This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Bundock EA, Corey TS, Andrew TA, et al., editors. Unexplained Pediatric Deaths: Investigation, Certification, and Family Needs [Internet]. San Diego (CA): Academic Forensic Pathology International; 2019.

Unexplained Pediatric Deaths: Investigation, Certification, and Family Needs [Internet].
Show detailsADELINE
“Adeline was 14 months old and my first and only child at the time of her death. When Adeline smiled, she did it not only with her mouth, but with her big, chocolate brown eyes. She was our world. When Adeline died, her pediatrician drove to the hospital as soon as she heard. She offered what seemed like a very odd suggestion at the time – to take pictures of us with Adeline in the hospital, despite the fact she was already gone. I didn’t understand how much I’d learn to love those pictures. Adeline just looked asleep, and they give me comfort that she left this world in no pain, peacefully.”
– Adeline’s Mom
Box
OUTLINE.
Cardiovascular diseases are important causes of sudden unexpected death in pediatrics (1, 2). Complete and thorough autopsies must be performed to recognize and diagnose cardiovascular etiologies. In infants and young children, undiagnosed congenital heart disease (CHD) along with potentially heritable cardiomyopathies and channelopathies can lead to sudden unexpected death (3). Therefore, dissection techniques should be modified to ensure that identifiable congenital malformations are not missed. In many instances of undiagnosed congenital heart disease, there will be some indication in the investigative report that the child was symptomatic. And, usually, the heart will appear abnormal during the initial examination of the organs at autopsy.
Any type of congenital heart disease can present for the first time at autopsy, including both simple (isolated atrial or ventricular septal defects, valvular abnormalities, patent ductus arteriosus, and coarctation of the aorta) and complex (tetralogy of Fallot, atrioventricular septal defects, complex lesions involving the great vessels, single ventricle heart disease, double inlet/outlet) lesions, depending on the age of the child at death (3–8). Additionally, undiagnosed coronary artery abnormalities, such as anomalous origins of the coronary arteries and coronary aneurysms (Kawasaki disease) can lead to sudden unexpected death in pediatrics (9–19).
An enlarged or dilated heart in a child should always make the examiner consider cardiomyopathy. However, the presence of a normal heart size and shape does not rule out the presence of a cardiomyopathy; many types of cardiomyopathy may still be present (20). Other clues that may point to a cardiomyopathy are hypertrophy of the interventricular septum or chamber walls or chamber dilatation. Cardiomyopathies that can cause sudden unexpected death in pediatrics include hypertrophic cardiomyopathy (21–23), restrictive/infiltrative cardiomyopathies (24–26), dilated cardiomyopathies (27, 28), and arrhythmogenic cardiomyopathy (29–35). There is debate regarding whether left ventricular noncompaction and primary endocardial fibroelastosis are true cardiomyopathies or whether they represent changes associated with other cardiomyopathies (36–41).
AUTOPSY-NEGATIVE CASES
Some of the most challenging cases are those in which no gross or microscopic cause of death is found after a complete autopsy (autopsy-negative cases). In these situations, additional dissections and more extensive histological sampling may reveal subtle causes of sudden unexpected death in pediatrics. Hypertrophic cardiomyopathy, arrhythmogenic cardiomyopathy, histiocytoid cardiomyopathy, and other forms of cardiomyopathy may have subtle findings that only manifest microscopically in children (42).
Pericardial, myocardial, or endocardial inflammation can result in sudden unexpected death in pediatrics and may not be apparent at the time of autopsy (43–47). Myocarditis can present at autopsy as a dilated or grossly normal-appearing heart. Myocarditis is defined as inflammation with associated cardiac myocyte necrosis, and borderline myocarditis is defined as significantly increased interstitial inflammation without definitive cardiac myocyte necrosis (47). The types of myocarditis (lymphocytic, eosinophilic, neutrophilic, giant cell) can be diagnosed with adequate histological sectioning at autopsy (47, 48).
In children over one year of age, if no definitive cause of death is found after microscopic examination, then examination of the cardiac conduction system – including the sinoatrial (SA) node, atrioventricular (AV) node, penetrating AV bundle of His, and proximal bundle branches – may be warranted (49–53). Inflammation and tumors (cystic AV nodal tumor) are known to cause sudden death, if they involve components of the cardiac conduction system. However, the significance of other lesions – such as fibrointimal hyperplasia of the SA or AV nodal arteries, increased fibrosis or adipose tissue in the nodes or AV bundles, persistent fetal dispersion of the AV node, cartilaginous metaplasia or fragmentation of the AV bundle – is unclear in the setting of sudden unexpected death in pediatrics (54).
In any case of sudden unexpected death in pediatrics, the pathologist should consider the possibility of a genetic disease, such as a heritable cardiomyopathy or a cardiac channelopathy (55–60). An appropriate specimen for subsequent DNA extraction and possible postmortem genetic testing (blood in EDTA or frozen heart) should be procured and saved. The family may be referred for cardiovascular screening and possible genetic counseling by clinicians specially trained in this area (61).
There are significant gaps in our knowledge of the onset, natural history, and clinical and pathological manifestations of many of the cardiovascular diseases that result in sudden unexpected death in pediatrics. Additionally, there are gaps in our understanding of when certain anatomical and pathological changes – such as alterations in coronary artery anatomy, increased heart size, fibrosis, and inflammation – become significant enough to be invoked as the cause and manner of death (62, 63).
Many variants of the normal cardiac anatomy have been identified, some more benign than others (9, 64). An anomalous origin of a coronary artery can cause sudden death (9, 64–66). Usually, the coronary ostia are situated within the sinuses of Valsalva, but they may occur in coronary ostia situated above the sinuses at the level of the sinotubular junction, or higher in the ascending aorta (9). There is thought to be an increased risk of sudden death if the coronary ostia are situated high enough in the aorta, so that there is an acute angle of take-off of the coronary arteries relative to the aorta. However, the angle of take-off is difficult to measure in the postmortem setting and it is unclear at what point a high coronary artery take-off increases the risk of sudden death. Another anatomic variant of unknown clinical significance is myocardial bridging or tunneling (67–70). In adults, it is not uncommon for the left anterior descending coronary artery to dive into the myocardium for a short distance, and this is considered a normal anatomic variant with no pathological significance. If the coronary artery is too deep within the myocardium and travels over a long distance, then there may be transient ischemia during systole (67). However, the features that differentiate pathologic myocardial bridging from a normal variant is unknown.
An enlarged heart (cardiomegaly) can be one of the first indications of a cardiomyopathy. However, determining the normal heart weight or weight range can be problematic in children (and adults). There is a direct correlation between heart weight and body weight. However, we do not have a clear understanding of what constitutes a pathologically enlarged heart in children. There are tables of normal, average, heart weights (with confidence intervals) for children with different body weights (71). However, there is neither any single, standard source for normal child heart weights nor any accepted, uniform method for determining a child’s heart weight at autopsy. The situation is even more complicated in obese children, in whom an increased heart weight is expected due to increased body weight. But in these cases, is the enlarged heart “normal” or is it pathologically enlarged? There is currently no answer to this question. Some consider enlarged hearts, whether the enlargement is expected based on body weight or pathological, to be more vulnerable to arrhythmias, while others consider an enlarged heart appropriate for increased body weight to be normal.
