NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Ramanan AV, Dick AD, Jones AP, et al. Adalimumab in combination with methotrexate for refractory uveitis associated with juvenile idiopathic arthritis: a RCT. Southampton (UK): NIHR Journals Library; 2019 Apr. (Health Technology Assessment, No. 23.15.)

Adalimumab in combination with methotrexate for refractory uveitis associated with juvenile idiopathic arthritis: a RCT.
Show detailsThe results reported in this chapter are based on the integrated analysis of the blinded and open-label phase for participants in the adalimumab group compared with the results from the blinded phase for participants in the placebo group. The last participant visit in the open-label phase took place on 29 June 2016.
Primary outcome
During the course of the open-label phase of the trial, there were three additional treatment failures that occurred in the adalimumab arm. One participant was classified as having a treatment failure because they had taken permitted concomitant medications against the acceptable criteria and two participants had sustained scores (as recorded at entry grade) that were still present after 6 months of therapy (see Appendix 3, Table 50).
There were a total of 17 (28.3%) treatment failures for the 60 participants in the adalimumab group and 17 (56.7%) failures for the 30 participants in the placebo group. Median time to treatment failure was 24.1 weeks (95% CI 14.7 weeks to 81 weeks) in the placebo group and not reached in the adalimumab group within the 18-month treatment period because fewer than half of the subjects experienced treatment failure at the conclusion of the study (Figure 8).
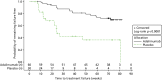
FIGURE 8
Primary outcome Kaplan–Meier plot for blinded and open-label phase.
The results of the log-rank test from SAS PROC LIFETEST (SAS Institute In, Cary, NC, USA) offered strong statistical evidence that the placebo and adalimumab groups differed with respect to time to treatment failure.
The HR indicated that treatment with adalimumab significantly decreased the hazard of treatment failure by 74% (HR 0.26, 95% CI 0.13 to 0.51; p < 0.0001), relative to placebo.
Test of proportional hazards assumption
The assumption of proportional hazards was tested by including an interaction between time and treatment group in the Cox proportional hazards model. There was no evidence (p = 0.1371) that the interaction was not zero and, therefore, no evidence that the HR was not constant over time.
Sensitivity analyses
There were no losses to follow-up during the course of the trial and, therefore, sensitivity analysis 8 was not conducted. The results of the other sensitivity analyses indicate that the original conclusion from the primary analysis was robust with regard to the changes that were made. The overall statistical significance of the sensitivity analyses did not change.
Additional analyses
Development of uveitis in non-study eye
There were no additional occurrences of this outcome during the open-label phase of the trial.
Time to treatment failure in both eyes
This analysis was not possible because only one participant (in the placebo group) failed in both eyes at different times.
Development of comorbidity from treatment failure
There were no additional occurrences of this outcome during the open-label phase of the trial. There were so few numbers in the two treatment groups of those who developed a comorbidity that any modelling, including the development of a comorbidity, was not possible.
Post hoc analyses
Time-to-treatment response
During the open-label phase of the trial, there were three participants in the adalimumab group who achieved treatment response, meaning that, overall, during the blinded and open-label phases, a total of 47 participants in the adalimumab group were classified as having a treatment response. The difference between the two groups was statistically significant (log-rank p-value = 0.003). The HR indicated that participants on adalimumab were just under three times more likely to achieve a treatment response than those on placebo (HR 2.96, 95% CI 1.40 to 6.27).
Proportion of responders/failures/no change
Proportion of responders/failures/no change at 3 months
During the open-label phase of the trial, there were no further occurrences of response at 3 months and the overall conclusion showed a significant difference between the treatment groups at 6 months.
Proportion of responders/failures/no change at 6 months
During the open-label phase of the trial, there were no further occurrences of response at 6 months and the overall conclusion showed a significant difference between the treatment groups at 6 months.
Area under the curve of anterior chamber cells in eligible eye
There was a significant difference in the median number of AC cells between the two groups from the overall data of –0.81 (95% CI –0.99 to –0.64; p < 0.0001) (results from eye level favouring the adalimumab group), with similar results obtained when the best or worst score was used for participants with two eligible eyes.
Secondary outcomes
Number of participants failing treatment
Seventeen participants in the adalimumab group (28.33%) and 17 participants in the placebo group (56.67%) were classified as having treatment failures. The risk of having a treatment failure was statistically significantly reduced by 54% (RR 0.46, 95% CI 0.26 to 0.83; p = 0.01) in the adalimumab group compared with placebo.
