NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Clinical Guideline Centre for Acute and Chronic Conditions (UK). Transient Loss of Consciousness (‘Blackouts’) Management in Adults and Young People [Internet]. London: Royal College of Physicians (UK); 2010 Aug. (NICE Clinical Guidelines, No. 109.)
We checked this guideline in March 2019. We found no new evidence that affects the recommendations in this guideline.

Transient Loss of Consciousness (‘Blackouts’) Management in Adults and Young People [Internet].
Show details3.1. Clinical questions
The clinical questions appropriate to this section are:
- Q2) In people who have experienced a TLoC, what aspects of patient history (including eye-witness accounts) are useful in discriminating between patients with syncope (cardiac, neurally mediated or orthostatic hypotension), epilepsy, psychogenic non-epileptic seizures and other causes of TLoC?
- Q3) In people who have experienced a TLoC, what aspects of physical examination are useful in discriminating between patients with syncope (cardiac, neurally mediated or orthostatic hypotension), epilepsy, psychogenic non-epileptic seizures and other causes of TLoC?
- Q4) In people who have experienced a TLoC, what routine laboratory tests are useful in discriminating between patients with syncope (cardiac, neurally mediated or orthostatic hypotension), epilepsy, psychogenic non-epileptic seizures and other causes of TLoC
- Q5) Which signs, symptoms and other features of presentation (e.g. patient history) are associated with an increased risk of a serious adverse event
- Q6) Which signs, symptoms and other features of presentation (e.g. patient history) are associated with an increased likelihood of spontaneous remission
- Q7) Can clinical decision tools or risk stratification tools be used to discriminate between patients who would benefit from admission and patients who can be safely discharged?
- Q9) When providing immediate care in the pre-hospital setting to a person who has experienced a TLoC, what aspects of the initial assessment should be performed in the pre-hospital setting?
- Q10) When is transfer to hospital by ambulance appropriate in the immediate care of a person who has experienced a TLoC and what discharge advice should be provided when transfer is not appropriate?
3.2. Interactive diagnostic simulation
In order to understand the context of initial stage assessment and to elicit GDG views in the early stages of guideline development, the GDG took part in an interactive diagnostic simulation exercise. A patient profile was shared with the GDG by an actor and four GDG members role-played a consultation. Different approaches to diagnosis were discussed, and the exercise and findings are reported in Appendix D5.
3.3. Reviews of diagnostic test accuracy: initial assessment
3.3.1. Introduction
There are two main reasons for evaluating patients who have had a TLoC: to m ake a diagnosis of the cause of TLoC and to determine the prognosis for the person with TLoC, i.e. to determine the risk of future adverse events.
Questions 2, 3, 4 and 8 (Section 3.1) illustrate the GDG’s first objective in this initial assessment stage: to use symptoms and tests either to predict or diagnose a cause for the TLoC or to state that there is no clear causal diagnosis at this stage (unexplained TLoC).
Knowing the likely cause also enables the clinician to determine the patient’s risk of death or adverse events or recurrence of the TLoC. It also determines the referral route for the patient: whether the patient should be admitted to a speciality department in which further tests can be carried out urgently (and if so, which speciality); whether it is referral to outpatient departments for further tests, or whether it safe to send the patient home with follow up in the community.
Questions 2 to 4 were intended to discriminate between:
TLoC itself is a symptom rather than a disease or condition, and because of its transitory nature, studies of diagnostic test accuracy can only investigate the causes of TLoC, rather than the event itself. This is further complicated by the fact that symptoms of the cause may not be present except during a TLoC.
There are numerous possible conditions that can give rise to syncope and the GDG divided this into three main categories, cardiac syncope, neurally mediated syncope and orthostatic hypotension (see glossary).
Clinical questions 2 to 4 can be answered either in terms of predictors for a particular cause of TLoC relative to all other causes, or the predictors for two different causes of TLoC can be compared directly.
The GDG’s second objective is illustrated by questions 5, 6 and 7, and is to determine directly predictors or combinations of predictors/risk stratification tools for adverse events, with a view to identifying patients at ‘high’, ‘moderate’ and ‘low’ risk. This, in turn, should determine the necessity of admission to speciality departments (with the appropriate degree of urgency) and should also indicate which patients can be safely discharged.
Questions 9 and 10 are addressed by all of the work in this chapter.
There are two ways in which we can consider predictors:
- Whether or not a particular sign/symptom predicts one target condition (either diagnosis or adverse events) compared to another. For example, whether coronary artery disease is a predictor for a cardiac cause of syncope rather than for non-cardiac syncope. In these analyses, the outcome is the likelihood ratio, which is the number of patients with the sign/symptom (e.g. coronary artery disease) in those who have the disease (e.g. cardiac cause of syncope), divided by the proportion with the sign/symptom in those without the disease (e.g. the non-cardiac syncope group).
- Whether having a particular sign/symptom puts a patient more at risk of the target condition (event or diagnosis) compared to not having that sign/symptom. For example, whether the patient is more at risk of a cardiac cause of syncope if they have coronary artery disease compared to not having CAD. In these analyses the outcome is the risk ratio (or odds ratio), which, for the RR, is the proportion of patients with the disease in those who have the sign/symptom divided by the proportion who have the disease in those who do not have the sign/symptom.
We are more likely to use the first method when we want to see if a particular sign or symptom enables us to distinguish between different causes of TLoC (the first three clinical questions listed at the start of this chapter). We are more likely to use the second method when we want to see if a high or a low score on a risk stratification tool or if the presence/absence of a particular sign/symptom predicts an adverse event (the fourth and fifth clinical questions listed).
There are four main ways in which these problems have been tackled in studies:
- Univariate analyses which examine the effect of a predictor without taking into account any other factors
- Multivariable analyses, in which all likely predictors are entered into an iterative regression analysis program in order to determine the effect, on the outcome concerned, of each predictor, taking into account the effects of all the others.
- The multivariable equation for predictors of a cause of TLoC or an event can be combined to form a model, or decision rule, that predicts the likelihood of that cause of syncope or event. Often authors determine the multivariable predictors in the decision rule in one population (derivation cohort) and validate the tool in a second population (validation cohort). We decided to exclude from this section, where possible, the test accuracy results for the derivation cohort (they are covered in the previous section).
- Finally, studies may examine a complex algorithm for diagnosis or prediction of risk categories.
Where the outcome considered is diagnosis of the cause of TLoC, the predictor is considered in the context of a reference standard, and the outcome measure is usually diagnostic test accuracy statistics (e.g. sensitivity and specificity). Where the outcome is an event, diagnostic test accuracy statistics may be provided, or the effect of predictors on the incidence of the event may be determined, giving outcomes as summary statistics such as odds ratios or relative risks.
3.3.2. Methods of the review
3.3.2.1. Selection criteria
The selection criteria given in the methods section were used, in combination with the following review specific criteria:
3.3.2.2. Types of participants
Adult patients who have had a TLoC presenting to emergency departments or general practice surgeries. Participants are not expected to have had any prior tests.
3.3.2.3. Reference standard
Diagnosis by expert clinician (following second stage tests); and follow up.
3.3.2.4. Comparator tests
Clinician decision making, or other tests.
3.3.2.5. Target condition
The target condition for these reviews was to be:
- the various causes of TLoC
- adverse events, which could be death only, death plus cardiac events, or any serious adverse event. The GDG defined a ‘serious adverse event’ to be death, any cardiac event, any cerebral event and serious injury. This combination of adverse events is equated to admission to hospital
3.3.2.6. Outcomes
Diagnostic test accuracy statistics
- Sensitivity and its 95% confidence interval
- Specificity and its 95% confidence interval
- Positive and negative predictive values
- Likelihood ratio (for this, the GDG considered the test to be good if it had a positive LR of more than 5 or a negative LR less than 0.2; the test was considered to be strong if the LR was greater than 10 or less than 0.1; however, if the confidence interval crossed 1 the findings were not considered to be a good or strong test)
- Pre- and post test probabilities
- Diagnostic odds ratio
3.3.3. Description of studies (Appendix D1)
Twenty-eight reports of 27 studies were included6,9,22,24,49,53–55,63,71,93,97,107,176–179,181,182,186,187,190,195,201,202,208,209,215; the Romme study186 was an additional report of the van Dijk study215. The Ammirati study9 reported a diagnostic algorithm, but did not give details of the initial stage evaluation and so this study was not considered further in this review. Two reports182,187 were included following stakeholder comments. Both of these were published after the guideline was submitted for consultation, however, the GDG decided to include them because they provided further evidence in an evidence-poor area. The Reed (2007) study181 was said to be a pilot for the Reed (2010) study182, but the former was concerned only with feasibility of recruitment and study method, rather than reporting pilot results. Thus the two Reed studies are independent. The Romme (2009) study187 states that it used data collected for the van Dijk (2008) study215, but aimed to validate the ‘Calgary Score’ derived in the Sheldon (2006) study201. A further study54 was identified from the reference list of the Romme (2009) study187.
3.3.3.1. Study Design
A summary of study design features across studies is given in the table and further details of individual studies in Appendix D1.
| Characteristics | Details |
|---|---|
| Design |
|
| Design 2 |
|
| Country of study |
|
| Funding and possible conflicts of interest |
|
| Sample size |
|
3.3.3.2. Population
A summary of population characteristics across studies is given in the table below and further details of individual studies in Appendix D1.
| Characteristics | Details |
|---|---|
| Setting |
|
| Prior tests |
|
| Age |
|
| Ethnicity |
|
| History of heart disease |
|
Type of TLoC
A summary of TLoC details across studies is given in the table below and further details of individual studies in Appendix D1.
| Characteristics | Details |
|---|---|
| Definition |
|
| Selection of patients |
|
| Inclusion of patients with epileptic seizures |
|
| Inclusion of psychogenic pseudosyncope or psychogenic non-epileptic seizures (PNES) | |
| Previous episodes of TLoC |
3.3.3.3. Index tests and reference standards
A range of index tests was investigated, ranging from aspects of patient history (predictors) to diagnostic algorithms. Additional details of the index tests are given in Appendix D1.
For the patient history items, some of the studies take the form of case control studies, in which ‘cases’ are one type of TLoC and ‘controls’ are another (as defined by the reference standard), and the study determined if a particular sign or symptom is predictive of one type of TLoC rather than the other.
For each index test or set of tests, we have described the reference standard used with that test. Summary descriptions of the index tests and reference standards are given at the start of the appropriate results sections.
3.3.4. Methodological quality
The methodological quality was assessed using QUADAS criteria (Appendix D2).
The following studies were found to be at risk of bias on the following criteria:
- Review bias (blinding): in six studies, it was unclear if the index test assessors were blinded to the reference standard results53,71,93,201,202 and Sarasin 2003190 (decision rule). In one study, the index test and reference standard were conducted by the same person53. In five studies it was unclear who conducted the follow up investigations for the reference standard49,71,178,179,181. In six studies the reference standard assessors were not blinded because the index test was part of the reference standard6,53,63,93,107,187.
Overall, the GDG considered that 24 tests in 15 studies were potentially or at risk of bias6,22,53,55,63,71,93,104,107,181,190,195,201,202 and Romme 2009187 (borderline risk). The three case control studies22,201,202 were considered to be most at risk. These studies were considered in sensitivity analyses.
3.3.5. Evidence for predictive factors for diagnosis
We report the evidence for predictors for one diagnosis over other.
Although some studies reported results for the different types of syncope separately, we decided it was more pragmatic to report the patient history predictors for a particular type of syncope versus not having that type of syncope, rather than having a head-to-head comparison of selected individual diagnoses. Values were calculated accordingly.