In grossly and microscopically normal hearts, channelopathies and cardiomyopathies should be considered as possible causes of death in children with no obvious cause of death following autopsy (2, 57, 72, 73). Most channelopathies will not have any histopathological signs, unless there is remodeling of the myocardium, producing fibrosis (58). In contrast, cardiomyopathies will often display histological changes, even if they are very subtle (42, 72). Little is known regarding the histopathological progression of cardiomyopathies in children. The microscopic changes most frequently seen in cardiomyopathy (fibrosis and inflammation) are often nonspecific (48, 62, 74), as are some of the other pathological changes that may be seen – left ventricular hypertrophy in the absence of fibrosis or cardiac myocyte disarray, fatty infiltration of the right ventricle in the absence of fibrosis, mild ventricular dilatation, isolated floppy mitral valve, and scattered foci of interstitial lymphocytes without cardiac myocyte necrosis (62, 74).
Increased interstitial fibrosis can be an early indication of a cardiomyopathy (62). However, assessing the degree and significance of interstitial fibrosis in the heart can be difficult. In certain parts of the heart, like the SA and AV nodes and cardiac conduction system, fibrosis is necessary for normal electrophysiology (75). However, abnormal fibrosis can cause disturbances in electrical conductance, leading to arrhythmias (50, 75–77). Performing special stains to highlight fibrosis, such as a Masson’s trichrome stain, can be helpful, but interpreting the significance of the fibrosis can be challenging (62). When accompanied by fibrosis, cardiac myocyte enlargement and disarray are histologic signs of hypertrophic cardiomyopathy (20, 23, 78–80). However, focal cardiac myocyte disarray can be seen in normal hearts, especially in areas of architectural distortion, such as around blood vessels and in the posterior interventricular septum (62). These focal changes can be misinterpreted as hypertrophic cardiomyopathy (62).
Arrhythmogenic cardiomyopathy can be used as clinical term to cover a diverse group of arrhythmogenic etiologies not explained by ischemic, hypertensive, or valvular heart disease (81). More commonly, the term is used to refer to the pathologic subtypes of arrhythmogenic right ventricular cardiomyopathy and arrhythmogenic left ventricular cardiomyopathy, which are rare genetic conditions that typically manifests later in life than other genetic cardiomyopathies (30, 32). The pathologic diagnosis of arrhythmogenic cardiomyopathy is made when there is fatty replacement with fibrosis in the right and/or left ventricular myocardium, and mutations in genes affecting cardiac myocyte junctions are present (30, 32, 35, 82, 83). Arrhythmogenic cardiomyopathy can present in children, but the gross and histological changes are not as well-understood as in adults and focal fatty replacement (without fibrosis) of the right ventricular wall (a normal finding) can be misinterpreted as arrhythmogenic cardiomyopathy (20, 62, 74).
Focal interstitial chronic inflammation can also be challenging to interpret (48, 62, 63). It is unclear how many chronic inflammatory cells are normally found in the hearts of children of various ages. The distinction between an increased number of interstitial lymphocytes and borderline lymphocytic myocarditis (without cardiac myocyte necrosis) is not well-defined (63). Some consider myocarditis to be overdiagnosed and there is a great deal of disagreement between pathologists over what constitutes a diagnosis of myocarditis, with or without obvious cardiac myocyte necrosis (63). Some pathologists diagnose myocarditis when there is an increase in lymphocytes, while others require definitive cardiac myocyte necrosis. While transient systemic immune responses in children may result in increased interstitial lymphocytes in the heart, this may also be a sign of a developing cardiomyopathy (20).
Another area of uncertainty in an apparently autopsy-negative case is when to examine the cardiac conduction system and what to assess. Forensic pathologists are taught to dissect and examine this system in sudden unexplained deaths of older children and adults with no significant autopsy findings (49–51). This is a labor-intensive technique that takes practice and experience. Understanding the normal gross and histologic anatomy of the cardiac conduction system is challenging. The challenge is amplified by the existence of normal anatomic variants that must be differentiated from pathological changes. Several studies have focused on examining the cardiac conduction system in infants, children, and young adults ((52, 54, 84, 85). However, it is still not entirely clear which conduction system changes constitute significant pathology, pathology that could result in sudden death. Some changes involving the conduction system are considered pathological and may result in sudden death. These include inflammation in the conduction system (as may be seen in myocarditis and pericarditis) and tumors, such as cystic AV nodal tumors (86). Cardiac conduction system changes of unknown significance include fibrointimal hyperplasia or fibromuscular dysplasia of the SA and AV nodal arteries, increased fibrosis in the SA node leading to sick sinus syndrome, persistent fetal dispersion of the AV node in the central fibrous body, cartilaginous metaplasia of the central fibrous body, fragmentation of the atrioventricular bundle (of His), and increased adipose tissue or inflammatory cells within various components of the cardiac conduction system (51, 54).
While postmortem imaging is routinely practiced in some offices, there is currently very little data regarding the benefit of various modalities, such as postmortem computed tomography, postmortem computed tomography with angiography, postmortem magnetic resonance imaging, and ultrasound in determining cause of death in children (87–98). Postmortem imaging has been used successfully to diagnose congenital anomalies prior to or in place of autopsy (87). Because the coronary and cerebral vasculature are difficult to assess at autopsy, it is possible that pre-autopsy use of postmortem computed tomography with angiography could have a role in the diagnosis of vascular anomalies in the brain and/or heart (89, 95).
EVALUATION OF THE CARDIOVASCULAR SYSTEM AT AUTOPSY
Prior to Autopsy
Prior to starting the postmortem examination in a case of sudden unexpected death in pediatrics, it is important to have information regarding the circumstances surrounding death and any pertinent medical and family history. This initial investigative information can be very helpful in guiding the postmortem examination (99).
In all cases of sudden unexpected death in pediatrics, postmortem imaging should be performed. At a minimum, plain radiographs should be obtained, which will show the relative size of the heart, position of the heart, and pulmonary changes that might be associated with heart disease, as well as other salient features, such as occult fractures, discussed elsewhere in this publication. If available, newer imaging modalities, such as postmortem computed tomography and postmortem magnetic resonance imaging can be considered. These may provide additional detail of any congenital anomalies or other disease processes that are present. Additionally, postmortem computed tomography with angiography is being investigated as a tool for assessing vascular anomalies in the great vessels, heart, brain, and lungs, anomalies that may cause sudden death.
Autopsy Examination
There are various methods for proper examination of the heart and great vessels at autopsy, including examining the heart in situ or as a heart-lung block, either fresh or after formalin fixation (74, 99–101). The goal is to be able to adequately assess the anatomy of the heart, lungs, and associated great vessels, so that any structural abnormalities can be diagnosed. The approach used may depend on the age of the child or any known prior history (42, 74, 100, 102, 103).
Examination of the heart should start with assessment of the epicardial surface for evidence of pericarditis, fibrofatty replacement, and any discoloration of the myocardium. Next, the epicardial coronary anatomy should be examined to assess for potential anomalies, including aneurysms or hemorrhage. To ensure that there is no anomalous origin of a coronary artery, the ascending aorta can be trimmed so that the coronary ostia can be observed by looking down into the aorta. If coronary artery abnormalities are observed, these should be investigated prior to opening the heart. Pre-autopsy, postmortem computed tomography with angiography could be helpful in showing the anatomy of the coronary arteries, especially if there are anatomic variations.