Safety, tolerability and compliance
Adverse events and serious adverse events
During the open-label phase of the trial, 63 AEs occurred in 12 participants and two SAEs occurred in two participants (one SAE was reported outside the 30-day window of treatment cessation). For completeness, the SAE that was reported outside the reporting time window has been reported in Appendix 3 (see Table 43) only and is not included in any of the total numbers. The SAE that was reported during follow-up was in relation to joint swelling of the right knee and the severity was classified as mild and judged ‘unlikely’ to be related to the study drug.
A total of 86 participants (out of 90) experienced at least one AE. A total of 682 AEs were reported in 60 participants (100%) in the adalimumab group and 114 AEs reported in 26 participants (86.7%) in the placebo group. The rate of AEs in the adalimumab group (9.86 per patient-year) was greater than that in the placebo group (7.21 per patient-year).
The most common AEs in the adalimumab group were classified as infections and infestations (85.0%); general disorders and administration-site conditions (55%); respiratory, thoracic and mediastinal disorders (55.0%); gastrointestinal disorders (48.3%); investigations (35.0%); nervous system disorders (31.7%); eye disorders (28.3%); musculoskeletal and connective tissue disorders (26.7%); and injury, poisoning and procedural complications (23.3%) (Table 19).
TABLE 19
Adverse events by treatment group (an integrated analysis of the blinded and open-label phases)
Laboratory parameters (haematological, biochemical analysis and urinalysis)
When the data from the open-label period were combined with the data from the blinded phase of the trial, there were no changes to the clinical conclusions of the analyses of the haematological, biochemical and urinalysis data.
Participant diaries and dosing records
On average, treatment compliance for the adalimumab group during the open-label phase of the study, was 84%, according to the participant diaries, and 87%, according to the accountability logs. Overall, treatment compliance for the adalimumab group during the blinded phase of the trial and the open-label phase of the study combined was 83%, according to the participant diaries, and 94%, according to the accountability logs.
The average compliance with MTX for the adalimumab group during the open-label phase of the trial (according to participant diaries) was 74%; the average compliance with MTX for the adalimumab group overall for both phases of the trial was 61%.
Use of corticosteroids over duration of study period
Total oral corticosteroid dose
One participant in the placebo group and five participants in the adalimumab group received oral corticosteroids during the course of the study. The five participants in the adalimumab group were on study treatment for a total of 6.03 years and the placebo participant was on study treatment for 0.17 years.
The total oral dose for the placebo group was 640 mg (which was 3767.74 mg standardised per patient-year) and 4267.5 mg for the adalimumab group (which was 707.70 mg standardised per patient-year). A rate ratio of 0.19 (95% CI 0.17 to 0.20) indicated that participants on placebo required more oral corticosteroids per patient-year than those on adalimumab and there was evidence at the 5% level of a statistically significant difference between the two groups (p < 0.0001).
Reduction in and rate of systemic corticosteroid dose from entry dose
Reduction in systemic corticosteroid dose from entry dose
Reduction in systemic corticosteroid dose from entry dose to 0 mg
This analysis was not able to be performed because the statistical algorithm did not converge.
Reduction in systemic corticosteroid dose from entry dose to < 5 mg
This analysis was not able to be performed because the statistical algorithm did not converge.
Rate of systemic corticosteroid dose from entry dose
The result of this analysis was the same as that of the total oral corticosteroid analysis.
Topical corticosteroid use (frequency) compared with entry use
Time to reduction to fewer than two drops in topical corticosteroid
The time to reduction to fewer than two drops per day was statistically significant in favour of adalimumab (HR 4.25, 95% CI 1.26 to 14.31; p = 0.02).
Time to reduction to zero drops in topical steroid (post hoc analysis)
The time to reduction to zero drops per day was statistically significant in favour of adalimumab (HR 4.26, 95% CI 1.49 to 12.2; p = 0.01).
Need for pulsed corticosteroid
During the open-label phase of the trial, there were no additional participants who required pulsed steroids. In total, one participant in the placebo group (3.33%) and two participants in the adalimumab group (3.33%) required pulsed corticosteroids during the course of the study. The RR showed that there was no evidence of a difference in the risk of requiring pulsed corticosteroids between the two treatment groups (RR 1.00, 95% CI 0.09 to 10.59; p > 0.99).
