NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1997.
Transfer of the RNA genome of a retrovirus from one cell to another requires its assembly within the structure of an infectious virion. Although most of the components of the retroviral particle have been identified (see Fig. 1 and Chapter 2), the molecular details of the assembly mechanisms are poorly understood. This may seem surprising since the number of distinct proteins contained in a retrovirus is small. What makes the process of assembly both difficult and interesting to study is its highly dynamic nature.
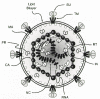
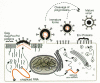
Figure 1
Model of a typical retroviral particle. The subviral locations of the virion components common to all retroviruses are shown.
An examination of the mature retroviral particle cannot fully explain the steps in assembly because these components are not those from which the particle was made. Rather, all of the structural proteins of the virion, with few exceptions, are derived from three polyproteins: Gag, Gag-Pro-Pol, and Env (Fig. 2). Each of these precursor proteins has special characteristics needed for specific steps in the assembly process, and each undergoes extensive changes along the way. In brief, the surface (SU) and transmembrane (TM) proteins found on the surface of the virion are initially synthesized as a single polypeptide, the Env glycoprotein, which is assembled into oligomeric complexes in the rough endoplasmic reticulum (RER), extensively modified, and then cleaved by a cell-encoded protease during transport to the surface of the cell. In some instances, further proteolytic processing of TM occurs after the particle is released. Likewise, the proteins found on the inside the virion (matrix, MA; capsid, CA; nucleocapsid, NC; protease, PR; reverse transcriptase, RT; integrase, IN) are initially linked within the Gag and Gag-Pro-Pol proteins. The Gag protein is sufficient for directing budding at the plasma membrane, and the Pro-Pol polyproteins are incorporated into the resulting particle because they are linked to Gag. Subsequent cleavage of the Gag and Gag-Pro-Pol proteins by the viral protease brings about new shapes and arrangements inside the nascent virion as the immature particle undergoes a metamorphosis into the mature, infectious retrovirus. Even the structure of the viral RNA, packaged into the virion through an interaction with Gag, changes during the budding and maturation process.
The production of an infectious retrovirus is thus not simply a matter of putting together a multitude of proteins and nucleic acids. Rather, it is a complex progression of many molecular interactions and rearrangements involving an unknown number of intermediate structures. This chapter summarizes what is currently known about the dynamic process of retroviral assembly. In a sense, it also describes the initial steps of virion disassembly, which can be thought to begin as soon as the assembly of polyproteins and RNA is completed; i.e., the immature particles that are initially formed are relatively stable entities (in nonionic detergents, for example), but noninfectious, whereas the mature virions created when the viral protease cleaves the Gag and Gag-Pro-Pol proteins are dramatically less stable, but infectious. Further uncoating events occur when the virion enters the next cell, as described in Chapter 4.
- Review HIV-1 replication.[Somat Cell Mol Genet. 2001]Review HIV-1 replication.Freed EO. Somat Cell Mol Genet. 2001 Nov; 26(1-6):13-33.
- Assembly and processing of avian retroviral gag polyproteins containing linked protease dimers.[J Virol. 1991]Assembly and processing of avian retroviral gag polyproteins containing linked protease dimers.Burstein H, Bizub D, Skalka AM. J Virol. 1991 Nov; 65(11):6165-72.
- Structure of the immature retroviral capsid at 8 Å resolution by cryo-electron microscopy.[Nature. 2012]Structure of the immature retroviral capsid at 8 Å resolution by cryo-electron microscopy.Bharat TA, Davey NE, Ulbrich P, Riches JD, de Marco A, Rumlova M, Sachse C, Ruml T, Briggs JA. Nature. 2012 Jul 19; 487(7407):385-9.
- Plasma membrane targeting of chimeric intracisternal A-type particle polyproteins leads to particle release and specific activation of the viral proteinase.[J Virol. 1997]Plasma membrane targeting of chimeric intracisternal A-type particle polyproteins leads to particle release and specific activation of the viral proteinase.Welker R, Janetzko A, Krausslich HG. J Virol. 1997 Jul; 71(7):5209-17.
- Review HIV-1: fifteen proteins and an RNA.[Annu Rev Biochem. 1998]Review HIV-1: fifteen proteins and an RNA.Frankel AD, Young JA. Annu Rev Biochem. 1998; 67:1-25.
- Synthesis, Assembly, and Processing of Viral Proteins - RetrovirusesSynthesis, Assembly, and Processing of Viral Proteins - Retroviruses
Your browsing activity is empty.
Activity recording is turned off.
See more...