Before removing the heart from the lungs, the pathologist must assess for normal connections of the great vessels to the heart, a normal, left-sided aortic arch, absence of vascular rings around the trachea or esophagus, absence of coarctation of the aorta (assessing the entire thoracic aorta), and closure or patency of the ductus arteriosus. If any congenital vascular anomalies are observed, it is best to leave the heart attached to the lungs and thoracic aorta, so that the anatomy is not disturbed and can be studied in detail after en block removal.
Dissection of the heart can be performed using several methods (42, 99–101). In infants without congenital heart disease, the preferred method is to open the heart along lines of blood flow (inflow-outflow method). This allows for adequate assessment of chamber anatomy and possible defects. In older children without apparent congenital heart disease, the combination of apical cross-sections and inflow-outflow dissection of the base can be performed, as in an adult heart. Other methods of dissection, such as four-chamber and left ventricular outflow dissection methods, utilized in other types of cases, are not recommended in cases of sudden unexpected death in pediatrics.
Once the heart is opened and all postmortem blood and postmortem thrombi are removed, the heart should be weighed. Measurements of wall thicknesses (left and right ventricle walls and interventricular septum) and valve circumferences (tricuspid, pulmonic, mitral, and aortic) may be recorded (Image 7.1) and compared to reference values (104–107). The heart should be examined for any endocardial or myocardial lesions or discolorations. Cardiac valve anatomy should be assessed, as well as any abnormalities involving the valve leaflets and papillary muscles. Photographs of any abnormalities should be taken.
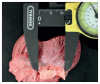
Image 7.1
Measurement of valve circumference.
Microscopic Examination
Histological sections should be taken in every case of sudden unexpected death in pediatrics that remains unexplained after autopsy (42, 99, 100). Sections from the left ventricle, right ventricle, interventricular septum, atria, and any grossly apparent lesions must be examined (Image 7.2). Ventricular sections should be taken at multiple levels (apex, middle, base). Atrial sections may include the right and left atrioventricular (AV) grooves to include the coronary arteries and interatrial septum (99). Consider taking sections from the cardiac conduction system, including the SA and AV nodal regions (Images 7.3 and 7.4), or sending relevant areas to a cardiac pathologist for evaluation.
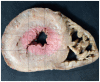
Image 7.2
Method for sampling heart for microscopic examination. Boxes indicate areas to be sampled.
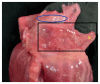
Image 7.3A
Dissection of the right atrium to sample the SA node. Oval indicates location of superior vena cava. Box indicates portion of right atrium to be fixed and sampled.
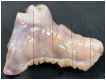
Image 7.3B
Dissection of the right atrium to sample the SA node. The right atrium is serially sectioned (at lines), yielding fragments to be embedded (see Image 7.3C).

Image 7.3C
Dissection of the right atrium to sample the SA node.
Microscopic examination of sections should include assessment for inflammation, infection, fibrosis, elastosis, and hamartomatous or neoplasia. When minor abnormalities are observed or suspected based on routine hematoxylin and eosin staining, special stains that highlight fibrosis (trichrome or pentachrome), elastosis (elastin), amyloid (Congo red), or infectious organisms (Gram, silver, periodic acid Schiff, acid-fast) may be of benefit. Because the myocardium of infants can be very cellular, immunohistochemical stains – such as CD3 (for T-cells), CD20 (for B-cells), and CD68 (for macrophages) – may be helpful.
Special Studies
In children, blood in EDTA and frozen heart tissue are the preferred samples for genetic testing. Blood on a filter paper card should always be retained and may be considered acceptable for DNA isolation by some laboratories (61). A small amount of myocardium can be frozen in liquid nitrogen or at ultra-low temperature for electron microscopy or molecular testing. In cases of possible or suspected metabolic disorders, oil red-O staining can be performed on frozen sections of fresh, frozen, or formalin-fixed tissue to assess for intracellular lipid accumulation. Finally, in certain cases, electron microscopy may be of benefit in diagnosing cardiomyopathies or storage disorders.
Interpretation of Findings
It should be recognized that there are diagnostic “gray zones” in which findings of uncertain significance exist on a continuum between normal and definite pathologic changes (42). Enlargement of the heart, heart chambers, chamber walls, or valves should raise the possibility of an underlying cardiomyopathy, with or without histological changes. Moreover, focal or mild histologic changes, such as inflammation or fibrosis, that are not diagnostic of a particular entity, may be suggestive of a developing cardiomyopathy. Finally, we should remember that some infants and children, just like adults, may die with conditions, rather than of conditions. In the end, historical information, scene findings, and autopsy findings should be interpreted together when formulating a reasonable and defensible opinion regarding cause and manner of death.
For questionable, unusual, or rare cardiac findings, one should consider consulting a pathologist with special expertise in cardiovascular pathology (74).
Pathologist Training
Forensic pathologists need to gain experience and be comfortable examining hearts from children of all ages so that they can recognize and diagnose various forms of cardiovascular disease and congenital heart disease and recognize more subtle abnormalities that may result in sudden unexpected death in pediatrics. The National Institutes of Health (NIH) hosts the 3D Print Exchange Heart Library, a collection of 3D printed hearts, focused on congenital heart disease (108), which may serve as a resource for forensic pathologists wishing to become more familiar with congenital heart defects.
CONCLUSION
In conclusion, thorough gross and histologic examination of the heart is important in determining the cause of sudden unexpected death in pediatrics (see recommendations in Table 7.1). There may be subtle findings, such as increased heart weight, inflammation, or fibrosis that may indicate underlying cardiac pathology. Additionally, the heart may show no pathological changes in “autopsy-negative” cases in which a channelopathy or cardiomyopathy may still be responsible for death.
Table 7.1
Procedural Guidance and Key Considerations for Evaluation of the Heart.
REFERENCES
- 1.
- Bar-Cohen Y, Silka MJ. Sudden cardiac death in pediatrics. Curr Opin Pediatr. 2008 Oct; 20(5):517–21. PMID: 18781113. https://doi
.org/10.1097/MOP .0b013e32830c9037. [PubMed: 18781113] - 2.
- Ackerman M, Atkins DL, Triedman JK. Sudden cardiac death in the young. Circulation. 2016 Mar 8; 133(10):1006–26. PMID: 26951821. PMCID: PMC5033115. https://doi
.org/10.1161/CIRCULATIONAHA .115.020254. [PMC free article: PMC5033115] [PubMed: 26951821] - 3.
- Serinelli S, Arunkumar P, White S. Undiagnosed congenital heart defects as a cause of sudden, unexpected death in children. J Forensic Sci. 2018 Nov; 63(6):1750–1755. PMID: 29601638. https://doi
.org/10.1111/1556-4029.13779. [PubMed: 29601638] - 4.
- Ottaviani G, Buja LM. Update on congenital heart disease and sudden infant/perinatal death: from history to future trends. J Clin Pathol. 2017 Jul; 70(7):555–62. PMID: 28450386. https://doi
.org/10.1136 /jclinpath-2017-204326. [PubMed: 28450386] - 5.