3.3.5.1. Patient history, physical examination, tests and decision rules, for diagnosis of epileptic seizures
A1. Patient history for diagnosis: epileptic seizures versus syncope
Two case control studies (Benbadis 199522 (n=108); Sheldon 2002202 (n=270)) and one cohort study (Hoefnagels 1991107 (n=94)) reported the value of patient history in distinguishing between epileptic seizures and syncope in selected patients.
Sheldon (2002)202
- Population – selected (patients were excluded if they had epileptic seizures not diagnosed by EEG, and if they had psychogenic non-epileptic seizures)
- Index test
- –
Patient characteristics (e.g. age)
- –
Medical history (e.g. coronary heart disease)
- –
TLoC history
- –
Predisposing/precipitating factors (e.g. hot/warm place; stress)
- –
Prodromal symptoms before TLoC (e.g. hallucinations, nausea)
- –
Signs and symptoms during TLoC (e.g. tongue biting)
- –
Prodromal symptoms after TLoC
- Case control design (patients included if they had a diagnosis according to preset criteria and if there was no reasonable diagnostic confusion; they were excluded if they had more than one plausible cause of syncope). Patients with an unclear cause of syncope were excluded from the analysis.
- Reference standard
- –
Diagnosis following secondary tests
- ⋄
Seizures were diagnosed on the basis of a suggestive EEG and causes of syncope were determined using a positive tilt test for vasovagal and orthostatic hypotension; ECG/electrophysiology for arrhythmias/heart block (and the diagnosis also included palpitations pre-syncope)
Benbadis (1995)22
- Index tests: tongue biting and lateral tongue biting
- Case control design
- Reference standard: secondary tests: EEG video monitoring; 12-lead ECG and Holter monitoring, tilt test and autonomic reflex examination. Final diagnoses were: 31% epileptic seizures; 27% pseudoseizures and 42% syncope.
Hoefnagels 1991107
- Population: patients referred to the neurology department (i.e. selected patients, non-seizure patients mainly had vasovagal syncope or hyperventilation)
- Index test: individual signs and symptoms before the event, after the event and during the event (as observed by an eye witness)
- Reference standard was eye witness observations of initial signs and symptoms (described below), that was not changed by follow up and secondary tests (including general and neurological examinations, routine laboratory tests, EEG and ECG; CT scan and 24h cardiac monitoring as appropriate). It was not stated what was the basis of deciding which signs and symptoms were predictive of seizures, but they were:
- –
If an eyewitness observed ‘more than a few’ movements during TLoC and identified clonic movements from a range imitated by the interviewer
- –
If an eyewitness observed automatisms, such as chewing or lip smacking, during TLoC
- –
If the patient was motionless and later reported an unequivocal aura, such as a strange smell
Firstly, univariate likelihood ratios across studies are reported for each sign and symptom – this is the likelihood that the sign or symptom predicts seizures rather than syncope. A likelihood ratio (LR) of more than 5 or less than 0.2 is considered a good test and a LR of more than 10 or less than 0.1 is considered a strong test.
Secondly, multivariable predictors obtained using regression analysis are given as odds ratios: they represent the odds that having a particular sign or symptom will predict epileptic seizures compared with the odds of not having that sign or symptom, independent of all the other predictors.
Signs and symptoms that are considered to be good and strong univariate predictors are shown in Table 1 as likelihood ratios with their 95% confidence intervals. Multivariable predictors for and against seizures are shown in Table 2. Full results are recorded in Appendix D3.
Table 1
Univariate predictors for epilepsy versus syncope.
We also give an evidence quality rating based on:
- Indirectness: Sheldon (2006)201 was restricted to patients who had an established diagnosis of TLoC; patients with epilepsy not diagnosed by EEG were excluded. Benbadis (1995)22 was in highly selected patients from an epilepsy clinic plus syncope patients of known cause. Hoefnagels (1991)107 included only referrals to a neurology department and the non-seizure patients mainly had vasovagal syncope or hyperventilation.
- Inconsistency between studies is indicated as a footnote
- Imprecision: for likelihood ratios, we defined imprecision as a confidence interval that crossed 5 or 0.2 for strong tests and 3 or 0.3 for a good test. If, for a good test, the lower confidence limit crossed 1 we did not include the study in the table). Imprecision is indicated with one or two asterisks (latter means very imprecise).
Additional significant weak univariate predictors for and against epileptic seizures are listed below, together with signs and symptoms with relatively narrow confidence intervals that are neither for nor against seizures. All were of low evidence quality unless otherwise stated.
- Weak significant univariate predictors for epileptic seizures: age less than 45 years; TLoC associated with stress; prodromal déjà vu; prodromal trembling; prodromal hallucinations (very low); prodromal preoccupation (very low); observed unresponsiveness; unusual behaviour; cannot remember behaviour; frothing at the mouth; duration of TLoC more than 5 minutes; sleepy post-TLoC; mood changes post-TLoC; muscle pain (2 studies)
- Weak significant univariate predictors against epileptic seizures: hypertension; self-reported high blood pressure; chest pain; pre-syncope with hot/warm place; pre-syncope after exercise; pre-syncopal spells; any presyncope; prodromal vertigo pre-TLoC (very low; 2 studies); dimming of vision pre-TLoC (very low); warmth pre-TLoC (very low); pale face during TLoC observed by witness;
- Non-significant signs and symptoms, in favour of neither: concussion in the past; sitting pre-TLoC; standing pre-TLoC; light-headedness pre-TLoC.
Two multivariable analyses were carried out in the Sheldon (2002) study202, based on significant univariate predictors at the p<0.05 level. Thirty-nine and 37 variables were included, depending on whether symptom burden predictors were included (i.e. the number of spells and the length of the TLoC history); they are listed in Appendix D3. The multivariable analyses were considered to be of low quality, mainly because of the case-control nature of the study, and also because the ratio of patients to covariables was a little low (7). The GDG considered there were no important confounders missing from the variables added to the regression analysis.
Some variables were independent of the model used: loss of consciousness with stress; head turning to one side during TLoC; unresponsiveness during TLoC; any presyncope, LoC with prolonged standing or sitting; diaphoresis before TLoC.
Other variables were sensitive to the model used (with or without symptom burden): waking with a cut tongue; unusual posturing; limb jerking; amnesia for abnormal behaviour; post ictal confusion; prodromal déjà vu (which was also not significant); number of spells more than 30.
A2. Patient history initial evaluation decision rules for diagnosis of epilepsy202,215
Two studies evaluated decision rules for the diagnosis of epilepsy202,215.
Sheldon (2002) 202 rules
- Population – selected, half the cohort in the study was used for validation of the rules
- Index test
- –
Initial evaluation decision rule based on symptoms alone, with positive and negative scoring items
- –
Rule consists of items that are significant predictors in a multivariable analysis (which included all items of patient history significant at the p<0.05 level)
- –
Scores are assigned according to the relative magnitude of the regression coefficients
- –
Rule 1: in the absence of knowledge of the numbers and historic duration of TLoC and lightheaded spells; Rule 2 in the presence of this knowledge.
- Case control design (patients included if they had a diagnosis according to preset criteria and if there was no reasonable diagnostic confusion; they were excluded if they had more than one plausible cause of syncope)
- Reference standard
- –
Diagnosis following secondary tests (see (A1) above)
| Rule 1 (no knowledge of symptom burden): scores | Rule 2 (knowledge of symptom burden: scores |
|---|---|
|
|
|
|
|
|
| |
| |
| |
|
|
|
|
|
|
| Patients classified as having a seizure if the total points score is 1 or more | Patients are classified as having a seizure if the total points score is 0 or more |
van Dijk (2008)215
- Population – unselected (several hospital departments)
- Index test – initial assessment based on ESC guidelines for people predicted to be ‘certain’ or ‘highly likely’ to have epilepsy.
- –
van Dijk (2008)215 did not give ‘certain’ and ‘highly likely’ definitions of epilepsy, and neither did the ESC guidelines from 2004 (appropriate for this study), but the latter states the following features to distinguish seizures from syncope; these appear to have been derived from the Hoefnagels (1991)107 study:
The Sheldon (2002)202 study reported the predictive ability of the two decision rules as ROC curves, giving pairs of sensitivity and specificity at particular point scores, for each of two rules, one with knowledge of previous TLoC and the other without that knowledge. The ROC curve is shown in Figure 3.1 for two rules predicting seizures, with different score thresholds; the sensitivity-specificity pairs were extracted from the authors’ graph.
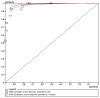
Figure 3.1
ROC curve for initial symptom score predicting epileptic seizures.
The authors recommended a cut-off point of ≥ 1 for the symptoms-only rule, which gave a sensitivity of 94% (95%CI 89 to 97) for both sensitivity and specificity in the validation cohort.
For the rule of symptoms plus knowledge about the number of episodes and the length of the history of TLoC, the authors recommended a cut-off point of ≥ 0, which gave a sensitivity of 92% (95%CI 86 to 96) and a specificity of 83% (95%CI 75 to 89) in the validation cohort.
The diagnostic test accuracy results for the initial assessment rules in Sheldon (2002)202 and van Dijk (2008)215 are shown in Appendix D3; a summary is given in Table 3.
Table 3
Diagnostic test accuracy results for the prediction of epilepsy * indicates imprecision.
The evidence quality for the Sheldon (2002)202 decision rules is low and we note that these rules are likely to overestimate the sensitivity and specificity because they were validated in a case control study. The evidence quality for the van Dijk (2008)215 study was considered to be moderate. The diagnostic yield is very low in the van Dijk (2008) study215.
3.3.5.2. Patient history, physical examination, tests and decision rules for diagnosis of vasovagal syncope
Patient history for the diagnosis of vasovagal syncope versus other types of syncope6,93,187,201
One case control study (Sheldon 2006201 (n=323)) and three prospective cohort studies (Alboni 20016 (n=337); Graf 200893 (n=212); Romme 2009187 (n=380)) reported the value of patient history in distinguishing between vasovagal syncope and other types of syncope in selected patients. All of the studies excluded patients with seizures to some degree: Sheldon (2006) 201 and Romme (2009)187 excluded those with known epilepsy; Graf (2008)93 excluded those with seizures and Alboni (2001)6 excluded those with a neurological or psychiatric cause.
- Population - all the studies had selected patients
- –
The Graf (2008) study93 was in people with unexplained syncope referred to a syncope clinic. It combined the results for people diagnosed with vasovagal syncope (23%) and psychogenic pseudosyncope (17%); the remaining patients had 9% cardiac syncope (7% tachyarrhythmia, 2% AV block); 3% orthostatic hypotension; 2% miscellaneous; 21% unexplained syncope
- –
The Sheldon (2006) study201 excluded patients with structural heart disease and did not analyse patients with syncope of unknown cause with a negative tilt test result. The remaining patients were: 56% tilt positive with no other diagnosis; 23% tilt negative with no other diagnosis and 21% with cardiac syncope or other NM syncope (complete heart block, SVT, idiopathic VT, aortic stenosis, Torsade-de-Pointe, VT, cough syncope, hypertensive carotid sinus syncope)
- –
The Alboni (2001) study6 reported on neurally mediated syncope (58%) - which comprised 10% ‘typical vasovagal’, 47% tilt-induced; 13% situational, 24% carotid sinus; 3% OHT; 3.5% adenosine sensitive syncope - cardiac syncope (23%); unexplained syncope (18%) and neurological/psychiatric syncope (1%).
- –
The Romme (2009) study187 sought to investigate the rule derived in the Sheldon (2006) study201, and, although Romme (2009)187 was not a case control study, in order to compare with Sheldon (2006)201, this study excluded 11% patients with a history of cardiomyopathy or myocardial infarction; 4% with epileptic seizures; and 11% with an unknown cause of syncope after 2 years. This left 55% with vasovagal syncope, 11% with other forms of NM syncope, 12% with orthostatic hypotension; 7% with cardiac syncope, and 6% with psychogenic pseudosyncope.