Optic and ocular
Number of participants having disease flares (as defined by worsening on Standardisation of the Uveitis Nomenclature criteria) following 3 months’ disease control
All events of disease flare following disease control took place in the blinded phase of the trial (i.e. there were no further events within the open-label phase).
Number of participants having disease flares within the first 3 months
One participant in the adalimumab arm failed treatment within the open-label phase of the study.
Overall, three participants in the placebo group (10%) and one participant in the adalimumab group (3%) had a disease flare in the first 3 months of treatment. There was no statistically significant evidence at the 5% level (p = 0.11) of a difference (RR 0.17, 95% CI 0.02 to 1.54) between the two groups.
Visual acuity measured by age-appropriate logarithm of the minimum angle of resolution assessment
The integrated analysis for the data on the logMAR score for participants in the trial split by treatment group and time point can be found in Table 20.
TABLE 20
The logMAR results for best/worst score by treatment group for each time point
The parameter estimates from the joint modelling for analyses 1 and 2 (for the blinded and open-label phase of the trial combined) are shown in Table 21.
TABLE 21
Model parameters for joint modelling of logMAR analyses 1 and 2
The results for integrated analyses 1 and 2 for the treatment effects (adalimumab) on the longitudinal logMAR are 0.01 (95% CI –0.06 to 0.02) and –0.02 (95% CI –0.07 to 0.02), respectively, implying that there is no significant difference between the treatments on logMAR.
These estimates are adjusted for the failure caused by treatment dropout from the trial.
Sensitivity analysis
The inferences of the sensitivity analyses for the data from the blinded phase of the trial combined with the open-label data were the same as those from the blinded phase alone.
Number of participants with resolution of associated optic nerve or macular oedema (as assessed by slit lamp biomicroscopy or optical coherence tomography, where available)
There were no further occurrences of optic nerve resolution or macular oedema in the open-label phase of the study.
Number of participants with disease control (defined as zero cells, with topical treatment for 3 and 6 months)
Three months
There were four participants in the adalimumab group who achieved disease control for 3 months during the open-label phase in at least one eligible eye. This meant that, overall, two participants in the placebo group (7%) and 27 in the adalimumab group (45%) had disease control for at least 3 months (RR 6.75, 95% CI 1.72 to 26.51; p = 0.0002).
The inferences for disease control in all eligible eyes were the same as those for disease control in one eligible eye.
Six months
There were five participants in the adalimumab group who achieved disease control for 3 months during the open-label phase in at least one eligible eye meaning that, overall, one participant (3%) in the placebo group and 22 participants in the adalimumab group (36.67%) had disease control in at least one of their eligible eyes (RR 11.00, 95% CI 1.56 to 77.74; p = 0.0003).
The inferences for disease control in all eligible eyes were the same as those for disease control in one eligible eye.
Number of participants entering disease remission (defined as zero cells, without topical treatment for 3 and 6 months)
Three months
There were no further cases of disease remission in the open-label phase.
Six months
There were no further cases of disease remission in the open-label phase.
Duration of sustaining inactive disease (zero cells in the anterior chamber, with or without topical treatment)
The difference in the total number of days that participants sustained inactive disease was statistically significant between the two treatment groups [16.31 days (SE 25.69 days) for the placebo group and 225.43 days (SE 18.15 days) for the adalimumab group], with participants in the adalimumab group spending approximately 209 (mean difference 208.80, 95% CI 143.91 to 273.69) more days with inactive disease than those in the placebo group (p < 0.0001).
Quality-of-life assessment
Childhood Health Questionnaire
The treatment effect on the longitudinal CHQ-PsS score (blinded and open-label phases) was 2.37 (95% CI –0.41 to 5.47), which implies that there is no difference between the treatments on the score (Table 22).
TABLE 22
Joint modelling results (random intercepts only) for the PsS and PhS summary score
The treatment effect on the longitudinal CHQ-PhS score (blinded and open-label phases) was 1.23 (95% CI –2.31 to 5.28), which implies that there is no difference between the treatments on this score (see Table 22). This estimate is adjusted for the failure due to dropout from the trial.
Sensitivity analysis
The inferences of the sensitivity analyses for the data from the blinded phase of the trial combined with the open-label data were the same as those from the blinded phase alone.
