By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gilbert SF. Developmental Biology. 6th edition. Sunderland (MA): Sinauer Associates; 2000.

Developmental Biology. 6th edition.
Show details“To be, or not to be: that is the question.” While we all are poised at life-or-death decisions, this existential dichotomy is exceptionally stark for embryonic cells. Programmed cell death, called apoptosis,* is a normal part of development. In the nematode C. elegans, in which we can count the number of cells, exactly 131 cells die according to the normal developmental pattern. All the cells of this nematode are “programmed” to die unless they are actively told not to undergo apoptosis. In humans, as many as 1011 cells die in each adult each day and are replaced by other cells. (Indeed, the mass of cells we lose each year through normal cell death is close to our entire body weight!) Within the uterus, we were constantly making and destroying cells, and we generated about three times as many neurons as we eventually ended up with when we were born. Lewis Thomas (1992) has aptly noted,
By the time I was born, more of me had died than survived. It was no wonder I cannot remember; during that time I went through brain after brain for nine months, finally contriving the one model that could be human, equipped for language.
Apoptosis is necessary not only for the proper spacing and orientation of neurons, but also for generating the middle ear space, the vaginal opening, and the spaces between our fingers and toes Saunders and Fallon 1966Roberts and Miller 1998;Rodriguez et al. 1997. Apoptosis prunes away unneeded structures, controls the number of cells in particular tissues, and sculpts complex organs.
Different tissues use different signals for apoptosis. One of the signals often used in vertebrates is bone morphogenetic protein 4 (BMP4). Some tissues, such as connective tissue, respond to BMP4 by differentiating into bone. Others, such as the frog gastrula ectoderm, respond to BMP4 by differentiating into skin. Still others, such as neural crest cells and tooth primordia, respond by degrading their DNA and dying. In the developing tooth, for instance, numerous growth and differentiation factors are secreted by the enamel knot. After the cusp has grown, the enamel knot synthesizes BMP4 and shuts itself down by apoptosis (see Chapter 13; Vaahtokari et al. 1996b).
In other tissues, the cells are “programmed” to die, and they will remain alive only if some growth or differentiation factor is present to “rescue” them. This happens during the development of mammalian red blood cells. The red blood cell precursors in the mouse liver need the hormone erythropoietin in order to survive. If they do not receive it, they undergo apoptosis. The erythropoietin receptor works through the JAK-STAT pathway, activating the Stat5 transcription factor. In this way, the amount of erythropoietin present can determine how many red blood cells enter the circulation.
One of the pathways for apoptosis was largely delineated through genetic studies of C. elegans. It was found that the proteins encoded by the ced-3 and ced-4 genes were essential for apoptosis, but that in the cells that did not undergo apoptosis, those genes were turned off by the product of the ced-9 gene (Figure 6.27A; Hengartner et al. 1992). The CED-4 protein is a protease activating factor that activates CED-3, a protease that initiates the destruction of the cell. Mutations that inactivate the CED-9 protein cause numerous cells that would normally survive to activate their ced-3 and ced-4 genes and die. This leads to the death of the entire embryo. Conversely, gain-of-function mutations of ced-9 cause CED-9 protein to be made in cells that would otherwise die. Thus, the ced-9 gene appears to be a binary switch that regulates the choice between life and death on the cellular level. It is possible that every cell in the nematode embryo is poised to die, and those cells that survive are rescued by the activation of the ced-9 gene.
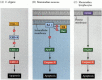
Figure 6.27
Apoptosis pathways in nematodes and mammals. (A) In C. elegans, the CED-4 protein is a protease activating factor that can activate the CED-3 protease. The CED-3 protease initiates the cell destruction events. CED-9 can inhibit CED-4 (and CED-9 can be (more...)
