NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine to increase live births in euthyroid women with thyroid antibodies trying to conceive: the TABLET RCT. Southampton (UK): NIHR Journals Library; 2019 Oct. (Efficacy and Mechanism Evaluation, No. 6.11.)

Levothyroxine to increase live births in euthyroid women with thyroid antibodies trying to conceive: the TABLET RCT.
Show detailsThis chapter reports the methods used to conduct the TABLET trial.
Trial design
The TABLET trial was a randomised, double-blind, placebo-controlled multicentre trial of levothyroxine in euthyroid women with TPOAbs, conducted to determine if levothyroxine can reduce miscarriage and premature births in women. The trial had a favourable ethics opinion from the South West 3 Multicentre Research Ethics Committee (reference number 11/SW/0036).
Eligibility (inclusion and exclusion)
Participants were recruited from early pregnancy units, recurrent miscarriage clinics and infertility clinics in the participating NHS hospitals across the UK. Participants had to meet the following eligibility criteria (see Recruitment for more details on the recruitment process):
- women trying to conceive
- a history of one or more miscarriage(s) or primary or secondary infertility
- aged 16–40 years at randomisation
- biochemically euthyroid [TSH 0.44–3.63 mlU/l; free thyroxine 4 (T4) 10.0–21.0 pmol/l using the appropriate analyser]
- TPOAb positive according to local laboratory reference ranges
- willing and able to give informed consent.
Participants could not be included if any of the following criteria were applicable:
- current treatment for any thyroid disorder [past treatment was considered on an individual basis (see below)]
- taking amiodarone or lithium therapy
- contraindications to levothyroxine therapy – thyrotoxicosis, hypersensitivity to levothyroxine, or any of its excipients
- participation in any other blinded, placebo-controlled trial of investigational medicinal products (IMPs) in pregnancy
- previous or current diagnosis of cardiac disease.
Women who had previously been treated for thyroid disorders were considered on a case-by-case basis. It was left to the discretion of the principal investigator whether or not a woman with a history of thyroid disorder could be safely offered participation in the trial. The rationale for exercising this discretion was because it was agreed that women who may have received very short-term treatment a long time ago and whom have since had normal thyroid function and not required treatment long term should not be automatically discounted from participation in the trial. These women would have been classified as ‘euthyroid’ for some time and it was not deemed clinically unsafe for these women to participate if they were TPO positive.
Recruitment
The TABLET trial recruited from three main populations: those with a history of one or two miscarriages, those with recurrent miscarriage (defined as three or more consecutive losses) and those under investigation or treatment for infertility. Recruitment was via a two-step process. Women were initially invited to be screened for TPOAbs and thyroid function tests (TFTs) and then those who were found to be positive for TPOAbs, with normal thyroid function, were introduced to the TABLET randomised controlled trial. Further details are given in the following sections.
Screening of potential participants
Potential participants were identified and approached by clinic doctors, nurses and research staff. The routes of initial approach for screening are shown in a flow diagram (Figure 3). All participants were clearly advised that participation in the trial was entirely voluntary with the option of withdrawing from the trial at any stage, and that participation or non-participation would not affect their usual care. All women were approached by staff who were appropriately trained in Good Clinical Practice and specifically trained in taking consent for this trial. To be invited for screening, the woman must have been willing and able to give informed consent and to provide a blood sample (of 10 ml) for thyroid antibody and thyroid function testing (TPOAbs and measurement of serum TSH and free T4).
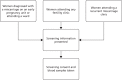
FIGURE 3
Routes of initial approach for screening.
The aim was to approach women at the optimum point, before their subsequent conception. For women who had a recent miscarriage, the initial approach was carried out after the miscarriage had been confirmed on the early pregnancy unit or when they were admitted to the ward for management. For women who were under infertility services, the initial approach was made at a routine clinic appointment and women with recurrent miscarriage were approached in the recurrent miscarriage clinics.
Some women were screened on an early pregnancy unit following an acute pregnancy loss, when it was their third (or more) miscarriage. These women were categorised into the recurrent miscarriage population. Potential participants were provided with a short screening patient information sheet and given time to consider their involvement.