- Index test
- –
Patient characteristics (e.g. age)
- –
Medical history (e.g. coronary heart disease)
- –
TLoC history
- –
Predisposing/precipitating factors (e.g. hot/warm place; stress)
- –
Prodromal symptoms before TLoC (e.g. hallucinations, nausea)
- –
Signs and symptoms during TLoC (e.g. tongue biting)
- –
Duration of TLoC
- –
Recovery after TLoC
- –
Prodromal symptoms after TLoC
- Study design varied:
- –
Case control design
- ⋄
Vasovagal syncope (tilt positive) versus ‘Secondary causes’ (84% cardiac)201
- –
Cohort studies
- ⋄
Neurally mediated (NM) syncope versus non-NM syncope in patients referred to a syncope unit6
- ⋄
Vasovagal syncope plus psychogenic pseudosyncope (Psy) versus other syncope in patients referred to a syncope clinic for unexplained syncope93
- ⋄
Vasovagal syncope versus non-vasovagal syncope in a subset (380/503) of patients presenting to neurology, cardiology, internal medicine, cardiac emergency room (up to 100 each) and the ED to (22%). Patients (25%) were excluded if they had a history of cardiomyopathy or myocardial infarction, epileptic seizures, or no diagnosis after 2 years187
- Reference standard
- –
Diagnosis following secondary tests
- ⋄
Initial evaluation plus other tests (unspecified)6
- ⋄
Positive tilt test for vasovagal syncope and orthostatic hypotension; ECG/electrophysiology for arrhythmias/heart block (diagnosis also included palpitations pre-syncope); EEG201
- ⋄
12-lead ECG, positive tilt test, supine and upright CSM, continuous blood pressure measurement, adenosine triphosphate and dinitrate isosorbide tests, hyperventilation test, psychiatrist evaluation, stress test, echocardiography, coronary angiography, electrophysiology93
- ⋄
Additional tests (echocardiography, 24h Holter monitoring, exercise test, tilt test, carotid sinus massage) or treatment. Final diagnosis using these and ESC criteria plus expert panel if disagreement187
Signs and symptoms that are considered to be good and strong univariate predictors are shown in Table 4. We also give an evidence quality rating based on:
Table 4
Univariate predictors for vasovagal syncope versus other causes of syncope.
- Indirectness: Sheldon (2006)201 was in patients who do not have structural heart disease or unexplained syncope. Graf (2008)93 and Alboni (2001)6 had indirect target conditions: respectively, vasovagal syncope or psychogenic pseudosyncope, and neurally mediated syncope.
- Inconsistency between studies is indicated as a footnote with possible explanations.
- Imprecision is defined as described in section 3.3.5.1.
Detailed results are reported in Appendix D3.
Additional significant weak univariate predictors for and against vasovagal syncope are listed below, together with signs and symptoms with relatively narrow confidence intervals that are neither for nor against vasovagal syncope. Only the two vasovagal syncope studies187,201 are reported, all were of low evidence quality. The Romme (2009) study187 is indicated with an ‘R’.
- Weak predictors for vasovagal syncope: age less than 50 years (R); frequency of TLoC - at least 4 in the past year (R); syncope after effort; stress pre-TLoC; auditory distortion pre-TLoC; nausea or vomiting pre-TLoC; diaphoresis pre-TLoC (2 studies); abdominal discomfort pre-TLoC; heart racing pre-TLoC; numbness/tingling pre-TLoC; cannot remember behaviour; unresponsive during TLoC; confusion after a spell; white or pale colour noted by bystander during TLoC; diaphoresis or warm feeling post-TLoC; mood changes post-TLoC; numbness/tingling post-TLoC; nausea or vomiting post-TLoC
- Weak predictors against vasovagal syncope: male gender (2 studies); frequency of TLoC - fewer than 1 in the past year (R); valvular heart disease; hypertension; less than 5 seconds warning; no memory about TLoC during syncope (R had no patients with an event); recovery duration of 1 minute or less (R)
- Not predictors either for or against vasovagal syncope (R): frequency of TLoC −2 to 3 in the past year
Three studies carried out multivariable analyses6,93,201.
The Alboni (2001) study6 conducted analyses for two groups of patients, those with and without suspected heart disease (following initial evaluation); each analysis was for the diagnosis of neurally mediated syncope (i.e. an indirect target condition for vasovagal syncope). The study included significant univariate predictors in the multivariable analyses: six and two variables were included for the groups, with and without suspected heart disease; they are listed in Appendix D3. The multivariable analyses were considered to be of low quality, mainly because of the selected population, and also because there were too few variables in the analysis. We considered there were some important confounders missing from the variables added to the regression analysis.
The Sheldon (2006) study201 carried out two multivariable analyses based on significant univariate predictors at the p<0.05 level. Thirty-six and 34 variables were included, depending on whether symptom burden predictors were included (i.e. the number of spells and the length of the TLoC history); they are listed in Appendix D3). The multivariable analyses were considered to be of low quality, mainly because of the case-control nature of the study. We considered there were no important confounders missing from the variables added to the regression analysis.
The Graf (2008) study93 carried out multivariable analyses based on significant univariate predictors at the p<0.001 level; 15 were included in the analysis. The multivariable analyses were considered to be of low quality because of the indirectness of the population (58% vasovagal syncope, 42% psychogenic pseudosyncope for the target condition). The GDG considered there were no important confounders missing from the list of variables in the analysis, and considered that some of the factors largely predicted psychogenic pseudosyncope (e.g. anxiety). The inclusion of these factors might confound the predictors for vasovagal syncope.
Multivariable predictors for and against vasovagal syncope are shown in Table 5. We note that there are no predictors common to more than one study, with the exception of age. Imprecision is indicated by an asterisk.
Table 5
Multivariable predictors for vasovagal syncope for each study.
Patient history initial evaluation score for diagnosis of vasovagal syncope (versus other types of syncope)6,93,187,201,215
Four studies evaluated a decision rule for the diagnosis of vasovagal syncope (Romme 2009187 (n=380); Sheldon 2006201 (n=323), van Dijk 2008215 (n=503)) or vasovagal syncope plus psychogenic pseudosyncope (Graf 200893 (n=65)).
- Population – all four studies had selected patients (as above)
- Index test
- –
Initial evaluation decision rules based on symptoms alone, with positive and negative scoring items
- –
Rules consisted of items that were significant predictors in multivariable analyses
- –
van Dijk (2008)215 evaluated an initial assessment scheme, based on the ESC guidelines
- ⋄
A ‘certain’ diagnosis of vasovagal syncope included: precipitating events such as fear, severe pain, emotional distress, instrumentation, or prolonged standing
- ⋄
A ‘highly likely’ diagnosis included: absence of cardiac disease; long history of syncope; after unpleasant sight, sound, smell, or pain; prolonged standing or crowded, hot places; nausea/vomiting associated with syncope; during/in the absorptive state after meal; after exertion
- –
Sheldon (2006)201 and Graf (2008)93 produced decision rules:
| Rule 1 (Sheldon 2006201 and Romme 2009187) - no knowledge of symptom burden: scores | Rule 2 (Graf 2008)93: scores for prediction of vasovagal syncope or psychogenic pseudosyncope |
|---|---|
|
|
| |
|
|
| |
|
|
| Apply formula
|
| |
| Patients classified as having vasovagal syncope if the total points score is −2 or more |
- Study design varied (as above)
- Reference standard
- –
Diagnosis following secondary tests (as above)
Sheldon (2006)201 reported sensitivity-specificity pairs for different cut-off points in the development sample and Graf (2008)93 evaluated their rule in the derivation cohort and further tested it in 65 newly included patients.
The ROC curve for the Sheldon (2006)201 rule is shown in Figure 3.2: the sensitivity-specificity pairs were extracted from the authors’ graph. The authors recommended a cut-off point of > −2, which gave a sensitivity of 89% (95%CI 85 to 93%) and a specificity of 91% (95%CI 83 to 96) after adjusting to represent an independent sample. The authors also reported that the score alone was not usually sufficient for a diagnosis of vasovagal syncope, and stated that, for such a diagnosis, the four risk factors of asystole, bifascicular block, SVT and diabetes usually needed to be absent. We note that this study was carried out in a highly selected case control population and these results should be considered with caution. The Romme (2009) study187 validated the Sheldon (2006)201 rule in a more representative cohort and found a sensitivity of 87% (95%CI 82 to 91) and a low specificity of 31% (95%CI 24 to 40%).

Figure 3.2
ROC curve for diagnosis of vasovagal syncope in patients without structural heart disease.
The Graf (2008) study93 reported a sensitivity of 84% (64–95) and a specificity of 50% (34–66) in their validation cohort for the diagnosis of vasovagal syncope or psychogenic pseudosyncope.
The van Dijk (2008) study215 considered the predictive ability of their ESC guidelines-based initial assessment scheme for people predicted to be ‘certain’ or ‘highly likely’ to have vasovagal syncope.
Full diagnostic test accuracy statistics are given in Appendix D3, with sensitivity, specificity and the likelihood ratios being summarised in Table 6 for each of these studies.
Table 6
Diagnostic test accuracy statistics for initial assessment rules for vasovagal syncope.
3.3.5.3. Patient history, physical examination, tests and decision rules, for diagnosis of psychogenic pseudosyncope215
One study215 investigated the ESC guidelines for the diagnosis of psychogenic pseudosyncope. Details of the study are given in Appendix D1.
The reference standard appeared to be a psychiatric diagnosis, although this was unclear, and it was assumed independent of the index test.
The index test was defined as follows:
Psychogenic pseudosyncope based on ESC guidelines
The definition of psychogenic pseudosyncope was unclear in the van Dijk paper215, simply stating the ESC guidelines were used. The ESC update33 (appropriate to this study) identifies the following indicators:
|
The ESC update of 2009145 (van Dijk is a member of the Task force for the 2009 edition) states the following indicators:
|
The results are summarised in Table 7: and reported in full in Appendix D3; imprecision is indicated with an asterisk.
Table 7
Diagnostic test accuracy statistics for psychogenic pseudosyncope.
3.3.5.4. Patient history, physical examination, tests and decision rules, for diagnosis of orthostatic hypotension cause of syncope215
One study215, examined the ESC guidelines for the diagnosis of orthostatic hypotension. Details of the study are given in Appendix D1. Blood pressure was measured in the supine position and after 3 minutes of upright position. The index test was defined as follows:
Orthostatic hypotension based on ESC guidelines
Certain diagnosis:
|
Highly likely diagnosis:
|
The GDG regarded the definition of a certain diagnosis as an indirect measure of orthostatic hypotension in that it did not accord with the widely accepted definition from the 1996 Consensus Statement of the American Autonomic Society and the American Academy of Neurology212: a decrease in systolic blood pressure of 20 mm Hg or more and/or decrease in diastolic blood pressure of 10 mm Hg or more within 3 minutes of standing.
The study appeared to have included the index test results as part of the reference standard, although this was unclear.
The results are summarised in Table 8 and reported in full in Appendix D3; imprecision is indicated with one or two asterisks.
Table 8
Diagnostic test accuracy statistics for orthostatic hypotension cause of syncope.
