ganglion and amacrine cell layers of the primate and murine retinas, and project to the suprachiasmatic nucleus (SCN) of the hypothalamus, the site of the circadian control of homeostatic functions generally (Figure 28.4A). These peculiar retinal photoreceptors contain a novel photopigment called melanopsin. Perhaps the most convincing evidence of the SCN's role as a sort of master biological clock is that its removal in experimental animals abolishes their circadian rhythm of sleep and waking. The SCN also governs other functions that are synchronized with the sleep-wake cycle, including body temperature (see Figure 28.3), hormone secretion, urine production, and changes in blood pressure. The cellular mechanisms of circadian control are summarized in Box B.

Figure 28.4
Anatomical underpinnings of circadian rhythms. (A) The hypothalamus, showing the location of the suprachiasmatic nucleus (SCN), which in mammals is the primary “biological clock.” The name “suprachiasmatic” derives from (more...)
Activation of the superchiasmatic nucleus evokes responses in neurons whose axons descend to the preganglionic sympathetic neurons in the lateral horn of the spinal cord (Figure 28.4B). These cells, in turn, modulate neurons in the superior cervical ganglia whose postganglionic axons project to the pineal gland (pineal means shaped like a pinecone) in the midline near the dorsal thalamus. The pineal gland synthesizes the sleep promoting neurohormone melatonin (N-acetyl-5-methoxytryptamine) from tryptophan, and secretes it into the bloodstream to help modulate the brainstem circuits that ultimately govern the sleep-wake cycle (see p. 615 ff.). Predictably, melatonin synthesis increases as light decreases and reaches it maximal level between 2:00 and 4:00 a.m. In the elderly, the pineal gland calcifies and less melatonin is produced, perhaps explaining why older people sleep fewer hours and are more often afflicted with insomnia.
- The Circadian Cycle of Sleep and Wakefulness - NeuroscienceThe Circadian Cycle of Sleep and Wakefulness - Neuroscience
- STX3 syntaxin 3 [Homo sapiens]STX3 syntaxin 3 [Homo sapiens]Gene ID:6809Gene
- 6809[uid] AND (alive[prop]) (1)Gene
- Zhx3 [Heterocephalus glaber]Zhx3 [Heterocephalus glaber]Gene ID:101705800Gene
- Slc17a3 [Cricetulus griseus]Slc17a3 [Cricetulus griseus]Gene ID:100755554Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...