Figure 4 shows the potential pathways that were followed by all of the screened participants. The co-ordinating midwife/nurse at each centre was responsible for contacting TPO-negative women to inform them that they were ineligible for the TABLET trial and provide reassurance about normal TFTs. A small number of asymptomatic women were identified as having abnormal TFTs via the screening process. It was advised that the local principal investigator would make decisions on further investigations and/or treatment for these women based on the degree of thyroid abnormality and local guidelines. If TPOAbs were positive and TSH and free T4 concentrations were within the normal range for the trial [see Eligibility (inclusion and exclusion) for limits], the woman was sent a TABLET trial participant information sheet and an appointment was arranged to discuss participation at a subsequent clinic visit at which final eligibility checks could be performed. For those who had recently suffered a miscarriage, the woman’s desire to conceive again was explored and only those who indicated that they intended to try again, within the next 12 months, were invited to participate. It was made clear to participants that they could withdraw from the trial at any time. Consent was confirmed in writing. This included consent for future evaluation of themselves and the child and the health records of both through the Office for National Statistics or equivalent.
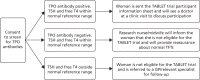
FIGURE 4
Screening of potential participants. GP, general practitioner.
Note on thresholds for thyroid function tests
Various assays for TPOAbs are available, each with different detection limits and thresholds for test positivity, which are pre-determined by the assay manufacturer. These variations are an accepted part of normal practice in the UK. Quality assurance for assays in the laboratories for all of the participating centres is provided by UK IMMQAS (Immunology Quality Services), which shows > 99% concordance in the classification of samples as either positive or negative for TPOAbs across all assays. Therefore, the TABLET trial protocol did not define a threshold for TPO positivity but, instead, accepted the classification provided by the laboratories servicing the participating centres. For TFT and free T4 testing, the participating site must have used an analyser approved by the Trial Management Group (TMG), and it must be routinely participating in the UK National External Quality Assessment Service (NEQAS) (Sheffield, UK) external quality assurance scheme.
The approved analysers were Elecsys®/Modular/Cobas® (F. Hoffmann-La Roche AG, Basel, Switzerland), Abbott ARCHITECT (Abbott Laboratories, Chicago, IL, USA) and Advia Centaur® (Siemens Healthineers AG, Munich, Germany). The euthyroid reference range of TSH 0.44–3.63 mlU/l and circulating free T4 10–21 pmol/l covered the central quartiles of all three assays and was in keeping with the non-pregnant reference range from the Roche manufacturer recommendations.26
The main aim of monitoring TFTs was to ensure the safety of the participant and the pregnancy according to the available evidence at the time. A significantly elevated level of TSH or a significantly elevated level of free T4 have been associated with adverse pregnancy outcomes and warrant treatment. There was no evidence of harm and treatment benefit for subclinical hyperthyroidism (an isolated lowering of TSH accompanied by a normal free T4) or isolated hypothyroxinaemia (low free T4 accompanied by a normal TSH). Thus, only upper limits of TSH and free T4 had been set to ensure safety in this trial.
Non-pregnant
We defined a similar (but not identical) upper limit to the TSH upper limit for eligibility for the non-pregnant recruits. This was justified by factoring in a 10% allowance for intra-individual variation over time and for interassay variations. For example, if we recruited a woman with a TSH of 3.63 mlU/l, she would not be withdrawn if her follow-up TSH was 3.9 mlU/l because of variations in the assay and the normal fluctuations of TSH and not because of a real difference in her thyroid function. Therefore, the agreed TSH level at follow-up was < 4.0 mlU/l and for free T4 it was < 25 pmol/l.
Pregnant
It was difficult to define one set of limits for all three assays during pregnancy because of the apparent differential exaggerated bias associated with different assays in the assessment of pregnancy samples. The limits also had to be similar to the current limits in use by some of the centres, as there would have been conflict in the management of trial and non-trial women. Based on a review of literature for the three assays, certain limits were proposed (see Appendix 2).