3.3.5.5. Patient history, physical examination, tests and decision rules, for diagnosis of cardiac syncope
Patient history for diagnosis of cardiac causes of syncope
Four prospective cohort studies reported the value of patient history in distinguishing between cardiac causes of syncope and other types of syncope (Alboni 20016 (n=337); del Rosso 200863 (n=260); Graf 200893 (n=317); Sarasin 2003190 (n=175)
- Population
- –
Three studies were in selected patients: Alboni (2001)6 – referrals to a syncope unit; Graf (2008)93 – referred for unexplained syncope; Sarasin (2003)190 – patients with a definite cause of syncope were excluded (i.e., those with a strongly suspected diagnosis of vasovagal syncope, situational syncope or orthostatic hypotension and people with abnormalities on 12-lead ECG). Del Rosso (2008)63 was in unselected patients
- –
The Sarasin (2003) study190 recorded results for cardiac arrhythmic syncope only
- –
The Graf (2008) study93 recorded results for ‘rhythmic syncope’, which included 66% cardioinhibitory CSS; the GDG therefore decided not to consider this study further for cardiac syncope
- –
del Rosso (2008)63 excluded non-syncope causes of TLoC and the other two studies had 1%6 and 13%190 with neurological or psychiatric causes of syncope.
- Index test
- –
Patient characteristics (e.g. age)
- –
Medical history (e.g. coronary heart disease)
- –
TLoC history
- –
ECG status
- –
Predisposing / precipitating factors (e.g. hot/warm place; stress)
- –
Prodromal symptoms before TLoC (e.g. hallucinations, nausea)
- –
Signs and symptoms during TLoC (e.g. incontinence)
- –
Duration of TLoC
- –
Recovery after TLoC
- –
Prodromal symptoms after TLoC
- Univariate and/or multivariable analyses carried out
- Study design varied:
- ⋄
Unselected patients presenting to ED. Cardiac syncope versus ‘other syncope’ (70% neurally mediated syncope; 10% orthostatic hypotension; 4% non-syncopal attacks; 3% unexplained)63
- ⋄
Cardiac syncope versus non-cardiac syncope (NM syncope 58%; 1% neurological/psychiatric; 18% unexplained) in patients referred to a syncope unit6
- ⋄
Cardiac arrhythmic syncope versus mainly unexplained syncope (organic heart disease 9%; vasovagal syncope 6%; seizures/psychiatric 13%; unknown 50%)190
- Reference standard
- –
Diagnosis following secondary tests
- ⋄
Initial ECG plus ECG monitoring or 24h Holter or during electrophysiological study63
- ⋄
Initial evaluation plus other tests (unspecified)6
- ⋄
Diagnostic tests performed and interpreted by cardiologists: echocardiography, ambulatory ECG (24h Holter or continuous-loop event recorder) and electrophysiological studies to detect arrhythmias in the presence of syncope or near syncope190
Signs and symptoms that are considered to be good and strong univariate predictors are shown in Table 9 as likelihood ratios with their 95% confidence intervals; non-significant likelihood ratios are not included. Multivariable predictors for and against cardiac syncope are shown in
Table 9
Univariate predictors for cardiac syncope versus other causes of syncope.
Table 10. Detailed results are reported in Appendix D3.
Table 10
Multivariable predictors for cardiac syncope for each study.
We also give an evidence quality rating based on:
- Indirectness: The GDG originally wished to determine the predictors of cardiac causes of syncope in an unselected population. In practice, the signs and symptoms could be used as predictors, either in the initial stage (unselected) or after referral for cardiological assessment (selected) and we did not downgrade the directness of the population on this basis.
- Inconsistency between studies is indicated as a footnote
- Imprecision: for likelihood ratios, we defined imprecision as in 3.3.5.1.
Three studies carried out multivariable analyses6,63,190
The Alboni (2001) study6 conducted analyses for all patients and then for two subgroups of patients, those with and without suspected heart disease (following initial evaluation based on history, physical examination or ECG abnormalities); each analysis was for the diagnosis of cardiac syncope. The multivariable analysis of all patients included only the non-syncope variables (age, gender and presence of suspected or certain heart disease), for which the presence of suspected or certain heart disease was the only significant factor. The subgroups’ multivariable analyses included significant univariate predictors in the multivariable analyses: six were included for the group with suspected heart disease, but there was only one significant univariate predictor for the group without suspected heart disease; covariables are listed in Appendix D3. The multivariable analyses were considered to be of low quality, mainly because there were too few variables in the analysis. We considered there were important confounders missing from the variables added to the regression analysis. The del Rosso (2008) study63 carried out multivariable analyses based on significant univariate predictors at the p<0.10 level; 14 were included in the analysis and are listed in Appendix D3. The multivariable analysis was considered to be of moderate quality. We did not think there were important confounders missing from the variables added to the regression analysis.
The Sarasin (2003) study190 carried out multivariable analysis for arrhythmic syncope based on significant univariate predictors; 5 were included in the analysis. The multivariable analyses were considered to be of moderate quality; they thought that most important predictors were included.
Multivariable predictors for and against cardiac syncope are shown in Table 10. Imprecision is indicated by an asterisk.
Patient history initial evaluation score for diagnosis of cardiac syncope or cardiac arrhythmias63,71,190,215
Four studies evaluated a decision rule for the diagnosis of cardiac or cardiac arrhythmic causes of syncope (del Rosso 200863 (n=256); Elseber 200571 (n=200); Sarasin 2003190 (validation cohort; n=267); van Dijk 2008215 (n=503))
- Population
- Index tests
| Rule 1 (EGSYS): initial evaluation decision rule based on symptoms and history for prediction of cardiac syncope63 | Rule 2 - Sarasin (2003) for prediction of cardiac arrhythmic syncope190 |
|---|---|
|
|
|
|
| |
| |
|
|
| |
| In a referral centre, patients are classified as having cardiac syncope if the total points score is 4 or more | Score one point for each of the above |
| Rule 3 – van Dijk (2008) based on ESC guidelines for cardiac syncope215 | |
|---|---|
Certain diagnosis:
| |
Highly likely diagnosis:
|
| Rule 4 (ACEP): initial evaluation decision rule based on ACEP guidelines for cardiac syncope (retrospective>71) A cardiac cause of syncope was equated with admission to hospital | |
|---|---|
| High risk – level B (corresponds to admission criteria); any one of the following: | Moderate risk – level C (consider admission); any one of the following: |
|
|
|
|
|
|
|
|
- Reference standard
- –
Diagnosis following secondary tests (including ECG)
- –
Elseber (2005)71: cardiac tests including initial ECG, plus Holter monitoring or event recording or electrophysiological testing, or cardiac catheterisation or echocardiography
- –
Follow up at 2 years plus further tests plus expert review leading to final diagnoses215
Del Rosso (2008)63 and Sarasin (2003)190 reported the percentage of patients having cardiac syncope and arrhythmias respectively for a given number of risk factors or given score, for both development and validation samples. The Elseber (2005) study71 reported the overall sensitivity and specificity for the ACEP guidelines in their validation sample.
The ROC curves for the del Rosso (2008) EGSYS rule63 and the Sarasin (2003)190 scoring system are shown in Figure 3.3 for the validation cohorts. Sensitivity-specificity pairs for each cut off score were calculated from the raw data, comparing the total number of patients with cardiac syncope who had more than the cut-off score versus the total number with cardiac syncope below or with that score.
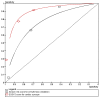
Figure 3.3
ROC curves for diagnostic rules for cardiac or arrhythmic causes of syncope.
The EGSYS score appears to be a better diagnostic test than the Sarasin (2003)190 risk score.
The authors in the del Rosso (2008) study63 reported diagnostic test accuracy statistics for two cut-off points, ≥3 points and >4 points, these are summarised in Table 11, along with values for the other studies. Full results are given in Appendix D3.
Table 11
Diagnostic test accuracy statistics for cardiac syncope.
3.3.6. Evidence for predictive factors for serious adverse events
We report the evidence for predictors for adverse events.
3.3.6.1. Patient history, physical examination, tests, decision rules, for predicting death
Patient history for a serious event: death within 12 months49,176
One study investigated signs and symptoms, physical examination and laboratory tests and ECG for their ability to predict death within 12 months (Colivicchi 200349; n=270), One additional study176 reported only one predictor, age over 65 years, for death within 30 days, 3 months and 6 months (n=1418).
- Population – unselected in both studies
- Index test
- Univariate and multivariable analyses carried out
- Reference standard
Signs and symptoms are reported as the relative risk of death for the symptom present versus not present, with their 95% confidence intervals. The results are given in Appendix D3 and significant risk factors, univariate and multivariable are summarised in Table 12.
Table 12
multivariable and univariate risk factors for death in people who have had a TLoC.
We also give an evidence quality rating based on:
- Indirectness: both studies were in unselected patients. However, the time of outcome measure is indirect: the GDG wished to know about death within 1–2 weeks.
- Limitations: Neither study was considered to have limitations
- Inconsistency between studies is indicated as a footnote
- Imprecision: for relative risks for mortality we defined imprecision in terms of a clinical important threshold of 1.25 or 0.75. Imprecision is indicated by one or two asterisks.
Likelihood ratios are also given in Appendix D3, but no symptom alone was a good or strong predictor for death.
The Colivicchi (2003) study49 carried out multivariable analysis for arrhythmic syncope based on significant univariate predictors; 8 were included in the analysis for 31 events. The multivariable analysis was considered to be of low quality because there were too few events per covariable and only one of the GDG’s key risk factors was present (age). The univariate risk factors listed in Table 12 are those entered in the multivariable analysis (i.e. the remainder were not significant independent risk factors).
We note that the multivariable predictors all have fairly small predictive abilities.
3.3.6.2. Decision rules for a serious event: death49,55,63,176
Four studies examined different risk stratification rules for death (Colivicchi 200349 (n=270); Crane 200255 (retrospective; n=208); del Rosso 200863 (n=256); Quinn 2008176 (n=1418)).
- Population
- –
Unselected for all studies
- –
The Crane (2002) study55 was a retrospective review of records.
- Index tests
| Rule 1 (EGSYS): initial evaluation decision rule for prediction of death63 | Rule 2 (OESIL‡ score): for prediction of death49 |
|---|---|
|
|
|
|
|
|
| |
| |
|
|
| In the ED, patients are classified as being at risk of death if the total points score is 4 or more. | Score one point for each of the above. Patients with more than 1 risk factor are considered at risk of death. |
| Rule 3 (San Francisco Syncope Rule) for prediction of death176 | Rule 4 (based on ACP guidelines): for prediction of all-cause mortality55 |
|---|---|
| High risk (admission indicated) – any one of:
|
| |
| |
| |
| Moderate risk (admission often indicated) – any one of:
|
| Any one of the above risk factors |
- Reference standard
- –
Follow up at 12 months in Colivicchi (2003)49 and Crane (2002)55
- –
Follow up at 21–24 months in del Rosso (2008)63
- –
Follow up: Quinn (2008)176 had two physicians consider if the death was related to TLoC, and results were reported for TLoC related and all-cause death at 6 months and 1 year and all cause death also at 30 days and 3 months.
- Target condition
- –
The GDG wished to determine which patients were at risk of a serious adverse event in the next 1–2 weeks, so they could identify people at higher risk who needed urgent referral. Therefore, the target condition for the studies was considered indirect
Colivicchi (2003)49 reported the percentage of patients who died as a function of the number of risk factors the OESIL score, for both development and validation samples; however there were insufficient data in the validation study and so the derivation cohort was used. The ROC curve for the Colivicchi (2003) OESIL scoring system49 is shown in Figure 3.4. Sensitivity-specificity pairs for each cut off score were calculated from the raw data.
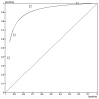
Figure 3.4
ROC curve for the OESIL score for death at 12 months.
Diagnostic test accuracy statistics for the various risk stratification tools are reported in Appendix D3 in full and summarised in Table 13.
Table 13
Diagnostic test accuracy for risk stratification tools for death.