- Polderman FN, Cohen J, Blom NA, et al. Sudden unexpected death in children with a previously diagnosed cardiovascular disorder. Int J Cardiol. 2004 Jun; 95(2–3):171–6. PMID: 15193816. https://doi
.org/10.1016/j .ijcard.2003.03.026. [PubMed: 15193816] - 6.
- Sanatani S, Wilson G, Smith CR, et al. Sudden unexpected death in children with heart disease. Congenit Heart Dis. 2006 May; 1(3):89–97. PMID: 18377551. https://doi
.org/10.1111/j .1747-0803.2006.00014.x. [PubMed: 18377551] - 7.
- Ilina MV, Kepron CA, Taylor GP, et al. Undiagnosed heart disease leading to sudden unexpected death in childhood: a retrospective study. Pediatrics. 2011 Sep; 128(3):e513–20. PMID: 21824887. https://doi
.org/10.1542/peds.2010-2307. [PubMed: 21824887] - 8.
- Jortveit J, Eskedal L, Hirth A, et al. Sudden unexpected death in children with congenital heart defects. Eur Heart J. 2016 Feb 14; 37(7):621–6. PMID: 26341891. https://doi
.org/10.1093/eurheartj/ehv478. [PubMed: 26341891] - 9.
- Steffensen TS, Spicer DE. Congenital coronary artery anomalies for the pathologist. Fetal Pediatr Pathol. 2014 Oct–Dec; 33(5–6):268–88. PMID: 25329249. https://doi
.org/10.3109/15513815 .2014.966182. [PubMed: 25329249] - 10.
- Basso C, Corrado D, Thiene G. Congenital coronary artery anomalies as an important cause of sudden death in the young. Cardiol Rev. 2001 Nov–Dec; 9(6):312–7. PMID: 11696258. https://doi
.org/10.1007 /s12024-009-9123-7. [PubMed: 11696258] - 11.
- De Giorgio F, Abbate A, Stigliano E, et al. Hypoplastic coronary artery disease causing sudden death. Report of two cases and review of the literature. Cardiovasc Pathol. 2010 Jul–Aug; 19(4):e107–11. PMID: 19616973. https://doi
.org/10.1016/j .carpath.2009.05.002. [PubMed: 19616973] - 12.
- Bishnoi RN, McMillan KN, Thompson WR. Unusual sudden cardiac death from an anomalous left coronary artery from the right sinus of Valsalva. Cardiol Young. 2014 Aug; 24(4):732–4. PMID: 23880063. https://doi
.org/10.1017 /S1047951113001005. [PubMed: 23880063] - 13.
- Hill SF, Sheppard MN. A silent cause of sudden cardiac death especially in sport: congenital coronary artery anomalies. Br J Sports Med. 2014 Aug; 48(15):1151–6. PMID: 24009012. https://doi
.org/10.1136 /bjsports-2013-092195. [PubMed: 24009012] - 14.
- Yajima D, Shimizu K, Oka K, et al. A case of sudden infant death due to incomplete Kawasaki disease. J Forensic Sci. 2016 Jan; 61 Suppl 1:S259–64. PMID: 26347043. https://doi
.org/10.1111/1556-4029.12929. [PubMed: 26347043] - 15.
- Shimizu C, Sood A, Lau HD, et al. Cardiovascular pathology in 2 young adults with sudden, unexpected death due to coronary aneurysms from Kawasaki disease in childhood. Cardiovasc Pathol. 2015 Sep–Oct; 24(5):310–6. PMID: 25791439. PMCID: PMC4547904. https://doi
.org/10.1016/j .carpath.2015.02.006. [PMC free article: PMC4547904] [PubMed: 25791439] - 16.
- Ponniah U. Coronary artery thrombus resulting in sudden cardiac death in an infant with Kawasaki disease and giant coronary artery aneurysms. Ann Pediatr Cardiol. 2013 Jul; 6(2):197–9. PMID: 24688247. PMCID: PMC3957459. https://doi
.org/10.4103/0974-2069.115281. [PMC free article: PMC3957459] [PubMed: 24688247] - 17.
- Parsons S, Lynch M. Kawasaki disease: A review of 2 cases of sudden death. Heart Lung Circul. 2015; 24(Suppl 3):S154. https://doi
.org/10.1016/j .hlc.2015.06.097. - 18.
- Rowley AH, Shulman ST. Recent advances in the understanding and management of Kawasaki disease. Curr Infect Dis Rep. 2010 Mar; 12(2):96–102. PMID: 21308505. PMCID: PMC3043557. https://doi
.org/10.1007 /s11908-010-0091-6. [PMC free article: PMC3043557] [PubMed: 21308505] - 19.
- Bryant V, George S. Sudden cardiac death in a normally developed male infant: an atypical presentation of Kawasaki disease. Virchows Arch. 2014 Aug; 465(1 Suppl 1):S196. https://doi
.org/10.1007 /s00428-014-1618-2. - 20.
- Dettmeyer RB, Kandolf R. Cardiomyopathies--misdiagnosed as sudden infant death syndrome (SIDS). Forensic Sci Int. 2010 Jan 30; 194(1–3):e21–4. PMID: 19931342. https://doi
.org/10.1016/j .forsciint.2009.10.010. [PubMed: 19931342] - 21.
- Bos JM, Towbin JA, Ackerman MJ. Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009 Jul 14; 54(3):201–11. PMID: 19589432. https://doi
.org/10.1016/j .jacc.2009.02.075. [PubMed: 19589432] - 22.
- Keren A, Syrris P, McKenna WJ. Hypertrophic cardiomyopathy: the genetic determinants of clinical disease expression. Nat Clin Pract Cardiovasc Med. 2008 Mar; 5(3): 158–68. PMID: 18227814. https://doi
.org/10.1038/ncpcardio1110. [PubMed: 18227814] - 23.
- Moak JP, Kaski JP. Hypertrophic cardiomyopathy in children. Heart. 2012 Jul; 98(14):1044–54. PMID: 22591735. https://doi
.org/10.1136 /heartjnl-2011-300531. [PubMed: 22591735] - 24.
- Niss O, Quinn CT, Lane A, et al. Cardiomyopathy with restrictive physiology in sickle cell disease. JACC Cardiovasc Imaging. 2016 Mar; 9(3):243–52. PMID: 26897687. PMCID: :PMC4788530. https://doi
.org/10.1016/j .jcmg.2015.05.013. [PMC free article: PMC4788530] [PubMed: 26897687] - 25.
- Walsh MA, Grenier MA, Jefferies JL, et al. Conduction abnormalities in pediatric patients with restrictive cardiomyopathy. Circ Heart Fail. 2012 Mar 1; 5(2):267–73. PMID: 22260945. https://doi
.org/10.1161 /CIRCHEARTFAILURE.111.964395. [PubMed: 22260945] - 26.
- Rivenes SM, Kearney DL, Smith EO, et al. Sudden death and cardiovascular collapse in children with restrictive cardiomyopathy. Circulation. 2000 Aug 22; 102(8):876–82. PMID: 10952956. https://doi
.org/10.1161/01.CIR.102.8.876. [PubMed: 10952956] - 27.
