A. Nuclei and Perinuclear Material
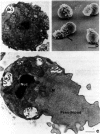
Nuclei of primary spermatocytes undergo two meiotic divisions to give rise to spermatid nuclei, which are haploid. The spermatid and spermatozoan nuclei are highly condensed (Fig. 2a,c) and stay in this state until they are within the oocyte during fertilization. (A condensed nucleus is also observed in most flagellated spermatozoa.) The nuclear division of secondary spermatocytes is rapid (∼2–5 minutes; Ward et al. 1981), and chromatin condensation apparently occurs as meiosis II is completed. However, unlike flagellated sperm, C. elegans spermatid and spermatozoan nuclei are not surrounded by an envelope or any type of membrane. The highly condensed spermatid chromatin is not associated with protamines (B. Nathans and S. Ward, unpubl.), unlike the chromatin of mammalian spermatozoa. However, a novel sperm-specific histone H1 in C. elegans is expressed during spermatogenesis (Sanicola et al. 1990), as is the case for a number of species that have flagellated sperm (see, e.g., Cole et al. 1986). It is possible that this histone H1 plays a part in formation of the highly condensed chromatin that characterizes sperm. The spherical nucleus has a closely applied layer of perinuclear material (forming a halo that surrounds the nucleus) in which the centriole pair is embedded (Fig. 2a,c). Ultrastructural analyses after cytochemical staining suggest that this material is enriched in RNA (Ward et al. 1981).
B. Plasma and Internal Membranes
Formation of spermatids involves dramatic rearrangements of the cytoplasm. At two points, sperm undergo an unequal division (Fig. 1A,D,D′). Several types of membrane-bounded organelles are confined to and segregate with spermatocytes as these cells separate from the rachis, despite a coenocytic association prior to division (Fig. 1A). The second unequal division is observed when spermatids bud from a common residual body (Fig. 1D,D′). This second division involves formation of a membrane boundary by accretion of vesicles at the site of division (Ward et al. 1981; Roberts et al. 1986); no microfilament-containing contractile ring can be detected and division is insensitive to cytochalasins (G. Nelson and S. Ward, unpubl.). These features of spermatid budding (Ward et al. 1981) resemble platelet formation from a megakaryocyte (Yamada 1957) or plant cell division (O'Brien and McCully 1969).
Table 1
spe genes discussed in this chapter.
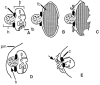
Some type of mechanism must ensure that cellular divisions are associated with the proper partitioning of cytoplasm and plasma membrane into the developing spermatid. For instance, recent data indicate that voltage-dependent ion channels in the plasma membrane are sorted so that most of these channels in spermatocytes are segregated into the residual body (Machaca et al. 1996). Intracellularly, the major mechanism employed to ensure proper cytoplasmic partitioning involves an unusual organelle called the fibrous body–membranous organelle complex (FB-MO; also referred to as the special vesicle) (Wolf et al. 1978; Ward et al. 1981), and this organelle appears to be peculiar to nematode sperm. The membranous organelle (MO) portion of the FB-MO is first observed after spermatogonial cells have initiated meiosis, but before they bud from the rachis (Figs. 1A and 3A). They first appear as vesicular swellings at the edges of the abundant Golgi complexes that are evident at this stage, and each cell forms a number of individual MOs. These vesicles are constricted at their base by a collar structure that forms an electron-dense necklace. As the MOs form, the fibrous body (FB) is first detected at their bases. The major constituent of the FB is the major sperm protein, or MSP (Fig. 3a). Roberts et al. (1986) examined developing MO membranes by monoclonal anti-MSP antibody labeling (Ward et al. 1986) and serial-section electron microscopy and showed that they are associated with MSP from their earliest identifiable stage.
As mentioned above, the FB-MO complexes are a major mechanism to prepackage proteins for delivery to spermatids during their budding division from the residual body. Their cargo includes proteins that will ultimately reside on the cell surface, as well as those that remain within the cytoplasm. As the FB-MO develops, it increases in size and soon has three distinct compartments (Fig. 3B). The head is a membrane vesicle that has an electron-dense interior that lacks internal membrane structure. An electron-dense collar separates the main body of the MO from the head, forming a noose-like structure that appears to constrict the membrane. The body of the MO has an internal membrane compartment continuous with the head. The body membrane folds around the FB but does not seal it off from the rest of the cytoplasm. The interior membrane of the FB is the only site in spermatocytes and developing spermatids where the MSP forms fibers that constitute the bulk of the FB. Another protein, sperm-specific protein SSP-10, also becomes associated with the MSP fibers (Sosnowski 1987). The FB-MO reaches its maximum size in budding spermatids where these structures (which fill ∼35% of the spermatid volume; Ward et al. 1981) are segregated along with mitochondria and the nucleus into the budding spermatid (Fig. 2a). In contrast, all ribosomes, actin, myosin, nearly all tubulin (except that in the centrosome), and many internal membranes are segregated into the residual body (Nelson et al. 1982; Ward 1986).