3.3.6.3. Patient history for a serious adverse event
Eight studies investigated signs and symptoms, physical examination and laboratory tests and ECG for their ability to predict serious adverse events, such as death or myocardial infarction (Birnbaum 200824 (n=743); Costantino 200854 (n=676); Grossman 200797 (n=362); Hing 2005104 (n=113); Quinn 2004179 (n=684); Reed 2007181 (n=99); Reed 2010182 (n=548); Sun 2007209 (n=477)).
Hing (2005)104 was primarily a retrospective study.
- Populations – unselected for all studies except Costantino (2008) 54.
- –
In Costantino (2008) 54, patients were excluded if:
- ⋄
they presented with conditions, primarily confirmed in the ED, that would have required hospital admission independently of whether they had TLoC, such as: myocardial infarction, acute pulmonary embolism, subarachnoidal haemorrhage, stroke, cardiac arrest, sustained bradycardia (< 35 bpm), complete atrioventricular block, sustained ventricular tachycardia
- ⋄
they had a referred non-spontaneous return to consciousness
- Index test
- Univariate and multivariable analyses carried out
- Reference standard
- Outcome/adverse events: the studies differed in their definitions of serious adverse events:
| Birnbaum 200824; Grossman 200797; Quinn 2004179; Sun 2007209; Reed 2007181; Reed 2010182 | Hing 2005104 | Costantino 200854 |
|---|---|---|
| Death | Death as a result of presumed cardiac causes | All-cause death |
| Myocardial infarction | Diagnosis or ongoing episodes of ischaemic heart disease requiring further investigation, including medication changes, admission to hospital, angiogram, etc | |
| Life threatening arrhythmia | Significant arrhythmia requiring treatment such as a pacemaker or medication | Need for pacemaker / ICD insertion or acute antiarrhythmia medication |
| Pulmonary embolism | ||
| Stroke, subarachnoid haemorrhage | ||
| Significant haemorrhage / anaemia needing transfusion | ||
| Any condition likely to cause a return to the ED or which did cause a return to the ED (not Reed 2010182) | Readmission to hospital for the same or similar symptoms | |
| Hospitalisation for related event | ICU admittance | |
| Procedural intervention to treat syncope cause181,182,209 | Major therapeutic procedures including:
| |
| Aortic dissection (only Sun 2007209) | ||
| New diagnosis of structural heart disease (only Sun 2007209) | ||
| Severe infection / sepsis (only Grossman 200797) |
Signs and symptoms are reported as the relative risk of adverse events for the symptom present versus not present. The results are given in Appendix D3 and significant univariate risk factors are summarised in Table 14; also reported are non-significant results where there is agreement between two or more studies. Results are reported as relative risks with their 95% confidence intervals, for the median value (or lowest value or 7 day value) in order to give an indication of the size of effect and precision. Lower quality evidence is reported only if there is no other. Disagreement between studies is indicated in Table 14, but where the disagreement was between 7 and 30 day studies, the former value was taken.
Table 14
Significant univariate risk factors for serious events at 1–2 weeks – low quality evidence is indicated, otherwise moderate quality.
We also give an evidence quality rating based on:
- Indirectness:
- –
The GDG wished to determine which patients were at risk of a serious adverse event in the next 1–2 weeks, so they could identify people at higher risk who needed urgent referral. Therefore, the target condition for three studies was considered indirect (Hing 2005104 (3–6 months; Reed 2007181 (3 months); Grossman 200797 (30 days))
- –
We recognised that the Costantino (2008) study54 reported for a different target condition, excluding people with conditions presenting in ED that would have required admission regardless of whether the person had TLoC. This study was not, however, treated as an indirect population.
- Limitations: the Hing (2005) study104 was retrospective and only 22% of eligible patients were recruited
- Inconsistency between studies is indicated as a footnote
- Imprecision: for likelihood ratios, we defined imprecision as in 3.3.6.1.
We have not reported the results for the Hing (2005) study104 in Table 14.
Three studies54,179,182 carried out multivariable analyses to determine the independent risk factors for short term serious adverse events including death. Two studies54,182 reported values for multivariable risk factors (given below). The Quinn (2004) study179 incorporated the multivariable risk factors in their risk stratification tool developed, but did not give separate results.
The Reed (2010) study182 carried out a multivariable analysis based on significant univariate predictors at the p<0.10 level; at least 8 were included in the analysis for 40 events and are listed in Appendix D3 (the full list was not stated). The multivariable analysis was considered to be of low quality, partly because there were insufficient events per covariable. The GDG noted that the BNP test covered their key risk factor for cardiovascular comorbidities, but noted that the other key risk factors, age and history of a cardiac disease, were not included.
The Costantino (2008) study54 examined multivariable risk factors for serious adverse events within 10 days, excluding patients with clinical conditions confirmed in ED that would have led to hospital admission independently of TLoC. Eight covariables for 41 events were included and are listed in Appendix D3. The multivariable analysis was considered to be of moderate quality, partly because there were insufficient events per covariable, but the GDG considered that 2/3 of their key risk factors were included.
The longer term analysis included nine covariables for 62 events and these are also listed in Appendix D3. The multivariable analysis was considered to be of moderate quality, partly because there were insufficient events per covariable, but the GDG considered that all of their key risk factors were included.
Multivariable predictors are shown in Table 15.
Table 15
Multivariate predictors for serious adverse outcomes. Evidence quality moderate unless otherwise stated; asterisk indicates imprecision
Age over 65 years was not a significant risk factor for the short term outcome in the Costantino (2008) study54, neither were heart failure; structural heart disease or COPD. However, two of these factors were significant for the longer term outcome. In the longer term analysis, hypertension, heart failure, COPD and abnormal ECG at presentation were not significant risk factors.
3.3.6.4. Decision rules for a serious adverse event24,97,104,177,178,181,195,209
Ten studies examined four different risk stratification rules for serious adverse events (Birnbaum 200824 (n=738); Cosgriff 200753 (n=113); Grossman 200797 (n=362); Hing 2005104 (n=100); Quinn 2005178 (n=684); Quinn 2006177 (n=767); Reed 2007181 (n=99); Reed 2010182 (n=549); Schladenhaufen 2008195 (retrospective; n=592); Sun 2007209 (n=477)).
- Population – unselected for all studies
- –
The Schladenhaufen (2008) study195 retrospectively determined the San Francisco Syncope Rule items and all patients were over 65 years
- –
The Quinn (2006) study177 excluded patients with outcomes diagnosed in the ED; three other studies carried out subgroup analyses excluding patients with outcomes diagnosed in the ED24,97,209.
- Index tests
| Rule 1 (San Francisco Syncope Rule): for prediction of adverse events24,53,177,178,181,209 | Rule 2 (OESIL‡ score): for prediction of adverse events104,181 |
|---|---|
| |
|
|
|
|
| |
| |
|
|
| Any one of the above. | Score one point for each of the above. Patients with more than 1 risk factor are considered at risk of adverse events. |
| Rule 3 (Boston Syncope Rule) – ESC guideline + San Francisco Syncope Rule + expert advice: for prediction of adverse events97 see Appendix D1 for more details | Rule 4 (ROSE rule): for prediction of adverse events182 |
|---|---|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| Any one of the above. | Any one of the above |
- Reference standard
- –
OESIL score
- ⋄
Follow up events (see Appendix D1) at 3 months181) and 3–6 months104
- ⋄
Identification of high risk group; equated with the need for admission to hospital / discharge
- –
San Francisco Syncope Rule: follow up events (See Appendix D1)
- –
Boston Syncope Rule: follow up events (See Appendix D1)
- ⋄
30 days and subsequent medical records97
- ⋄
Identification of high risk group; equated with the need for admission to hospital / discharge
- –
Rose Rule: follow up events (See Appendix D1)
- ⋄
1 month182
- ⋄
Identification of high risk group; equated with the need for admission to hospital / discharge
One study181 compared two index tests in the same patients: the San Francisco Syncope Rule versus the OESIL score.
Hing (2005)104 and Reed (2007)181 each reported the number of patients who had an adverse event as a function of the risk points score, in 99 and 100 patients respectively, allowing a combined ROC curve to be constructed (Figure 3.5). The SFSR was reported by seven studies in different populations and the sensitivity-specificity pairs are also plotted on the ROC curve.
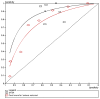
Figure 3.5
ROC curve for risk stratification tools for adverse events.
We also examined the evidence quality, based on:
- Indirectness:
- Limitations: the Schladenhaufen (2008) study195 was retrospective; the Cosgriff (2007) study53 had an unacceptable follow up rate of 79%; the Reed (2007) study181 had a population skewed towards more serious risk patients and the Hing (2005) study104 had a retrospective reference standard and only 22% of those eligible were recruited.
- Inconsistency between studies is indicated as a footnote
- We considered imprecision around the diagnostic test accuracy statistics.
There is clearly heterogeneity among the SFSR studies. In the absence of the studies with limitations, a slightly improved result was found (Figure 3.6), but overall the evidence for this rule is of low quality.
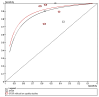
Figure 3.6
sensitivity analysis for San Francisco Syncope Rule.
The diagnostic test accuracy statistics for each of the risk stratification rules are given in Appendix D3 and summarised in Table 16. A range of values is reported for the SFSR studies (based on the studies without limitations) and the optimum OESIL score from the ROC curve (a score of more than 1) is used.
Table 16
Decision rules for adverse outcomes.
Risk stratification tools for recurrence of syncope
One study (Hing 2005104; n=100) also reported the number of patients with recurrence of syncope after 3 to 6 months follow up. The diagnostic test accuracy of the OESIL score for this outcome was reported, by the risk points score, and the ROC curve is given in Figure 3.7. The summary curve is very close to the diagonal, indicating that this is not a good test for recurrence of syncope.

Figure 3.7
Risk stratification tools for the recurrence of syncope.
3.4. Health Economics
None of the health economic evidence identified in our search was relevant to the initial assessment. None of the clinical questions relating to the initial assessment were prioritised for further economic analysis, and therefore the GDG considered the likely cost-effectiveness of associated recommendations by making a qualitative judgement on the likely balance of costs, health benefits and any potential harms. These considerations are discussed in the evidence to recommendations sections below (3.6.1 and 3.6.2).
3.5. Evidence Statements
The evidence is summarised as follows:
3.5.1. Diagnosis of epileptic seizures versus non-seizures (syncope)
3.5.1.1. Signs and symptoms of epileptic seizures
There was low- and very low- quality evidence from three studies for univariate and multivariable predictors for epilepsy in selected patients.
| Signs and symptoms that are predictors for epilepsy: Multivariable predictors are indicated by M1 and M2 for the two Sheldon (2006) models; strong and good univariate predictors by SU and GU (and weak significant univariate predictors by U, where appropriate); and the evidence quality is given |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[A ‘strong’ univariate predictor is a likelihood ratio of more than 10 and a ‘good’ predictor is more than 5. Multivariable predictors are independent risk factors.]
| Signs and symptoms that are predictors against epilepsy being the cause of the TLoC: |
|
|
|
|
|
|
|
|
|
|
3.5.1.2. Decision rules for Epilepsy
There was low quality evidence from one case control study with two decision rules, and from one cohort study32 of initial evaluation based on the ESC guidelines (2001)
| Rule 1:TLoC is classified as due to epilepsy if the total symptom score is 1 or more, calculated by summing the following, if present: |
|
|
|
|
|
|
|
|
|
|
| Rule 2: TLoC is classified as due to epilepsy if the total symptom score is 0 or more, calculated by summing the following if present: |
|
|
|
|
|
|
| ESC guidelines (moderate quality study) presence of: |
|
|
|
|
|
|
|
The sensitivity and specificity of rule 1 were high (94% each, with little uncertainty) and were high (92%) and moderately high (83%) for rule 2, with little uncertainty. The sensitivity was moderate (73%) with much uncertainty, and the specificity (100%, with little uncertainty) for the ESC initial assessment.