- Dettmeyer R, Schmidt P, Kandolf R, Madea B. Evolution of dilated cardiomyopathy (DCM) from idiopathic hypertrophic cardiomyopathy (IHCM) vs. inflammatory dilated cardiomyopathy (DCMi): a rare case of sudden death in an 8-year-old boy. Pathol Res Pract. 2004; 200(5):411–5. PMID: 15239350. https://doi
.org/10.1016/j .prp.2004.03.005. [PubMed: 15239350] - 28.
- McNally EM, Puckelwartz MJ. Genetic variation in cardiomyopathy and cardiovascular disorders. Circ J. 2015; 79(7):1409–15. PMID: 26040335. PMCID: PMC4605546. https://doi
.org/10.1253/circj.CJ-15-0536. [PMC free article: PMC4605546] [PubMed: 26040335] - 29.
- El Demellawy D, Nasr A, Aloawmi S. An updated review on the clinicopathologic aspects of arrhythmogenic right ventricular cardiomyopathy. Am J Forensic Med Pathol. 2009 Mar; 30(1):78–83. PMID: 19237863. https://doi
.org/10.1097/PAF .0b013e318187379e. [PubMed: 19237863] - 30.
- Pilichou K, Thiene G, Bauce B, et al. Arrhythmogenic cardiomyopathy. Orphanet J Rare Dis. 2016 Apr 2; 11:33. PMID: 27038780. PMCID: PMC4818879. https://doi
.org/10.1186 /s13023-016-0407-1. [PMC free article: PMC4818879] [PubMed: 27038780] - 31.
- Shukla R, McPartland J, Kokai G, Mendelsohn S. Arrhythmogenic left ventricular cardiomyopathy and desmoplakin gene mutation. Pediatr Dev Pathol. 2011 Mar; 14(2):164. https://doi
.org/10.2350/11-02-0989-MISC .1. - 32.
- Basso C, Corrado D, Marcus FI, et al. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009 Apr 11; 373(9671): 1289–300. PMID: 19362677. https://doi
.org/10.1016 /S0140-6736(09)60256-7. [PubMed: 19362677] - 33.
- Hamilton RM. Arrhythmogenic right ventricular cardiomyopathy. Pacing Clin Electrophysiol. 2009 Jul; 32 Suppl 2:S44–51. PMID: 19602162. https://doi
.org/10.1111/j .1540-8159.2009.02384.x. [PubMed: 19602162] - 34.
- Herren T, Gerber PA, Duru F. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: a not so rare “disease of the desmosome” with multiple clinical presentations. Clin Res Cardiol. 2009 Mar; 98(3):141–58. PMID: 19205777. https://doi
.org/10.1007 /s00392-009-0751-4. [PubMed: 19205777] - 35.
- McRae AT 3rd, Chung MK, Asher CR. Arrhythmogenic right ventricular cardiomyopathy: a cause of sudden death in young people. Cleve Clin J Med. 2001 May; 68(5): 459–67. PMID: 11352326. https://doi
.org/10.3949/ccjm.68.5.459. [PubMed: 11352326] - 36.
- Takahashi S, Kanetake J, Moriya T, Funayama M. Sudden infant death from dilated cardiomyopathy with endocardial fibroelastosis. Leg Med (Tokyo). 2008 Sep; 10(5):277–80. PMID: 18442941. https://doi
.org/10.1016/j .legalmed.2008.03.001. [PubMed: 18442941] - 37.
- Ker J, Du Toit-Prinsloo L, Van Heerden WF, Saayman G. Subendocardial fibrosis in left ventricular hypertrabeculation-cause or consequence? Clin Med Insights Cardiol. 2011 Feb 2; 5:13–6. PMID: 21344021. PMCID: PMC3041236. https://doi
.org/10.4137/CMC.S6507. [PMC free article: PMC3041236] [PubMed: 21344021] - 38.
- Cohen PJ, Prahlow JA. Sudden death due to biventricular non-compaction cardiomyopathy in a 14-year-old. Forensic Sci Med Pathol. 2015 Mar; 11(1):92–8. PMID: 25549957. https://doi
.org/10.1007 /s12024-014-9637-5. [PubMed: 25549957] - 39.
- Ker J, Toit-Prinsloo LD, van Heerden WF, Saayman G. Sudden infant death syndrome and left ventricular hypertrabeculation-hidden arrhythmogenic entity? Clin Med Insights Cardiol. 2010 Sep 17; 4:85–7. PMID: 20981130. PMCID: PMC2956473. https://doi
.org/10.4137/CMC.S5933. [PMC free article: PMC2956473] [PubMed: 20981130] - 40.
- Ursell PC. Noncompaction in the fetus and neonate: an autopsy study. Am J Med Genet C Semin Med Genet. 2013 Aug; 163C(3):169–77. PMID: 23720434. https://doi
.org/10.1002/ajmg.c.31367. [PubMed: 23720434] - 41.
- Lad SK, Amonkar G. Sudden death in a case of isolated left ventricular noncompaction: an autopsy case report. Am J Forensic Med Pathol. 2015 Dec; 36(4):249–50. PMID: 26332644. https://doi
.org/10.1097/PAF .0000000000000191. [PubMed: 26332644] - 42.
- Basso C, Aguilera B, Banner J, et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017 Dec; 471(6): 691–705. PMID: 28889247. PMCID: PMC5711979. https://doi
.org/10.1007 /s00428-017-2221-0. [PMC free article: PMC5711979] [PubMed: 28889247] - 43.
- Kearney MT, Cotton JM, Richardson PJ, Shah AM. Viral myocarditis and dilated cardiomyopathy: mechanisms, manifestations, and management. Postgrad Med J. 2001 Jan; 77(903):4–10. PMID: 11123385. PMCID: PMC1741887. https://doi
.org/10.1136/pmj.77.903.4. [PMC free article: PMC1741887] [PubMed: 11123385] - 44.
- Cooper LT Jr, Keren A, Sliwa K, et al. The global burden of myocarditis: part 1: a systematic literature review for the Global Burden of Diseases, Injuries, and Risk Factors 2010 study. Glob Heart. 2014 Mar; 9(1):121–9. PMID: 25432122. https://doi
.org/10.1016/j .gheart.2014.01.007. [PubMed: 25432122] - 45.
- Weber MA, Ashworth MT, Risdon RA, et al. Clinicopathological features of paediatric deaths due to myocarditis: an autopsy series. Arch Dis Child. 2008 Jul; 93(7):594–8. PMID: 18263694. https://doi
.org/10.1136/adc.2007.128686. [PubMed: 18263694] - 46.
- Osculati A, Visonà SD, Ventura F, et al. Sudden, unexpected death of a 15-year-old boy due to pancarditis: A case report and possible etiopathogenesis. Medicine (Baltimore). 2016 Aug; 95(35):e4586. PMID: 27583870. PMCID: PMC5008554. https://doi
.org/10.1097/MD .0000000000004586. [PMC free article: PMC5008554] [PubMed: 27583870] - 47.
- Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol. 1987 Jun; 18(6):619–24. PMID: 3297992. https://doi
.org/10.1016 /S0046-8177(87)80363-5. [PubMed: 3297992] - 48.
- Edgecombe A, Veinot J. Myocarditis at post-mortem examination: a forensic perspective. Acad Forensic Pathol. 2011 Sep; 1(2): 216–23. https://doi
.org/10.23907/2011.028. - 49.