Childhood Health Assessment Questionnaire
The overall treatment effect (blinded and open-label phases) on the longitudinal CHAQ was –0.14 (95% CI –0.32 to 0.003), which implies that there is no difference between the treatments on CHAQ (Table 23). This estimate is adjusted for the failure due to dropout from the trial. However, the p-value (0.08) is close to the margin of statistical significance.
TABLE 23
Random intercepts model (overall data): CHAQ
Sensitivity analysis
The inferences of the sensitivity analyses for the data from the blinded phase of the trial combined with the open-label data were the same as those from the blinded phase alone.
American College of Rheumatology Pedi core set criteria at ACR 30, 50, 70, 90 and 100
The results for the joint modelling of ACR Pedi and time to treatment failure can be seen in Table 24. None of the improvements on ACRs is significantly different between treatments. All estimates are adjusted for the failure caused by dropout from the trial.
TABLE 24
Parameter estimates for ACR 30, 50, 70, 90 and 100
Sensitivity analysis
The inferences of the sensitivity analyses for the data from the blinded phase of the trial combined with the open-label data were the same as those from the blinded phase alone.
Number of participants undergoing disease flare, in remission on and/or off medication for their juvenile idiopathic arthritis, and with minimum disease activity
Number of participants undergoing disease flare
During the open-label phase of the trial, there were no additional occurrences of participants undergoing a disease flare; therefore, the results of the combined analyses are the same as those reported during the blinded phase.
Number of participants in remission on and/or off medication for their juvenile idiopathic arthritis
Number of participants in remission on medication for their juvenile idiopathic arthritis
Seven participants (12%) in the adalimumab group and 17 (57%) participants in the placebo group could not be included in the analysis because they had not been on medication for the required amount of time (6 months).
Ten (19%) of those in the adalimumab analysis population achieved remission; none of the placebo participants did. The risk of having remission while on medication in the adalimumab group was greater than for those on placebo but was not statistically significant (RR 5.40, 95% CI 0.30 to 87.40; p = 0.19).
Number of participants in remission off medication for their juvenile idiopathic arthritis
There were 45 (75%) participants in the adalimumab group and 21 (70%) in the placebo group who could not be included in the analysis because they had not been off medication for the required amount of time (12 months).
No participants in either the adalimumab group or the placebo group achieved remission off medication for their JIA.
Number of participants with minimum disease activity
During the open-label phase of the trial, 22 (37%) participants in the adalimumab group (this was three more than the result from the blinded phase alone) and four participants in the placebo group had at least one case of minimum disease activity during the course of the trial. The RR was 2.70 (95% CI 1.03 to 7.12) and the associated p-value from the chi-squared test was 0.03, indicating that there was a statistically significant difference between the two groups.
Number of participants requiring change in biological or disease-modifying antirheumatic drug therapy as a result of failure to respond from arthritis
There were no further cases of participants requiring a change in biological or DMARD therapy as a result of failure to respond from arthritis in the open-label phase.
Juvenile Arthritis Disease Activity Score
The parameter estimates for each of the joint models of JADAS 10, 27 and 71 can be seen in Table 25. The treatment effect on the longitudinal JADAS 10, 27 and 71 was non-significant at the 5% level, implying that there is no difference between the treatments on the scores. However, all three p-values are close to the margin of statistical significance.
TABLE 25
Parameter estimates from joint modelling (random intercepts only) for JADAS 10, 27 and 71
Sensitivity analysis
The inferences of the sensitivity analyses for the data from the blinded phase of the trial combined with the open-label data were the same as those from the blinded phase alone.
- Primary outcome
- Additional analyses
- Post hoc analyses
- Secondary outcomes
- Sensitivity analysis
- Quality-of-life assessment
- Number of participants undergoing disease flare, in remission on and/or off medication for their juvenile idiopathic arthritis, and with minimum disease activity
- Number of participants requiring change in biological or disease-modifying antirheumatic drug therapy as a result of failure to respond from arthritis
- Juvenile Arthritis Disease Activity Score
- Clinical effectiveness results: open-label phase - Adalimumab in combination wit...Clinical effectiveness results: open-label phase - Adalimumab in combination with methotrexate for refractory uveitis associated with juvenile idiopathic arthritis: a RCT
Your browsing activity is empty.
Activity recording is turned off.
See more...