3.5.2. Diagnosis of vasovagal syncope versus other forms of syncope
3.5.2.1. Signs and symptoms of vasovagal syncope
There was low- and very low- quality evidence from four studies investigating vasovagal syncope in selected patients; two studies had indirect target conditions of vasovagal syncope or psychogenic pseudosyncope93 and neurally mediated syncope6, which showed the following:
| Signs and symptoms that are predictors for vasovagal syncope |
| Multivariable predictors are indicated by: |
| M1 for Sheldon (2006)201 without structural heart disease or unknown causes |
| M2 for Alboni (2001)6 heart disease patients; M3 for Alboni (2001)6 without heart disease |
| M4 for Graf (2008)93 in unexplained syncope; Strong & good univariate predictors by SU & GU |
| Predictors for VVS / Psychogenic pseudosyncope by V/P & neurally mediated syncope by NM |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Signs and symptoms that are predictors against vasovagal syncope |
|
|
|
|
|
|
|
|
|
|
|
3.5.2.2. Decision rules
There was low- and moderate-quality evidence from four studies investigating three decision rules for vasovagal syncope; one study had an indirect target condition of vasovagal syncope or psychogenic pseudosyncope93; two studies validated the Sheldon (2006) rule201 in a selected 201 and a relatively unselected 186 population; one study investigated an initial evaluation scheme based on the 2001 ESC guidelines32:
| Rule 1: TLoC is classified as a vasovagal syncope if the total symptom score is -2 or more, calculated by summing the following if present201: |
|
|
|
|
|
|
|
The study noted that the last bullet of arrhythmia abnormalities all had to be absent (as well as positive symptoms) in order to have a diagnosis of vasovagal syncope. People with epilepsy were excluded.
| ESC guidelines – presence of: |
|
|
We note that this study included patients with epilepsy (2%).
| Rule 2 (classified as VVS or psychogenic pseudosyncope if score is 0 or above), TLoC is classified as a vasovagal syncope or psychogenic pseudosyncope if the total symptom score is 0 or more, calculated by summing the following, if present: |
|
|
|
| Then apply the formula: |
We note that this study excluded people with epilepsy.
The sensitivity and specificity of the Sheldon (2006) rule201 differed across the two populations: being moderately high (89% and 91%), with little uncertainty in the selected population (low quality evidence), and moderately high (87%) and low (31%) in the relatively unselected population (moderate quality evidence).
The sensitivity and specificity were high (98% and 100%; moderate quality evidence) with little uncertainty for the ‘certain diagnosis’ of the ESC guidelines initial assessment scheme. When a ‘highly likely’ diagnosis was also included, the sensitivity and specificity remained high (98 and 95% respectively, with little uncertainty).
The sensitivity was moderate (84%), and the specificity moderately low (50%), with some uncertainty, for the Graf (2008) rule93 for vasovagal syncope or psychogenic pseudosyncope (low quality evidence).
3.5.3. Decision rules for a diagnosis of psychogenic pseudosyncope versus other forms of syncope
There was low-quality evidence from one study of the ESC guidelines for the diagnosis of psychogenic pseudosyncope. The paper was unclear on the definition of psychogenic pseudosyncope and it was assumed that the guidance in the ESC guidelines should be used33,145.
Factors contributing to a diagnosis of psychogenic pseudosyncope included a high frequency of attacks (many in a day); lack of a recognisable trigger; eyes usually closed; long period of lying on the floor, young age.
The sensitivity was 86% with much uncertainty around the estimate and the specificity was 100% with very little uncertainty.
3.5.4. Decision rules for a diagnosis of orthostatic hypotension cause of syncope versus other forms of syncope
There was very low quality evidence from one study investigating the ESC guidelines for the diagnosis of orthostatic hypotension as the cause of syncope. The ESC guideline definition reported in the paper for a ‘certain diagnosis’ was: a decrease in systolic blood pressure of 20 mm Hg or a decrease of systolic blood pressure to below 90 mm Hg, following supine and three minute upright blood pressure measurements. The GDG regarded this as an indirect measure of orthostatic hypotension in that it did not accord with the widely accepted definition of the Consensus Statement of 1996212.
The ‘certain’ diagnosis category gave very high sensitivity (100%), but with much uncertainty and very high specificity (99%), with little uncertainty. The addition of patients with a highly likely diagnosis decreased the sensitivity to 89%, with only minor improvements in precision, and the specificity remained at 98%.
3.5.5. Diagnosis of cardiac or arrhythmic causes of syncope versus other forms of syncope
3.5.5.1. Signs and symptoms of cardiac or arrhythmic causes of syncope
There was mainly low- and very low- quality evidence from univariate analyses in two studies investigating cardiac causes of syncope6,63 and in one study investigating cardiac arrhythmic causes of syncope192; the del Rosso (2008) study63 was in unselected patients and the other studies had selected populations. Multivariable predictors were mainly moderate- and low- quality evidence.
| Signs and symptoms that are predictors for a cardiac cause of syncope or a cardiac arrhythmic cause: |
| M1: multivariable for del Rosso (2008)63 |
| M2: multivariable for Alboni (2001) heart disease patients6 |
| M3: multivariable for Alboni (2001) without heart disease6 |
| M4: multivariable for Alboni (2001) all patients excluding non-syncope risk factors6 |
| M5: multivariable for Sarasin (2003) in patients with unexplained syncope190 |
| SU and GU: strong and good univariate predictors |
| Card and cardiac: predictors for cardiac cause; Arr_C: arrhythmic causes |
|
|
|
|
|
|
|
|
|
|
| Signs and symptoms that are predictors against cardiac or cardiac arrhythmic syncope: |
|
|
|
|
3.5.5.2. Decision rules for cardiac syncope
There was low- and moderate- quality evidence from four studies investigating decision rules for cardiac syncope or cardiac arrhythmic syncope, three studies in selected patients. Two of the studies investigated an initial evaluation scheme based on syncope guidelines (ESC in one study and ACEP in another retrospective study):
| Rule 1 (del Rosso 200863; EGSYS score): TLoC is classified as a cardiac syncope and equated with the need for admission if the total symptom score is 3 or more, calculated by summing the following, if present: |
|
|
|
|
|
|
| Rule 2 (Sarasin 2003190): TLoC is classified as cardiac arrhythmic syncope if the patient has any one of the following: |
|
|
|
| Rule 3: ESC guidelines (certain and highly-likely diagnoses): TLoC is classified as cardiac syncope if the patient has any of the following: |
|
|
|
|
|
| Rule 4: ACEP recommendations: TLoC is classified as cardiac syncope, which is equated with admission to hospital, if the patient has any one of the following: |
| ACEP level B (high risk, admit to hospital): |
|
|
|
|
|
|
| ACEP level C (moderate risk; consider admission to hospital) |
|
|
|
|
For cardiac syncope:
- –
EGSYS (low quality evidence): sensitivity high (91%), with some uncertainty; specificity moderate (69%), with little uncertainty
- –
ESC guidelines: sensitivity moderate (71%), with large uncertainty, specificity high (100%), with little uncertainty for the ‘certain diagnosis’ (low quality evidence). Inclusion of a ‘highly likely’ diagnosis gave similar sensitivity and specificity and the uncertainty was reduced (moderate quality).
- –
ACEP guidelines: sensitivity high (100%) and the specificity moderately high (81%), with little uncertainty, for level B in a retrospective study (low quality evidence). When level C patients were also included, the sensitivity was unchanged but the specificity reduced (33%).
For cardiac arrhythmic syncope:
- –
Sarasin score: sensitivity high (96%), with little uncertainty, and specificity moderately low (42%) (low quality evidence).
ROC curves comparing the EGSYS score and the Sarasin rule suggested that the most reliable test of these two was the EGSYS score.
3.5.6. Risk factors and decision rules for death within 12 months
3.5.6.1. Features that are risk factors for death
There was low-quality evidence from two studies recording death at an indirect time (12 months and limited evidence for 30 days).
| Signs and symptoms that are predictors for a risk of death within 12 months: Multivariable predictors are indicated by M; significant univariate risk factors by SigU |
|
|
|
|
|
|
|
3.5.6.2. Decision rules for death within 12 months
There was low-, very low- and moderate-quality evidence from four studies examining different risk stratification rules for death in an unselected population; one study was retrospective:
| OESIL score (Colivicchi 200349); the score was predictive of death if there were at least two of the following: |
|
|
|
|
| San Francisco Syncope Rule (Quinn 2008176); the score was predictive of death at 30 days, 3, 6 and 12 months if there was any one of: |
|
|
|
|
|
| ACP guidelines (Crane 200255, retrospective) – the group at high risk of death was identified with admission criteria: |
|
|
|
|
| ACP guidelines – the moderate risk group was identified with considering admission |
|
|
|
|
|
|
Diagnostic test accuracy statistics, including the ROC curve, suggested that the most reliable test was the OESIL score, closely followed by the San Francisco syncope rule; both rules had moderate quality evidence, although at an indirect time (mainly 6 and 12 months), high sensitivity (97 and 93% respectively), but only moderate specificity (73 and 53%). There was low-quality evidence at an indirect time from one UK study, which evaluated the American College of Physicians (ACP) guidelines. The high- and moderate-risk groups combined had a sensitivity of 100% and a specificity of 53%.
3.5.7. Risk factors and decision rules for a serious adverse event within 7–14 days
A ‘serious event’ is defined in most of the studies in this section as: death, myocardial infarction, arrhythmia, pulmonary embolism, stroke, subarachnoid haemorrhage, significant haemorrhage / anaemia needing transfusion; procedural intervention to treat cause of syncope; any condition likely to cause a return to the ED or which did cause a return to the ED; hospitalisation for a related event.
The Costantino (2008) study54 excluded patients with conditions primarily confirmed in the ED, that would have required hospital admission independently of whether they had TLoC, such as: myocardial infarction, acute pulmonary embolism, subarachnoidal haemorrhage, stroke, cardiac arrest, sustained bradycardia (< 35 bpm), complete atrioventricular block, sustained ventricular tachycardia. The events recorded in this study were death and major therapeutic procedures or early readmission.
3.5.7.1. Risk factors for a serious adverse event
There was low- and moderate-quality evidence from six studies in unselected patients showing that the following features were statistically significant risk factors for a serious event within 7–14 days;
| Univariate and multivariable risk factors for a serious event 7–14 days |
| 2 studies investigated multivariable predictors, indicated by: M1 (Costantino 200854 – different adverse events) and M2 (Reed 2010182); Significant univariate risk factors are indicated by SigU. We state when the confidence interval for the RR (or OR) lies wholly or almost wholly > 2. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
There was moderate quality evidence in one study54 for multivariable analyses comparing short term events (up to 10 days) and longer term (11 days to 1 year).
The short term events predictors included:
|
The longer term events predictors included:
|
3.5.7.2. Decision rules for a serious adverse event
Ten studies reported four decision rules for serious adverse events at 1–2 weeks. The evidence was very low quality for the OESIL score (2 studies at 3 months); low quality for the San Francisco Syncope Rule (6 studies, 3 without limitations); moderate quality for the Boston Syncope Rule (1 study at 30 days) and moderate quality for the ROSE Rule (1 study at 1 month).
| San Francisco Syncope Rule (6 studies) for predicting adverse events. Patients were considered at risk if any one of the following was present: |
|
|
|
|
|
| Boston Syncope Rule (1 study) at 30 days. Patients were considered at risk if any one of the following was present: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| OESIL score (two low-quality studies) at 3 months: patients were considered at risk if they two or more of: |
|
|
|
|
For the San Francisco Syncope Rule at 7 days, the sensitivity ranged from 74–96% across the studies, with little uncertainty in the point estimates and the specificity ranged from 57 to 62%, with little uncertainty.