Randomisation
Eligibility criteria were confirmed prior to obtaining consent, and demographic and prognostic factors on the Randomisation Notepad were gathered. Following this, the woman could be randomised into the trial. Randomisation was conducted through a secure online randomisation service provided by the University of Birmingham Clinical Trials Unit (BCTU). Following this, trial and bottle numbers were allocated.
Randomisation method and stratification variables
Participants were randomised individually in an equal (1 : 1) ratio of levothyroxine to placebo. A ‘minimisation’ procedure using a computer-based algorithm was used to avoid chance imbalances in important stratification variables. Strata used in the minimisation were:
- maternal age (< 35 years, ≥ 35 years)
- number of previous miscarriages (0, 1 or 2, ≥ 3)
- initial TSH concentration (≤ 2.5 mlU/l, > 2.5 mlU/l)
- women who were having infertility treatment (yes/no).
For logistical reasons, the randomisation was also minimised by centre.
Treatment allocations
Levothyroxine
The IMP was levothyroxine; 50 µg of levothyroxine sodium as an encapsulated tablet was taken once daily after randomisation and preconceptually, and continued to the end of any pregnancy or until 12 months post randomisation if pregnancy did not occur. It was assumed that, for the majority of participants, pregnancy would occur within 1 year of randomisation and that the pregnancy may continue to term up to 42 weeks. Thus, the treatment period ranged from 42–44 weeks to 94 weeks for term pregnancies.
It was advised that the IMP should be taken orally before breakfast and ingested with water (milk, iron supplements, calcium supplements and antacids can impair the absorption of levothyroxine and it was advised to not take these at the same time).
The choice of 50 µg per day was made after a careful review of the existing literature, an extensive survey of endocrinologists as well as obstetricians with an interest in maternal medicine, a review of the host organisation’s obstetric–endocrine practice database and a review of other related evidence.
Placebo
The placebo was a placebo tablet, encapsulated in the same format as the IMP to be identical in colour, shape and weight. The treatment regime was exactly the same as in the levothyroxine group.
Excluded medications
The use of amiodarone and lithium were included in the exclusion criteria for the study as these medications can independently affect thyroid function. Women were advised to stop taking the TABLET trial treatment if these drugs were indicated during the trial. Oral contraceptives may alter the pharmacodynamics of thyroxine, so women were also advised to stop trial treatment if these were taken.
Drug supply and dispensing
Interventions were supplied by Sharp Clinical Services (Rhymney, UK; formerly Bilcare UK Ltd), which procured the trial drug and manufactured the placebo tablet, overencapsulated the IMP and placebo, and dispensed them into containers accordingly. This company had no role in the design of the trial, the collection, analysis, interpretation of the data or the writing of the report.
A hospital pharmacist prepared the trial treatment bottle for dispensing. Each trial treatment bottle contained 13 weeks’ supply for use by one participant. Each subsequent trial treatment bottle contained a further 13 weeks’ supply (see Figure 6 and Scheduled trial appointments).
Blinding
Participants, investigators, research midwives/nurses and other attending clinicians all remained blind to the trial drug allocation for the duration of the trial.
In the case of any serious adverse events (SAEs), management and care of the women was initiated as though the woman was taking levothyroxine. Cases that were considered serious, unexpected and possibly, probably or definitely related [i.e. possible suspected unexpected serious adverse reactions (SUSARs)] were unblinded only at the trial office by the trial co-ordinator. The attending clinician and local principal investigator were not made aware of the actual trial drug. If a participant was withdrawn from the trial as a result of abnormal TFTs (see Thyroid hormone monitoring and criteria for stopping trial treatment) and if the drug allocation was required for the continued medical management of the withdrawn participant, clinicians were advised to contact the TABLET trial office or use the online access to gain unblinding information. Any instances of this were recorded on the trial database.
Scheduled trial appointments
Trial participants returned to the randomising hospital at two further intervals while trying to conceive and for routine antenatal appointments. At each visit, blood samples were taken for TSH and free T4 level (see Thyroid hormone monitoring and criteria for stopping trial treatment). If conception did not take place by the end of the 12th month, the woman was asked to perform a pregnancy test and ensure that it was negative prior to stopping trial medication (Figure 5).