- Michaud K, Romain N, Taroni F, et al. Evaluation of a simplified method of the conduction system analysis in 110 forensic cases. Forensic Sci Int. 2002 Nov 5; 130(1): 13–24. PMID: 12427445. https://doi
.org/10.1016 /S0379-0738(02)00269-4. [PubMed: 12427445] - 50.
- Gulino SP. Examination of the cardiac conduction system: forensic application in cases of sudden cardiac death. Am J Forensic Med Pathol. 2003 Sep; 24(3):227–38. PMID: 12960658. https://doi
.org/10.1097/01 .paf.0000083453.43318.74. [PubMed: 12960658] - 51.
- Cohle SD, Suarez-Mier MP, Aguilera B. Sudden death resulting from lesions of the cardiac conduction system. Am J Forensic Med Pathol. 2002 Mar; 23(1):83–9. PMID: 11953502. https://doi
.org/10.1097 /00000433-200203000-00018. [PubMed: 11953502] - 52.
- Paz Suárez-Mier M, Aguilera B. Histopathology of the conduction system in sudden infant death. Forensic Sci Int. 1998 May; 93(2–3):143–54. PMID: 9717265. https://doi
.org/10.1016 /s0379-0738(98)00046-2. [PubMed: 9717265] - 53.
- Wu C, Lin P, Maleszewski JJ. Evaluation of the cardiac conduction system in a blended (medical-forensic) autopsy practice: A review of 215 cases (1994–2013). Lab Investig. 2015 Feb; 95 Suppl 1:80A–1A.
- 54.
- Matturri L, Ottaviani G, Ramos SG, Rossi L. Sudden Infant Death Syndrome (SIDS): a study of cardiac conduction system. Cardiovasc Pathol. 2000 May–Jun; 9(3):137–45. PMID: 10989312. https://doi
.org/10.1016 /S1054-8807(00)00035-1. [PubMed: 10989312] - 55.
- Tester DJ, Wong LCH, Chanana PJ, et al. Cardiac genetic predisposition in sudden infant death syndrome. J Am Coll Cardiol. 2018 Mar 20; 71(11):1217–27. PMID: 29544605. https://doi
.org/10.1016/j .jacc.2018.01.030. [PubMed: 29544605] - 56.
- Tester DJ, Medeiros-Domingo A, Will ML, et al. Cardiac channel molecular autopsy: insights from 173 consecutive cases of autopsy-negative sudden unexplained death referred for postmortem genetic testing. Mayo Clin Proc. 2012 Jun; 87(6):524–39. PMID: 22677073. PMCID: PMC3498431. https://doi
.org/10.1016/j .mayocp.2012.02.017. [PMC free article: PMC3498431] [PubMed: 22677073] - 57.
- Tester DJ, Ackerman MJ. Cardiomyopathic and channelopathic causes of sudden unexplained death in infants and children. Annu Rev Med. 2009 Jan; 60:69–84. PMID: 18928334. https://doi
.org/10.1146/annurev .med.60.052907.103838. [PubMed: 18928334] - 58.
- Loporcaro CG, Tester DJ, Maleszewski JJ, et al. Confirmation of cause and manner of death via a comprehensive cardiac autopsy including whole exome next-generation sequencing. Arch Pathol Lab Med. 2014 Aug; 138(8):1083–9. PMID: 24298987. https://doi
.org/10.5858/arpa .2013-0479-SA. [PubMed: 24298987] - 59.
- Tester D, Medeiros-Domingo A, Ackerman MJ. Post-mortem cardiac channel genetic testing for autopsy negative sudden unexplained death. Heart Rhythm. 2009 May; 6(5 Suppl 1):S137.
- 60.
- Tester DJ, Ackerman MJ. The role of molecular autopsy in unexplained sudden cardiac death. Curr Opin Cardiol. 2006 May; 21(3):166–72. PMID: 16601452. https://doi
.org/10.1097/01 .hco.0000221576.33501.83. [PubMed: 16601452] - 61.
- Ackerman MJ, Tester DJ, Driscoll DJ. Molecular autopsy of sudden unexplained death in the young. Am J Forensic Med Pathol. 2001 Jun; 22(2):105–11. PMID: 11394742. https://doi
.org/10.1097 /00000433-200106000-00001. [PubMed: 11394742] - 62.
- Papadakis M, Raju H, Behr ER, et al. Sudden cardiac death with autopsy findings of uncertain significance: potential for erroneous interpretation. Circ Arrhythm Electrophysiol. 2013 Jun; 6(3):588–96. PMID: 23671135. https://doi
.org/10.1161/CIRCEP .113.000111. [PubMed: 23671135] - 63.
- Bonsignore A, Palmiere C, Buffelli F, et al. When is myocarditis indeed the cause of death? Forensic Sci Int. 2018 Apr; 285:72–6. PMID: 29453007. https://doi
.org/10.1016/j .forsciint.2018.01.027. [PubMed: 29453007] - 64.
- Davis JA, Cecchin F, Jones TK, Portman MA. Major coronary artery anomalies in a pediatric population: incidence and clinical importance. J Am Coll Cardiol. 2001 Feb; 37(2):593–7. PMID: 11216984. https://doi
.org/10.1016 /S0735-1097(00)01136-0. [PubMed: 11216984] - 65.
- Kaczkowska BA, Sacchi TJ. Anomalous aortic origin of coronary artery and sudden cardiac death: consideration of diagnostic and treatment approaches. J Gen Intern Med. 2013 Jun; 28 Suppl 1:S292–3.
- 66.
- Brothers JA. Coronary artery anomalies in children: what is the risk? Curr Opin Pediatr. 2016 Oct; 28(5):590–6. PMID: 27379803. https://doi
.org/10.1097/MOP .0000000000000399. [PubMed: 27379803] - 67.
- Ceauşu M, Ionescu RA, Mălinescu B, et al. Sudden cardiac death due to triple myocardial bridging associated with atypical coronary topography. Rom J Morphol Embryol. 2013; 54(3 Suppl):833–7. PMID: 24322036. [PubMed: 24322036]
- 68.
- Hostiuc S, Curca GC, Dermengiu D, et al. Morphological changes associated with hemodynamically significant myocardial bridges in sudden cardiac death. Thorac Cardiovasc Surg. 2011 Oct; 59(7):393–8. PMID: 21448858. https://doi
.org/10.1055/s-0030-1270703. [PubMed: 21448858] - 69.
- Shenoy M, Bolos M, Hira R, et al. Myocardial bridging and coronary thrombus - when is a bridge not a bridge? J Gen Intern Med. 2010 Jun; 25 Suppl 3:S531. https://doi
.org/10.1007 /s11606-010-1338-5. - 70.
- Sunnassee A, Shaohua Z, Liang R, Liang L. Unexpected death of a young woman: is myocardial bridging significant?--A case report and review of literature. Forensic Sci Med Pathol. 2011 Mar;7(1):42–6. PMID: 20697843. https://doi
.org/10.1007 /s12024-010-9175-8. [PubMed: 20697843] - 71.
- Kitzman DW, Scholz DG, Hagen PT, et al. Age-related changes in normal human hearts during the first 10 decades of life. Part II (Maturity): a quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin Proc. 1988 Feb; 63(2):137–46. PMID: 3276974. https://doi
.org/10.1016 /S0025-6196(12)64946-5. [PubMed: 3276974] - 72.