For the Boston Syncope Rule at 30 days for a single study, the sensitivity was 97% and the specificity 62%, both had little uncertainty around the estimates.
For the OESIL Rule at 3 months, the sensitivity was 78 or 91%, with some uncertainty, and the specificity was 64 or 49%, with little uncertainty.
For the ROSE Rule at 1 month for a single study, the sensitivity was 87%, with some uncertainty, and the specificity was 66%, with little uncertainty.
3.6. Evidence to Recommendations
3.6.1. Information-gathering and initial decision making (recommendations 1.1.1.1 - 1.1.3)
The GDG considered all the evidence from the initial stage assessment. The guideline covers three main points of initial patient contact; the ambulance service, the emergency department and the GP surgery. Although these areas have differences, particularly in referral patterns, the GDG decided at the outset to write the recommendations such that each area could be covered by a single recommendation, with clarifying comments being added where appropriate, rather than giving three separate pathways.
It was clear from the evidence that there are two distinct types of diagnostic information about the person with TLoC that it is important to capture:
- The TLoC event itself: the symptoms experienced by the person having the TLoC and the observations made by any eye-witnesses, before during and after TLoC. This information is likely to be gathered at the initial consultation at the point of contact, but the GDG noted that sometimes it is necessary to contact any eyewitnesses at a later stage.
- History-taking, clinical examination and subsequent tests: History-taking includes the person’s medical history, including their current health status, drug therapy, past medical history and family history. Initial tests may require equipment, in particular a 12-lead ECG, and may include laboratory tests on a blood sample.
The GDG were mindful that information obtained at the initial assessment is critical in establishing whether a TLoC has occurred, making an initial diagnosis and directing patients along the correct care pathway. The GDG considered it likely that recommendations to improve the quality of information available to clinicians would be highly cost-effective, given that a lack of good quality information could result in patients receiving inappropriate subsequent care which may be costly, ineffective and possibly harmful.
The GDG recognised at the outset that people who had a serious injury as a result of a suspected TLoC could be in need of urgent treatment. They noted that injury was fairly common in people having TLoC, and drew on additional information19 that recorded 29% of patients with TLoC presenting to a general hospital ED had physical injury secondary to TLoC and 5% had severe trauma (causing skull or other major bone segments fracture; intracranial haemorrhage; internal organ lesions requiring urgent, specific treatment; retrograde amnesia or focal neurologic defect).
The GDG were also aware that TLoC can, rarely, be caused by acute hydrocephalus, such as in tumours of the third ventricle (colloid cysts) and in patients with cerebrospinal fluid shunts who develop blocking of the shunt. These patients may have dilated unreactive pupils and respiratory arrest or impairment during an attack, and such episodes constitute a neurological emergency. The GDG therefore decided to make a recommendation covering both of these issues (recommendation 1.1.1.1). Health care professionals should use clinical judgement to determine appropriate management and the urgency of treatment for people with suspected TLoC who had an injury or who had not made a full recovery of consciousness. This ‘appropriate management’ could equally include further investigation of the TLoC (all subsequent recommendations).
The GDG determined that the next stage in the patient pathway was to find out as much information as possible about the TLoC event. Recommendation 1.1.1.2 therefore sets out the information that should be collected at the first point of contact. This list was based on the predictors described in the evidence. Part of recommendation 1.1.3.1 emphasises the need to take a record of this information from all sources, including the person, any witnesses and paramedics. The GDG also considered, in recommendation 1.1.1.4, the impact on the witnesses of observing somebody having TLoC, and they were particularly concerned when that witness was a child or young person or a person with learning disabilities and/or communication difficulties.
The GDG decided that, before moving on to take the more detailed clinical history, it was important to decide on the basis of the initial information, whether the person had lost consciousness. If they had not, then that person would not be covered by the guideline and should be managed in other ways. However, the GDG noted that, sometimes, the person is not aware, or denies, that they have lost consciousness, therefore in order to exclude someone from the guideline, it is necessary to be definite that the person did not have TLoC; people in whom there is uncertainty should be assumed to have had TLoC. Recommendation 1.1.1.4 describes the steps that should be taken.
3.6.2. Obtaining patient history, clinical examination, and initial tests (recommendations 1.1.2.1 and 1.1.2.2)
The GDG described in recommendation 1.1.2.1 items of patient history that should be obtained, features that should be determined by clinical examination and general tests that should be carried out to aid diagnosis. The GDG also recognised that some people would have underlying conditions that might have caused TLoC, such as hypoglycaemia, and recommended that the health care professional carry out relevant additional tests (recommendation 1.1.2.2). A 12-lead ECG should also be obtained (see section 4.8).
3.6.3. Making a judgement based on initial assessment
Decision-making based on evidence was on the following:
- people at increased risk of death or serious adverse events in the immediate future (and who require urgent referral to specialist departments)
- people who can safely be sent home from hospital or who need not be taken to hospital by ambulance crews or referred by GPs.
- the diagnosis of the cause of TLoC, especially vasovagal syncope, orthostatic hypotension, epileptic seizures and cardiac syncope.
3.6.3.1. Red flag recommendations (1.1.4.1 and 1.1.4.2)
Quality of the evidence
There was moderate- and low-quality evidence from the review on risk factors and decision rules for serious adverse events; mainly low-quality evidence from the review on risk factors and decision rules for death; and moderate- and low-quality evidence on univariate and multivariable predictors and decision rules for a cardiac cause of syncope.
GDG discussion
The GDG wished to determine who was at high risk of a serious event and who should be referred for urgent assessment (that is, within 24 hours). This is how ‘red flags’ are defined in the guideline. Serious events could be death, cardiovascular, or cerebrovascular.
In considering red flags, the GDG focussed on the evidence for short term adverse outcomes (up to 2 weeks). They also noted that a diagnosis of a cardiac cause of syncope has been identified with higher risk and admission to hospital. Although several of the studies aligned high risk with hospital admission, the GDG concluded that a decision to admit the patient should be left to clinical judgement, but that the recommendations should indicate the urgency of the need for further investigation or treatment. The GDG were mindful of the costs of urgent hospital admission and of other urgent referral, and the potential impact of hospitalisation on the individual’s quality of life. They therefore felt that it was important to target urgent referral to those people who were most likely to experience a serious adverse event in the days following TLoC.
The GDG considered the decision rules for a diagnosis of cardiac syncope or cardiac arrhythmic syncope, preferring to use the predictors for the former.
The GDG identified that it was important to minimise the number of false negatives (i.e. requiring a test of high sensitivity), because failing to identify people who had a cardiac cause of syncope could have serious consequences. Preferably, the test should have high specificity to avoid over-referral.
For a diagnosis of a cardiac cause of syncope, the GDG considered the Sarasin (2003) rule190 and the ACEP guidelines (level B) study71. However, both of these studies were retrospective and the GDG had some concerns about the evidence quality. The GDG also took into account the consistent univariate and multivariate signs and symptoms predicting cardiac syncope, namely: suspected heart disease, history of congestive heart disease, abnormal ECG, syncope while supine, syncope during effort and dyspnoea pre-TLoC. The GDG did not feel confident in the risk factors, palpitations pre-TLoC and blurred vision or the time between first and last TLoCs. The GDG was also concerned to include a family history of sudden death as an important risk factor: they recognised this as a relatively rare, though serious, occurrence that might not be sufficiently prevalent to be detected in a multivariable analysis – family history of sudden death appeared in the two guidelines tested as ‘moderate risk’. The GDG noted that there was heterogeneity across the multivariable analyses for the risk factor, age over 65 years, and identified that even when this risk factor was significant, there was uncertainty around the estimate.
The GDG then considered the reviews of predictors and decision rules for death and for serious adverse events. The GDG emphasised that the most relevant target condition was serious adverse events within 7–14 days. They took into consideration the Costantino (2008) study54 which showed that multivariable predictors for death, major therapeutic procedures or early re-admission were very different for longer term follow up (11 days to one year), compared to short term events (up to 10 days). As a result, the GDG decided to regard as indirect evidence the review for risk factors for death at up to 12 months and the studies reporting risk factors or decision rules for serious events at three months and, to a lesser extent, at one month. This meant that the OESIL and San Francisco Syncope Rules for death and the OESIL score for serious adverse events were treated with caution.
The GDG decided not to recommend using the San Francisco Syncope Rule because it only had moderate-high sensitivity (74 – 96%) and moderate specificity (57 – 62%). The ROSE rule for serious events at one month was regarded as slightly indirect evidence and had only moderately high sensitivity (87%) and specificity (66%). The remaining rule, the Boston Syncope Rule was regarded as slightly indirect at one month, and the GDG noted this was validated in only one study; however, the sensitivity was high (97%) and the specificity moderate (62%).
The GDG therefore decided to also take into account the significant univariate and multivariable predictors about which they were confident. These included: congestive heart failure, abnormal ECG, breathlessness, systolic blood pressure below 90 mm Hg, respiratory rate more than 24 breaths per minute, pulse rate less than 50 bpm or more than 110 bpm, chest pain, any one of râles; abnormal heart sounds; carotid bruits and heart murmur; haematocrit less than 30%, a rectal examination showing faecal occult blood, a GI bleed; haemoglobin 90 g/l or less; the absence of symptoms pre-TLoC and trauma.
The GDG noted that age over 65 years was a significant univariate predictor, but did not feature in the short term multivariable analyses, and concluded that it could be a confounder for other factors. Nevertheless the GDG were concerned, from their clinical experience, about the risks of adverse events in people over 65 years who had no warning before TLoC.
The GDG took into account the Costantino (2008) study54 which separated out (and excluded) the patients who had conditions confirmed in ED that would have led to hospital admission independently of TLoC. These conditions included myocardial infarction, acute pulmonary embolism, subarachnoidal haemorrhage, stroke, cardiac arrest, sustained bradycardia (< 35 bpm), complete atrioventricular block, and sustained ventricular tachycardia.
In a similar way, the GDG decided to separate the predictors for short term adverse events and those for a diagnosis of a cardiac cause of syncope into two main groups: (1) those identifying people for whom TLoC is secondary to a condition that requires immediate treatment, and (2) those for people who had TLoC and also have other signs and symptoms, that together mean that the patient requires urgent attention.
For the latter category, the GDG noted that, although the absence of prodromal symptoms was a multivariable independent predictor for short term adverse events in one study54, the odds ratio was relatively small with some uncertainty, and did not appear to be supported by other studies. The GDG also noted that, although most people with cardiac syncope and potential high risk of death will have no prodromes and that people with vasovagal syncope are most likely to have prodromes, older people with vasovagal syncope do not always have prodromes. The GDG decided that the risk factor, absence of prodromal symptoms, although an indicator of a high risk category, was not sufficiently strong to use independently to determine people in need of urgent referral, and decided to add a weak recommendation combining age with no prodromal symptoms (recommendation 1.1.4.2).
The GDG also noted that some of the predictors in the other studies fell into this category of conditions independently requiring urgent attention, for example, a GI bleed, chest pain and abnormal vital signs. If people who had TLoC did have conditions that required immediate treatment, they should be managed according to the needs for that condition, with the appropriate degree of urgency (recommendation 1.1.4.1).
The GDG concentrated on defining the risk factors that, together with TLoC, made the person at high risk of an adverse event (recommendation 1.1.4.2). In doing so, the GDG chose an upper age limit of 40 years for family history of sudden cardiac death, based on the NSF guidance. This limit is pragmatic: the GDG noted that, with increasing age, coronary heart disease overtakes other, mostly inherited, conditions as the commonest cause of sudden cardiac death.