How FB-MOs segregate into developing C. elegans spermatids is unknown, but in Ascaris, FB-MOs localize near the centrosomes, and spermatids form around the haploid nucleus that is very close to the centrosome after completion of the second meiotic division (T. Roberts, pers. comm.). Reorganization of the FB occurs once spermatids bud from the residual body. The double membrane surrounding the MSP fibers is retracted and folds in on itself to form a compact, bilobed MO (Fig. 3C). The MSP fibers from the FB depolymerize and the MSP is scattered throughout the cytoplasm, as detected by antibody labeling (Roberts et al 1986). This basic protein (pI 8.6; Klass and Hirsh 1981; Burke and Ward 1983) renders the spermatid more electron-dense following depolymerization of the FB, but it has no known role during this developmental stage. The MO localizes below the plasma membrane (Fig. 3D).
MOs have a secretory function during spermiogenesis. Well before fertilization, the MO head fuses with the plasma membrane and releases its contents onto the cell surface (Figs. 2c and 3E). Much of this material stains with fluorescent wheat-germ agglutinin and is removed by proteases, suggesting that it is glycoprotein (Ward et al. 1983). The collar structure of the MO forms a permanent fusion pore with the plasma membrane, and the interior of the MO body is open to the cell's exterior. The MSP, which had scattered throughout the spermatid cytoplasm, concentrates in the single pseudopod and forms 2-nm filaments that appear to play a part in spermatozoan motility (see below).
C. Mutants with Organelle Defects
1. Chromosome Segregation Defects
The spe-6 and spe-26 genes have been shown to be involved in proper chromosome segregation (Varkey et al. 1993, 1995). Mutants defective in spe-6 contain spermatocytes that arrest meiotic nuclear divisions at diakinesis. DAPI staining of spe-6 mutant testes reveals terminal spermatocytes that contain condensed meiotic chromosomes, usually located near the center of the cell. Mutants lacking spe-26 form normal-appearing primary spermatocytes that are incapable of forming spermatids. The mutant cells arrest as terminal spermatocytes that frequently contain four DAPI-positive regions, suggesting that the nuclear divisions of meiosis have occurred. However, about half of the examined spe-26 terminal spermatocytes contain 5–12 regions of chromatin, indicating that aberrant meiotic nuclear divisions are also common. The spe-26 gene encodes a protein that has homology with a family of actin-binding proteins, suggesting that mutant defects in meiosis might be due to improper function of microfilaments (Varkey et al. 1995).
2. Defects in Perinuclear Material
The fer-2 , fer-3 , fer-4 (Ward et al. 1981), and spe-11 mutations (Hill et al. 1989; Browning and Strome 1996) cause defects in the structure and organization of the perinuclear halo. A perinuclear halo is absent in fer-2 , fer-3 , and fer-4 mutants, but it is replaced by an accumulation of large tubules. The aberrant ultrastructure of the perinuclear halo is evident from its first appearance during development of fer-4 sperm, whereas the fer-2 perinuclear material initially is almost wild type in appearance, becoming progressively more tubular in appearance. In addition to perinuclear defects, fer-2 , fer-3 , and fer-4 sperm also have defects in the pseudopod, which are presumably responsible for their defects in both motility and fertilization (see below). It is unclear how the perinuclear defects might be related to these pseudopod defects (Ward et al. 1981). In contrast, the only evident cytological defects in spe-11 sperm are in the perinuclear region (Hill et al. 1989). The most penetrant spe-11 mutant lacks most of the perinuclear halo, except for a small amount surrounding the centriolar region. Mutant spe-11 spermatozoa are competent to enter the oocyte but result in a paternal-effect lethal phenotype (Kemphues and Stone, this volume), suggesting that the perinuclear region is essential for embryonic viability.
3. FB-MO Defects
Mutant spe-4 , spe-5 , spe-6 , spe-10 , spe-17 , fer-1 , and fer-6 sperm all exhibit defects in FB-MO morphogenesis and/or function. The most striking defects are found in sperm lacking spe-4 or spe-6 , which never make spermatids and always arrest as cells that, by Nomarski optics, are similar to spermatocytes, except that they contain four haploid nuclei. Such cells are called “terminal spermatocytes” (L'Hernault and Arduengo 1992; Varkey et al. 1993). The earliest defects are observed in spe-6 mutant sperm, which never make an identifiable FB containing MSP filaments (Varkey et al. 1993). Instead, antibody staining reveals that MSP is present throughout the terminal spermatocyte cytoplasm. This result suggests that spe-6 lacks the normal site(s) required for the nucleated assembly of MSP filaments normally found in the FB. Developing MOs become distended and vacuolated in spe-6 mutants and are scattered throughout the cytoplasm.