The CED-3 and CED-4 proteins form the center of the apoptosis pathway that is common to all animals studied. The trigger for apoptosis can be a developmental cue such as a particular molecule (such as BMP4 or glucocorticoids) or the loss of adhesion to a matrix. Either type of cue can activate the CED-3 or CED-4 proteins or inactivate the CED-9 molecules. In mammals, the homologues of the CED-9 protein are members of the Bcl-2 family of genes. This family includes Bcl-2, Bcl-X, and similar genes. The functional similarities are so strong that if an active human BCL-2 gene is placed into C. elegans embryos, it prevents normally occurring cell deaths in the nematode embryos (Vaux et al. 1992). In vertebrate red blood cell development (mentioned above), the Stat5 transcription factor activated by erythropoietin functions by binding to the promoter of the Bcl-X gene, where it activates the synthesis of that anti-apoptosis protein (Socolovsky et al. 1999).
The mammalian homologue of CED-4 is called Apaf-1 (apoptotic protease activating factor-1), and it participates in the cytochrome c-dependent activation of the mammalian CED-3 homologues, the proteases caspase-9 and caspase-3 (Shaham and Horvitz 1996; Cecconi et al. 1998; Yoshida et al. 1998). The activation of the caspases causes the autodigestion of the cell. Caspases are strong proteases, and they digest the cell from within. The cellular proteins are cleaved and the DNA is fragmented.†
While apoptosis-deficient nematodes deficient for CED-4 are viable (despite their having 15% more cells than wild-type worms), mice with loss-of-function mutations for either caspase-3 or caspase-9 die around birth from massive cell overgrowth in the nervous system (Figure 6.28; Kuida et al. 1996, 1998; Jacobson et al. 1997). Mice homozygous for targeted deletions of Apaf-1 have severe craniofacial abnormalities, brain overgrowth, and webbing between their toes.
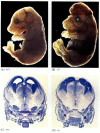
Figure 6.28
Disruption of normal brain development by blocking apoptosis. In mice in which caspase-9 or Apaf-1 has been knocked out, normal neural apoptosis fails to occur. In caspase 9-deficient mice, the overproliferation of brain neurons is obvious on a morphological (more...)
In mammals, there is more than one pathway to apoptosis. The apoptosis of the lymphocytes, for instance, is not affected by the deletion of Apaf-1 or caspase-9, and works by a separate pathway initiated by the CD95 protein (Figure 6.27B,C) Different caspases may be functioning in different cell types to mediate the apoptotic signals (Hakem et al. 1998; Kuida et al. 1998).
WEBSITE
6.7 The uses of apoptosis. Apoptosis is used for numerous processes throughout development. This website explores the role of apoptosis in such phenomena as Drosophila germ cell development and the eyes of blind cave fish. http://www.devbio.com/chap06/link0607.shtml
Box
Cell-Cell Interactions and Chance in the Determination of Cell Types.
Footnotes
- *
Apoptosis (both “p”s are pronounced) comes from the Greek word for the natural processes of leaves falling from trees or petals from flowers. It is active and can be evolutionarily selected. The other type of cell death, necrosis, is a pathological death caused by external factors such as inflammation or toxic injury.
- †
There is some evidence (see Barinaga 1998a,b; Saudou et al. 1998) that activation of the apoptosis pathway in adult neurons may be responsible for the pathology of Alzheimer's disease and stroke. Fragmentation of DNA is one of the major ways that apoptosis is recognized, and it is seen in regions of the brain affected by these diseases. Its fragmentation into specific sized pieces (protected and held together by nucleosomes) may be caused by the digestion of poly(ADP-ribose) polymerase (PARP) by caspase-3 (Lazebnik et al. 1994). PARP recognizes and repairs DNA breaks. For more on other pathways leading to apoptosis, see Green 1998.
- The Cell Death Pathways - Developmental BiologyThe Cell Death Pathways - Developmental Biology
- 5450[uid] AND (alive[prop]) (1)Gene
- POU2AF1 POU class 2 homeobox associating factor 1 [Homo sapiens]POU2AF1 POU class 2 homeobox associating factor 1 [Homo sapiens]Gene ID:5450Gene
- MYH9-DT MYH9 divergent transcript [Homo sapiens]MYH9-DT MYH9 divergent transcript [Homo sapiens]Gene ID:105377199Gene
- LOC105374511 [Homo sapiens]LOC105374511 [Homo sapiens]Gene ID:105374511Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...