- Corrado D, Basso C, Thiene G. Sudden cardiac death in young people with apparently normal heart. Cardiovasc Res. 2001 May; 50(2):399–408. PMID: 11334844. https://doi
.org/10.1016 /S0008-6363(01)00254-1. [PubMed: 11334844] - 73.
- Basso C, Carturan E, Pilichou K, et al. Sudden cardiac death with normal heart: molecular autopsy. Cardiovasc Pathol. 2010 Nov–Dec; 19(6):321–5. PMID: 20381381. https://doi
.org/10.1016/j .carpath.2010.02.003. [PubMed: 20381381] - 74.
- de Noronha SV, Behr ER, Papadakis M, et al. The importance of specialist cardiac histopathological examination in the investigation of young sudden cardiac deaths. Europace. 2014 Jun; 16(6):899–907. PMID: 24148315. https://doi
.org/10.1093/europace/eut329. [PubMed: 24148315] - 75.
- Aranega A, De La Rosa AJ, Franco D. Cardiac conduction system anomalies and sudden cardiac death: insights from murine models. Front Physiol. 2012 Jun 14; 3:211. PMID: 22783196. PMCID: PMC3390691. https://doi
.org/10.3389/fphys.2012.00211. [PMC free article: PMC3390691] [PubMed: 22783196] - 76.
- Nerantzis CE, Koulouris SN, Marianou SK, et al. Histologic findings of the sinus node and the perinodal area in street heroin addicts, victims of sudden unexpected death. J Forensic Sci. 2011 May; 56(3):645–8. PMID: 21361943. https://doi
.org/10.1111/j .1556-4029.2011.01717.x. [PubMed: 21361943] - 77.
- Nerantzis CE, Couvaris CM, Pastromas SC, et al. Histological findings of the atrioventricular conductive system in street heroin addicts, victims of sudden unexpected death. J Forensic Sci. 2013 Jan; 58 Suppl 1: S99–104. PMID: 23083062. https://doi
.org/10.1111/j .1556-4029.2012.02304.x. [PubMed: 23083062] - 78.
- Ahmad M, Afzal S, Malik IA, et al. An autopsy study of hypertrophic cardiomyopathy. J Pak Med Assoc. 2003 Oct; 53(10): 459–62. PMID: 14696885. [PubMed: 14696885]
- 79.
- Towbin JA. Hypertrophic cardiomyopathy. Pacing Clin Electrophysiol. 2009 Jul; 32 Suppl 2:S23–31. PMID: 19602159. https://doi
.org/10.1111/j .1540-8159.2009.02381.x. [PubMed: 19602159] - 80.
- Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017 Sep 15; 121(7):749–70. PMID: 28912181. PMCID: PMC5654557. https://doi
.org/10.1161/CIRCRESAHA .117.311059. [PMC free article: PMC5654557] [PubMed: 28912181] - 81.
- Towbin JA, McKenna WJ, Abrams DJ, et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019 May 9. pii: S1547–5271(19)30438–2. PMID: 31078652. https://doi
.org/10.1016/j .hrthm.2019.05.007. [PubMed: 31078652] - 82.
- d’Amati G, Leone O, di Gioia CR, et al. Arrhythmogenic right ventricular cardiomyopathy: clinicopathologic correlation based on a revised definition of pathologic patterns. Hum Pathol. 2001 Oct; 32(10): 1078–86. PMID: 11679942. https://doi
.org/10.1053/hupa.2001.28232. [PubMed: 11679942] - 83.
- Thiene G, Basso C, Calabrese F, et al. Pathology and pathogenesis of arrhythmogenic right ventricular cardiomyopathy. Herz. 2000 May; 25(3):210–5. PMID: 10904840. https://doi
.org/10.1007/s000590050008. [PubMed: 10904840] - 84.
- Opeskin K, Thomas A, Berkovic SF. Does cardiac conduction pathology contribute to sudden unexpected death in epilepsy? Epilepsy Res. 2000 Jun; 40(1):17–24. PMID: 10771254. https://doi
.org/10.1016 /S0920-1211(00)00098-X. [PubMed: 10771254] - 85.
- Zack F, Rodewald AK, Blaas V, Büttner A. Histologic spectrum of the cardiac conducting tissue in non-natural deaths under 30 years of age: an analysis of 43 cases with special implications for sudden cardiac death. Int J Legal Med. 2016 Jan;130(1): 173–8. PMID: 26526026. https://doi
.org/10.1007 /s00414-015-1287-y. [PubMed: 26526026] - 86.
- Vinter S, Isaksen C, Vesterby A. Sudden cardiac death in a young woman: tumor of the atrioventricular (AV) node or citalopram intoxication? Am J Forensic Med Pathol. 2005 Dec; 26(4):349–51. PMID: 16304469. https://doi
.org/10.1097/01 .paf.0000188200.66211.8b. [PubMed: 16304469] - 87.
- Arthurs OJ, Hutchinson JC, Sebire NJ. Current issues in postmortem imaging of perinatal and forensic childhood deaths. Forensic Sci Med Pathol. 2017 Mar; 13(1):58–66. PMID: 28083782. PMCID: PMC5306347. https://doi
.org/10.1007 /s12024-016-9821-x. [PMC free article: PMC5306347] [PubMed: 28083782] - 88.
- Oyake Y, Aoki T, Shiotani S, et al. Postmortem computed tomography for detecting causes of sudden death in infants and children: retrospective review of cases. Radiat Med. 2006 Aug; 24(7):493–502. PMID: 17058143. https://doi
.org/10.1007 /s11604-006-0061-y. [PubMed: 17058143] - 89.
- Michaud K, Grabherr S, Jackowski C, et al. Postmortem imaging of sudden cardiac death. Int J Legal Med. 2014 Jan; 128(1):127–37. PMID: 23322013. https://doi
.org/10.1007 /s00414-013-0819-6. [PubMed: 23322013] - 90.
- Proisy M, Marchand AJ, Loget P, et al. Whole-body post-mortem computed tomography compared with autopsy in the investigation of unexpected death in infants and children. Eur Radiol. 2013 Jun; 23(6): 1711–9. PMID: 23242003. https://doi
.org/10.1007 /s00330-012-2738-1. [PubMed: 23242003] - 91.
- Oncel G, Oncel D. Anomalous origin of the left coronary artery from the pulmonary artery: diagnosis with CT angiography. J Clin Imaging Sci. 2013 Jan 30; 3(1):4. PMID: 23607073. PMCID: PMC3625882. https://doi
.org/10.4103/2156-7514.106618. [PMC free article: PMC3625882] [PubMed: 23607073] - 92.
- Clarke N, Paterson A. Post mortem CT as an adjunct to radiographic skeletal survey in the assessment of sudden unexpected death in infancy and childhood. Pediatr Radiol. 2016 May;46 Suppl 1:S173–4. https://doi
.org/10.1007 /s00247-016-3579-x. - 93.
- McGraw EP, Pless JE, Pennington DJ, White SJ. Postmortem radiography after unexpected death in neonates, infants, and children: should imaging be routine? AJR Am J Roentgenol. 2002 Jun; 178(6):1517–21. PMID: 12034631. https://doi
.org/10.2214/ajr .178.6.1781517. [PubMed: 12034631] - 94.