FIGURE 5
Thyroid drug supply and monitoring timelines.
Compliance and treatment withdrawal
Compliance monitoring
Compliance was evaluated by two methods, first by pill-counting. Women were asked to bring completed, partially used and unused treatment bottles to the trial centres at follow-up visits. The research nurse would receive the empty/partially used/unused treatment bottles at the local centres and document the number of remaining pills (if any) in the database for each trial participant. Second, the participant was asked how often they took the capsules at each monitoring and resupply visit, and asked again when they completed the trial. The categories of compliance were as follows: 0%, never; 1–24%, hardly any; 25–49%, some; 50–74%, most; 75–99%, almost always; 100%, every day. Good compliance was defined as ≥ 75% usage.
Participant withdrawal from treatment
A participant was considered for withdrawal from the trial treatment if, in the opinion of the investigator or the care providing clinician or clinical team, it was medically necessary to do so. Participants could also voluntarily withdraw from treatment at any time; however, given that withdrawn patients can bias clinical trial results, women were encouraged to allow data collection to continue even if trial treatment ceased.
Thyroid hormone monitoring and criteria for stopping trial treatment
If a woman developed overt or subclinical hypothyroidism with TSH concentrations above the decision limit for the specified analyser, or overt hyperthyroidism with a free T4 above the decision limit for the specified analyser, she was discontinued from trial medication and treated according to local clinical guidelines.
Withdrawal from trial
Participants could voluntarily withdraw their consent to trial participation at any time. If a participant did not return for a scheduled visit, attempts were made to contact her and, when possible, review compliance and AEs. We made an attempt to document all reasons for self-withdrawal. If a participant explicitly withdrew consent to have any further data recorded, their decision was respected and recorded on the electronic data capture system. All communication surrounding the withdrawal was noted in the patient’s records and no further data collected for that patient.
Outcomes and assessment
Primary outcome measures
The primary outcome was the proportion of women who had a live birth at or beyond 34 completed weeks of gestation. This proportion was calculated with the denominator totalling all women randomised, and the numerator totalling women who conceived within 1 year of randomisation and went on to give live birth at or beyond 34 weeks of gestation. Women who failed to conceive within 1 year, or who became pregnant but had a miscarriage, ectopic pregnancy, termination, or gave birth before 34 weeks or experienced a stillbirth were included in the denominator but not the numerator.
Secondary outcome measures
Secondary outcomes were as follows:
- clinical pregnancy at 7 weeks
- ongoing pregnancy at 12 weeks
- miscarriage at < 24 weeks
- stillbirth (intrauterine death at ≥ 24 weeks)
- ectopic pregnancy
- termination (and reasons)
- live birth at < 34 weeks
- time from conception to pregnancy end (any reason)
- mode of initiation of labour (spontaneous/induced)
- mode of delivery (vaginal/operative vaginal/caesarean)
- gestation at delivery (weeks)
- time from conception to live birth
- gestation at delivery of < 28 weeks/< 34 weeks/< 37 weeks
- birthweight (g)
- birthweight adjusted for gestational age and sex (centiles)
- birthweight adjusted for gestational age, sex, parity, maternal body mass index (BMI) and ethnicity (centiles)
- small for gestational age and sex (proportion < 10th centile)
- small for gestational age, sex, parity, maternal BMI and ethnicity (birthweight proportion < 10th centile)
- large for gestational age and sex (proportion ≥ 90th centile)
- large for gestational age, sex, parity, maternal BMI and ethnicity (birthweight proportion ≥ 90th centile)
- Apgar (appearance, pulse, grimace, activity and respiration) score at 1 minute/5 minutes
- serum TSH concentration (mlU/l; log-transformed) at each assessment time
- serum free T4 level (pmol/l) at each assessment time
- subclinical/overt hypothyroidism
- subclinical/overt hyperthyroidism
- maternal antenatal complications (hyperemesis gravidarum/gestational diabetes/pre-eclampsia or eclampsia/obstetric cholestasis/preterm pre-labour rupture of membranes/intrauterine growth restriction/others)
- intrapartum complications (shoulder dystocia/others)
- maternal postnatal complications [admission to a high-dependency unit (HDU) or intensive care unit/abnormal thyroid test within 4 weeks/referred to a psychiatrist or started on antidepressants/others]
- neonatal complications (early neonatal death, defined as death within 7 days after delivery/late neonatal death, defined as death beyond 7 days and before 28 days post delivery/admission to neonatal unit or special care baby unit, or active resuscitation, within first 28 days/surfactant use/mechanical ventilation/intermittent positive pressure ventilation/continuous positive airway pressure/oxygen use/congenital abnormalities/hypoxic ischaemic encephalopathy/retinopathy of prematurity/respiratory distress syndrome/pneumothorax/intraventricular haemorrhage (grade 3 or 4)/necrotising enterocolitis/early infection/others)
- reported symptoms that participant is concerned about at each assessment time
- SAEs.