Ultrastructural analyses reveal that spe-4 mutants have defects in FB-MO morphogenesis. Although examination of wild-type spermatocytes has revealed that the FB normally develops in intimate association with the MO, in spe-4 mutants, the FB and MO seem to develop as discrete structures. It is not known if there is a transient association between the FB and MO during their development or if there is an alternative pathway for formation of the FB in spe-4 mutants. Nevertheless, the end result is a terminal spermatocyte that contains distended MOs and separate FBs. The spe-4 gene encodes an integral membrane protein that appears to be localized to the FB-MO during sperm development (Arduengo et al. 1994). The precise biochemical function of SPE-4 is unknown, although this protein shows homology with the presenilins (Levy-Lahad et al. 1995; Rogaev et al. 1995; Sherrington et al. 1995) and the C. elegans SEL-12 protein (Levitan and Greenwald 1995). The presenilins are integral membrane proteins implicated in the development of Alzheimer's disease, and sel-12 mutations were identified as a suppressors of lin-12 . The SEL-12 protein facilitates reception of intercellular signals by LIN-12 (see Greenwald, this volume).
Mutations in spe-5 , spe-10 , spe-17 , fer-1 , and fer-6 all affect FB-MO morphogenesis, but allow formation of at least a few spermatids. These spermatids usually have ultrastructural defects in their FB-MOs, although they occasionally develop into spermatozoa that successfully fertilize oocytes. The spe-5 , spe-10 , and spe-17 mutants show defects in FB-MO morphogenesis in spermatocytes. In spe-5 , defects are first evident in primary spermatocytes. The MO portion of the FB-MO appears to be distended and vacuolated, and in most cases, spermatids do not form and cells arrest in a manner reminiscent of spe-4 mutants described above. Occasionally, spermatids with vacuolated MOs form, and some of these differentiate into functional spermatozoa. Putative null mutants are slightly self-fertile (L'Hernault et al. 1988; K. Machaca and S. L'Hernault, unpubl.).
The spe-17 FB-MOs assemble in a manner that appears superficially normal except that the membranes appear to be studded with ribosomes (Shakes and Ward 1989b); wild-type FB-MO membranes are never associated with ribosomes (Wolf et al. 1978; Ward et al. 1981). Normally, ribosomes are segregated to the residual body during the second meiotic division, but, in spe-17 , ribosomes associated with the FB-MO are carried into the spermatid. The FB appears to disassemble normally, but many MOs do not fuse with the cell surface during spermiogenesis. Nevertheless, many spe-17 spermatozoa are able to crawl in vitro (Shakes and Ward 1989b) and a few can fertilize oocytes in vivo (L'Hernault et al. 1993). The spe-17 -encoded protein is not homologous to any known protein, and it does not appear to be an integral membrane protein (L'Hernault et al. 1993).
Morphogenesis of FB-MOs appears to be superficially normal in spe-10 mutants until spermatids start to form (Shakes and Ward 1989b). In this mutant, the membrane around the FB appears to retract prematurely and the membrane-free fibrous body ends up in the residual body. The MO is segregated into the spermatid without an attached FB, and it becomes distended and vacuolated. The spe-10 mutants do not have any MOs that fuse with the cell surface during spermiogenesis, and spe-10 spermatozoa do not crawl.
In contrast to the above-mentioned mutants, many fer-6 spermatids contain FBs that fail to disassemble. The membrane that normally surrounds the FB is apparently retracted into the MO during spermatid budding, but the naked MSP fiber bundles fail to disassemble and can be observed in the cytoplasm of cells that have extended pseudopods. Additionally, fer-6 spermatozoa contain many MOs that fail to fuse with the plasma membrane (Ward et al. 1981).
The latest FB-MO defects are shown by fer-1 mutants, which appear indistinguishable from wild type until spermiogenesis (Ward et al. 1981). During spermiogenesis, the MOs in fer-1 mutants do not fuse with the cell surface. The resulting spermatozoa have abnormally short pseudopods (see Fig. 1G) and do not crawl. These observations, together with those from spe-10 mutants, suggest that MO fusion with the cell surface is an essential prerequisite for proper spermatozoan motility. MO fusions introduce permanent fusion pores into the plasma membrane, probably insert MO-derived membrane components into the plasma membrane (Roberts et al. 1986), and release extracellular matrix constituents onto the cellular surface (Fig. 2c) (Ward et al. 1981). However, no MO-derived component has been shown to have a direct function in motility.
Publication Details
Copyright
Publisher
Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY)
NLM Citation
Riddle DL, Blumenthal T, Meyer BJ, et al., editors. C. elegans II. 2nd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1997. Section II, Organelle Morphogenesis During Spermatogenesis.