- de Lange C, Vege A, Stake G. Radiography after unexpected death in infants and children compared to autopsy. Pediatr Radiol. 2007 Feb; 37(2):159–65. PMID: 17200844. https://doi
.org/10.1007 /s00247-006-0364-2. [PubMed: 17200844] - 95.
- Gorincour G, Sarda-Quarello L, Laurent PE, et al. The future of pediatric and perinatal postmortem imaging. Pediatr Radiol. 2015 Apr; 45(4):509–16. PMID: 25828354. https://doi
.org/10.1007 /s00247-014-3266-8. [PubMed: 25828354] - 96.
- Cohen MC, Offiah A, Sprigg A, Al-Adnani M. Vitamin D deficiency and sudden unexpected death in infancy and childhood: a cohort study. Pediatr Dev Pathol. 2013 Jul–Aug; 16(4):292–300. PMID: 23600989. https://doi
.org/10.2350/13-01-1293-OA.1. [PubMed: 23600989] - 97.
- Prodhomme O, Seguret F, Martrille L, et al. Organ volume measurements: comparison between MRI and autopsy findings in infants following sudden unexpected death. Arch Dis Child Fetal Neonatal Ed. 2012 Nov; 97(6):F434–8. PMID: 22447988. https://doi
.org/10.1136 /fetalneonatal-2011-301309. [PubMed: 22447988] - 98.
- Ross SG, Thali MJ, Bolliger S, et al. Sudden death after chest pain: feasibility of virtual autopsy with postmortem CT angiography and biopsy. Radiology. 2012 Jul; 264(1):250–9. PMID: 22570504. https://doi
.org/10.1148/radiol.12092415. [PubMed: 22570504] - 99.
- Matshes EW, Trevenen C. Infant heart dissection in a forensic context: babies are not just small adults. Acad Forensic Pathol. 2011 Sep; 1(2):156–65. https://doi
.org/10.23907/2011.021. - 100.
- Sheppard MN. Approach to the cardiac autopsy. J Clin Pathol. 2012 Jun; 65(6):484–95. PMID: 22287688. https://doi
.org/10.1136 /jclinpath-2011-200366. [PubMed: 22287688] - 101.
- Gulino SP, Burns K, Gunther WM, Mac-Leod H. Improving forensic pathologic investigation of sudden death in the young: tools, guidance, and methods of cardiovascular dissection from the Sudden Death in the Young Case Registry. Acad Forensic Pathol. 2018 Jun; 8(2):347–91. https://doi
.org/10.1177/1925362118782077. [PMC free article: PMC6490127] [PubMed: 31240048] - 102.
- Cohle SD, Sampson BA. The negative autopsy: sudden cardiac death or other? Cardiovasc Pathol. 2001 Sep–Oct; 10(5): 219–22. PMID: 11673059. https://doi
.org/10.1016 /S1054-8807(01)00093-X. [PubMed: 11673059] - 103.
- Noronha SV, Ohta-Ogo K, Behr ER, et al. The importance of expert cardiac pathology for the investigation of sudden cardiac death; results from a fast track cardiac pathology service in the UK. Eur Heart J. 2011 Aug;32 Suppl 1:17. https://doi
.org/10.1093/eurheartj/ehr322. - 104.
- Evetts AM, Shkrum MJ, Tugaleva E. A new reference source for postmortem body measurements and organ weights in neonates and infants: a statistical analysis based on sudden death classification (part 2). Am J Forensic Med Pathol. 2018 Dec; 39(4):285–303. PMID: 29794804. https://doi
.org/10.1097/PAF .0000000000000401. [PubMed: 29794804] - 105.
- Fracasso T, Vennemann M, Pfeiffer H, Bajanowski T. Organ weights in cases of sudden infant death syndrome: a German study. Am J Forensic Med Pathol. 2009 Sep; 30(3):231–4. PMID: 19696576. https://doi
.org/10.1097/PAF .0b013e318187e0f2. [PubMed: 19696576] - 106.
- Thompson WS, Cohle SD. Fifteen-year retrospective study of infant organ weights and revision of standard weight tables. J Forensic Sci. 2004 May; 49(3):575–85. PMID: 15171179. https://doi
.org/10.1520/JFS2003288. [PubMed: 15171179] - 107.
- Pryce JW, Bamber AR, Ashworth MT, et al. Reference ranges for organ weights of infants at autopsy: results of >1,000 consecutive cases from a single centre. BMC Clin Pathol. 2014 Apr 28; 14:18. PMID: 24822034. PMCID: PMC4017708. https://doi
.org/10.1186/1472-6890-14-18. [PMC free article: PMC4017708] [PubMed: 24822034] - 108.
- NIH 3D print exchange [Internet]. Bethesda (MD): National Institutes of Health; c2019. Heart library; [cited 2019 Jul 22]. Available from: https://3dprint
.nih.gov /collections/heart-library
- *
The views expressed in this manuscript are those of the authors and do not reflect official positions of the National Institutes of Health.
- Vojvodinascore--local system for cardiac operative risk evaluation.[Med Pregl. 2013]Vojvodinascore--local system for cardiac operative risk evaluation.Mihajlović B, Dejanović J, Mihajlović B, Popović D, Panić M, Bjeljac I. Med Pregl. 2013 Mar-Apr; 66(3-4):139-44.
- Negative impact of cardiac evaluation before vascular surgery.[Vasc Med. 2000]Negative impact of cardiac evaluation before vascular surgery.Krupski WC, Nehler MR, Whitehill TA, Lawson RC, Strecker PK, Hiatt WR. Vasc Med. 2000; 5(1):3-9.
- Rationale and design of the CAREFUL study : The yield of CARdiogenetic scrEening in First degree relatives of sudden cardiac and UnexpLained death victims <45 years.[Neth Heart J. 2010]Rationale and design of the CAREFUL study : The yield of CARdiogenetic scrEening in First degree relatives of sudden cardiac and UnexpLained death victims <45 years.Hendrix A, van der Werf C, Bots ML, Birnie E, van der Smagt JJ, Borleffs CJ, Vink A, van Weert HC, Doevendans PA, Wilde AA, et al. Neth Heart J. 2010 Jun; 18(6):286-90.
- Review Efficacy of pre-participation cardiac evaluation recommendations among athletes participating in World Athletics Championships.[Eur J Prev Cardiol. 2020]Review Efficacy of pre-participation cardiac evaluation recommendations among athletes participating in World Athletics Championships.Dahlström Ö, Adami PE, Fagher K, Jacobsson J, Bargoria V, Gauffin H, Hansson PO, Andersson C, Bermon S, Timpka T. Eur J Prev Cardiol. 2020 Sep; 27(14):1480-1490. Epub 2019 Oct 24.
- Review Recent advances in preoperative cardiac evaluation.[Curr Pharm Des. 2012]Review Recent advances in preoperative cardiac evaluation.Priebe HJ. Curr Pharm Des. 2012; 18(38):6182-94.
- Evaluation for Cardiac Diseases - Unexplained Pediatric DeathsEvaluation for Cardiac Diseases - Unexplained Pediatric Deaths
Your browsing activity is empty.
Activity recording is turned off.
See more...