Outcome assessment details
The timing of scheduled hospital assessments is described in Scheduled trial appointments. Details of how outcome measures were generated are given in Table 1.
TABLE 1
Outcome assessment details
Relevant trial data were transcribed directly onto a secure web-based database. All personal information was treated as strictly confidential. Source data comprised the research clinic notes, hospital notes, hand-held pregnancy notes and laboratory results. Women were encouraged to report pregnancies, miscarriages or other pregnancy losses, deliveries and AEs that occurred between clinic visits or that were presented at non-participating hospitals to the research midwife. Self-reports were verified against clinical notes.
Definition of the end of the trial
The interventional phase of the trial ended when the last participant delivered her baby, suffered a pregnancy loss or completed 12 months of treatment without becoming pregnant. The observational phase of the trial ceased when the 28-day follow-up had been completed for the baby of the last participant recruited who became pregnant. The primary analysis was scheduled to occur after all randomised women had completed the primary and secondary outcomes (up to 28 days of neonatal life following a maximum of 12 months preconception, i.e. up to a maximum of approximately 2 years post randomisation) and the corresponding outcome data were entered into the trial database and validated as being ready for analysis.
Notes on adverse events and serious adverse events
All AEs, from the first administration of trial treatment until the end of the pregnancy or 12 months of trial participation without pregnancy (whichever was later), whether observed directly or reported by the participant, were collected and recorded. Trial participants were asked about the occurrence of AEs and SAEs at each trial visit. All SAEs were recorded and faxed to BCTU within 24 hours of the research staff becoming aware of the event. The local principal investigator (or other nominated clinician) had to assign seriousness, severity, causality and expectedness to the SAE before reporting. SAEs categorised by the local investigator as both suspected to be related to the trial drug and unexpected were classified as SUSARs, and were subject to expedited reporting.
All relevant trial documentation, including the screening information sheet, screening consent form, randomisation information sheet, randomisation consent form, trial schema and the SAE form, can be found in the trial protocol.
Statistical considerations
Sample size
We planned to randomise 900 women (450 in each arm). To detect a minimally important difference of 10% in live birth at or beyond 34 weeks of gestation (from 55% to 65%), at p = 0.05 and power of 80%, 380 women needed to be randomised to the levothyroxine arm and 380 women to the placebo arm (760 in total). Including a worse-case scenario attrition rate of 15%, the total number of participants required was 900.
The minimally important difference of 10% was defined following consultations with health-care practitioners, patients and representatives of patient bodies for the progesterone in recurrent miscarriages (PROMISE) trial.27 However, it should be noted that this difference was smaller than that expected from the existing literature, which showed that the risk of miscarriage alone is halved with levothyroxine therapy (RR 0.48, 95% CI 0.25 to 0.92). Hence, assuming an expected absolute difference of 15% in live births beyond 34 weeks of gestation, 900 participants (after accounting for 15% attrition) would provide a power of 99%.
The 55% baseline live birth rate in the control group was based on the assumption that 10% of women would fail to conceive within 1 year28 and a further 35% would either miscarry or have a preterm birth.2
Statistical analysis
A comprehensive statistical analysis plan was drawn up prior to any analysis and provided to the independent Data Monitoring Committee (DMC) and Trial Steering Committee (TSC) for review. Full details of the statistical analysis can be found in the statistical analysis plan.
In summary, categorical baseline data were summarised with frequencies and percentages. Normally distributed continuous variables were summarised as means with standard deviations (SDs); for continuous variables that were not normally distributed, medians with interquartile ranges (IQRs) were presented. In the first instance, participants were analysed in the treatment group to which they were randomised, irrespective of compliance with the treatment protocol. All estimates of differences between groups are presented with 95% two-sided CIs. p-values from two-sided tests at the 5% significance level are also included.
For the primary outcome (live birth at ≥ 34 weeks of gestation), the population was all randomised participants. A log-binomial model was used to generate RRs along with 95% CIs, adjusting for the minimisation parameters. Statistical significance of the treatment group parameter was determined through examination of the associated chi-squared statistic.
Analysis was performed as per the primary outcome for the other binary outcomes. For maternal pregnancy outcomes (such as miscarriage and stillbirth), the analysis population was all women who went on to achieve confirmed pregnancy. For all other secondary maternal and neonatal outcomes, the analysis population was those with live births at ≥ 24 weeks of gestation. For secondary neonatal outcomes and complication rates, twin babies were both counted in the analysis population. For time from conception to pregnancy end, and time from conception to birth, a Cox proportional hazards model was employed, adjusting for the minimisation variables. A chi-squared test was used to test the statistical significance of the treatment group parameter. For continuous outcomes [e.g. birthweight, birthweight centiles, TSH (following a log-transformation) and free T4 values], a linear regression model was used, adjusting for the same minimisation parameters. Here, a F-test was used to test the statistical significance of the estimated treatment group parameter generated from the restricted maximum likelihood estimates. The proportion and percentage of participants experiencing any SAE were presented by group. Statistical significance was determined by a chi-squared test.
A sensitivity analysis was performed on the primary outcome and the outcome of miscarriage at < 24 weeks of gestation to test the impact of any missing data. This assumed that all participants lost to follow-up had a negative outcome (i.e. preterm < 34 week birth or miscarriage). The number of missing data was very limited for these outcomes (< 1%), so no further sensitivity analysis was performed.
Pre-planned subgroup analyses (limited to the primary outcome measure and miscarriage rate) were completed in the following: (1) maternal age: (< 35 years, ≥ 35 years), (2) number of previous miscarriages (0, 1 or 2, ≥ 3), (3) initial TSH concentration (≤ 2.5 mlU/l, > 2.5 mlU/l), (4) women undergoing infertility treatment (yes, no), (5) ethnicity (black, white, Chinese, South Asian, other), (6) TPO baseline level (‘very high’, taken as ≥ 50th percentile, and ‘high’, taken as < 50th percentile) and (7) BMI (< 25 kg/m2, ≥ 25 kg/m2). The effects of these subgroups were examined by adding the subgroup by treatment group interaction parameters to the log-binomial model; a chi-squared test was used to test the statistical significance of this parameter.
Interim analyses of effectiveness and safety end points were performed on behalf of the Data and Safety Monitoring Committee on an approximately 6-monthly basis during the period of recruitment. These analyses were performed with the use of the Haybittle–Peto principle;29 hence, no adjustment was made in the final p-values to determine significance.
Trial oversight
Trial oversight was provided by a TSC (chaired by Professor Jane Norman, University of Edinburgh) and a DMC (chaired by Professor John Lazarus, University of Cardiff).
The TSC provided independent supervision for the trial, providing advice to the chief and co-investigators and the sponsor on all aspects of the trial throughout the trial. The DMC adopted the DAMOCLES charter30 to define its terms of reference and operation in relation to oversight of the TABLET trial. The DMC met on an approximately 6-monthly basis during the period of recruitment. The patient safety and treatment efficacy aspects were reviewed at each meeting and a decision to continue or stop trial recruitment was based on the criteria defined prior to the trial starting.
- Methods for the randomised trial - Levothyroxine to increase live births in euth...Methods for the randomised trial - Levothyroxine to increase live births in euthyroid women with thyroid antibodies trying to conceive: the TABLET RCT
Your browsing activity is empty.
Activity recording is turned off.
See more...