3.6.3.2. Recommendations for an uncomplicated faint (recommendation 1.1.4.3)
Quality of the evidence
There was low- and very-low quality evidence from the review on univariate and multivariable predictors and low- and moderate- quality evidence for decision rules for vasovagal syncope.
GDG discussion
The GDG considered it important to identify those people who have experienced an uncomplicated faint, which is not associated with any increased risk of serious adverse events, in order to prevent further unnecessary investigations which would be inconvenient for the person, costly and unlikely to result in any change in clinical management.
The GDG considered the evidence for decision rules and noted that the Sheldon (2006) rule201 did not perform well in a population representative of the guideline, having low specificity, which would result in people being incorrectly assessed to have had vasovagal syncope, when they might have more serious causes of TLoC.
The GDG decided to focus on the evidence for the population with pure vasovagal syncope, and based their recommendations on the univariate and multivariable predictors of vasovagal syncope, together with the factors included in the ESC guidelines study. The GDG noted that the evidence also required cardiac syncope predictors to be absent and made this clear in their recommendation.
The multivariable evidence showed the vasovagal predictors were independent so only one was necessary for a diagnosis of uncomplicated faint. Based on their consensus experience, the GDG expanded the posture factor to cover any previous similar episodes in which TLoC has been prevented by lying down. Although the multivariable predictor for prodromes was specifically ‘sweating and feeling warm pre-TLoC’, the GDG also took account of the weak univariate evidence for other prodromal factors and decided to recommend prodromal symptoms more generally. After the DVLA, the GDG adopted the mnemonic, ‘the 3Ps’ to enable easy recall of the factors.
In addition, the GDG noted, from their consensus experience, that situational syncope can be diagnosed on the basis of initial assessment, and added recommendation 1.1.4.4.
3.6.3.3. Recommendations for orthostatic hypotension (recommendation 1.2.1.1)
Quality of the evidence
There was low to very low-quality evidence from one study on the predictors for orthostatic hypotension based on the ESC guidelines. There was much uncertainty in the estimates of diagnostic test accuracy and the GDG regarded the definition of orthostatic hypotension as being indirect because it differed from the 1996 Consensus Statement212.
GDG discussion
The study reported indicators for both ‘certain’ and ‘highly likely’ diagnoses of orthostatic hypotension, following supine and three-minute upright blood pressure measurements. The GDG noted the very high point estimate for the sensitivity (100%) and very high specificity (99%) for the certain diagnosis, but also took into account the high degree of uncertainty surrounding the sensitivity. The GDG therefore lacked confidence in the evidence.
The GDG also drew on their experience and noted that there are different definitions of orthostatic hypotension, with a range of definitions used in the recent literature. In the absence of a full literature review of orthostatic hypotension, including in people who have not necessarily had TLoC, the GDG decided to state in their recommendation the basic method of measuring orthostatic hypotension (supine followed by three minutes of repeated measurements in an upright position). This approach should be taken only for people who are suspected, on the basis of history, to have orthostatic hypotension, and who do not have features suggesting an alternative diagnosis.
The GDG did not consider it desirable to routinely carry out supine and standing blood pressure measurements, which could be time consuming. The GDG recognised that some people who had a suggestive history of orthostatic hypotension would not necessarily have positive results on this simple test, but rather than recommending alternative approaches that they had not reviewed, preferred to refer the person with suspected orthostatic hypotension for further specialist cardiovascular assessment. [Alternative approaches might involve tilt testing with beat-to beat blood pressure monitoring in order to detect transient initial orthostatic hypotension or delayed orthostatic hypotension].
The GDG noted that orthostatic hypotension can be caused by some medications, and indicated in their recommendation that if the condition is diagnosed, causes including drug therapy should be investigated. When describing further management following a diagnosis, the GDG took into consideration their concerns that a person with low blood pressure should be treated accordingly and not be sent home, possibly to be alone. This aspect is covered by the NICE Falls guideline150 and the GDG wished to cross refer to this guidance.
3.6.4. Recording information and transfer of patients and records
The GDG noted from their discussions that different clinicians may be involved; for example, there may be initial contact with the ambulance service, but then the person is transferred to the Emergency Department or discharged home. The GDG considered that there was a risk that important information could be lost when different clinicians are involved, and therefore decided to recommend that the initial information is recorded clearly and that a copy of the record is transferred with the person who had a TLoC (recommendation 1.1.3.1).
If the person with TLoC had a clear diagnosis of uncomplicated faint or situational syncope, they should be discharged home, provided there were no other social or clinical causes for concern. The GDG wished to encourage people to see their GP if they had called an ambulance or attended the ED and were later discharged. The health care professional should give a copy of the patient record and ECG report to the patient (recommendation 1.1.4.5).
The GDG made one recommendation specific to the ambulance service (recommendation 1.1.4.6), namely that all people who had TLoC should be taken to the ED unless they clearly had a diagnosis of an uncomplicated faint or situational syncope. This recommendation did not discrimate the degree of urgency.
3.6.4.1. Recommendation for a diagnosis of psychogenic pseudosyncope
Quality of the evidence
There was low-quality evidence from one study on indicators for psychogenic pseudosyncope, based on the ESC guidelines. There was much uncertainty in the estimates of diagnostic test accuracy.
GDG discussion
The GDG did not carry out a full review of the literature on psychogenic pseudosyncope or psychogenic non-epileptic seizures (PNES), outside diagnostic test accuracy studies. They considered that this topic should be dealt with as a separate guideline and were aware that this may be taken up by NICE at a later date. Meanwhile, the GDG recognised that some guidance in the TLoC guideline was needed for people with suspected psychogenic pseudosyncope or PNES and made a recommendation accordingly (recommendation 1.4.1.1).
The GDG did not feel sufficiently confident in the evidence from the review of a single study to use signs and symptoms to make a differential diagnosis of psychogenic pseudosyncope or PNES at the initial stage, preferring to carry out other investigations first, and then consider the possibility of psychogenic pseudosyncope or PNES later in the diagnostic pathway. The GDG gave some indications for suspecting psychogenic forms of TLoC, noting that the distinction between epilepsy and non-epileptic seizures is complex and requires specialist assessment, usually neurological.
The GDG noted that there is some evidence on the use of tilt testing for the diagnosis of psychogenic pseudosyncope, but had not reviewed the evidence for this topic.
Recommendation 1.4.1.1 is based on the GDG’s experience, with limited supporting evidence from the van Dijk (2008) study215.
3.6.4.2. Recommendation for referral to a specialist in epilepsy (recommendation 1.2.2.1)
Quality of the evidence
There was low-quality evidence for three decision rules for predicting epilepsy: One of the decision rules had high sensitivity (94%) and specificity (94%), but was validated in a selected population. The other study in an unselected population had only moderate sensitivity (73%) with uncertainty around this estimate; the specificity was 100%. Three studies reported data on signs and symptoms as univariate predictors of epilepsy as the cause of the TLoC: one study also gave multivariable predictors. The evidence quality for each of these predictors was low or very low, reflecting study limitations, a lack of representativeness of the population, inconsistency between studies and imprecision.
GDG discussion
The GDG considered the benefits of referring people with features that are suggestive of epilepsy to an epilepsy specialist in order to obtain an accurate diagnosis and appropriate treatment. Given the much lower prevalence of epilepsy in comparison to syncope, they were also mindful of the likely costs and possible harms that could result from directing patients with syncope along the wrong diagnostic pathway. They were therefore keen to ensure that referrals to an epilepsy specialist are targeted at those patients with features that are suggestive of epilepsy and without features suggestive of syncope.
The GDG did not feel confident to recommend either of the Sheldon decision rules202 because the study excluded people with an unexplained cause of TLoC. In the study examining the ESC guidelines, the GDG considered that there was too much uncertainty around the estimates to recommend the ESC guidelines. The GDG therefore examined individual predictors from the univariate and multivariable analyses to help them make recommendations.
Usually it would be desirable to base judgements on independent multivariable predictors for risk factors, but these varied with the model used and the GDG considered that, for signs and symptoms, strong or good univariate predictors would be equally useful. The GDG interpreted the low-and very low quality evidence in the light of their experience, particularly because they were concerned that the main study excluded patients with epileptic seizures that were not supported by EEG, and they were not very confident in the results from the case-control studies.
The GDG also noted that, although the main study stated that it excluded people with psychogenic non-epileptic seizures, it did not say how this was diagnosed. The GDG considered that the multivariable risk factor, TLoC with emotional stress, was more likely to be a predictor for psychogenic non-epileptic seizures, and therefore decided not to include this factor in their recommendation for epileptic seizures.
The GDG concurred with the multivariable risk factor, ‘witnessed amnesia for abnormal behaviour’, and clarified the time it should occur, noting from their experience that before, during and/or after an epileptic seizure, eyewitnesses have reported unusual behaviour of which the person has no recollection. This is distinguished from abnormal behaviour which the person does recall, which is not likely to be epileptic, but more likely to be emotional in nature. The GDG noted that, during syncope, people often shake or groan or posture, and often recall this partially.
The GDG emphasised in this recommendation that limb jerking should be prolonged for epilepsy to be suspected and noted that brief limb jerking can also be manifested during vasovagal syncope. As part of their consensus discussion, the GDG watched a video of an experimental study demonstrating induced syncope.
Regarding tongue biting, the GDG considered the very low quality evidence from a case control study in a highly selected population in addition to the main study. The former study suggested lateral tongue biting was an even stronger predictor than tongue biting generally, but there was much imprecision, and the GDG were more confident to use the non-specific ‘tongue biting’ symptom as an indicator of epilepsy.
Regarding the often cited ‘urinary incontinence’ as an indicator of epilepsy, the GDG noted the difference between univariate predictors in two of the studies, one significant for ‘bedwetting’ and one not significant for ‘urinary incontinence’. The absence of either term in multivariable analysis and the very low quality of the evidence reinforced the GDG’s lack of confidence in this indicator for epilepsy.
The GDG also decided to give an indication of features that health care professionals should consider more likely to be caused by syncope than epileptic seizures, and based their recommendation on the very low quality evidence and their consensus discussion. The GDG’s consensus, based on the evidence, is given in recommendation 1.2.2.1.
Finally, the GDG wished to reinforce the recommendation from the NICE guideline on epilepsy on not using an electroencephalogram routinely in the investigation of TLoC.
3.7. Recommendations
1.1. Initial assessment
1.1.1. Gathering information about the event and initial decision-making
- 1.1.1.1.
If the person with suspected transient loss of consciousness (TLoC) has sustained an injury or they have not made a full recovery of consciousness, use clinical judgement to determine appropriate management and the urgency of treatment.
- 1.1.1.2.
Ask the person who has had the suspected TLoC, and any witnesses, to describe what happened before, during and after the event. Try to contact by telephone witnesses who are not present. Record details about:
- circumstances of the event
- person’s posture immediately before loss of consciousness
- prodromal symptoms (such as sweating or feeling warm/hot)
- appearance (for example, whether eyes were open or shut) and colour of the person during the event
- presence or absence of movement during the event (for example, limb-jerking and its duration)
- any tongue-biting (record whether the side or the tip of the tongue was bitten)
- injury occurring during the event (record site and severity)
- duration of the event (onset to regaining consciousness)
- presence or absence of confusion during the recovery period.
- 1.1.1.3.
When recording a description of the suspected TLoC from the patient or a witness, take care to ensure that their communication and other needs are taken into account. This is particularly important when communicating with a child or young person, or person with special communication needs.
Determining whether the person had TLoC
- 1.1.1.4.
Use information gathered from all accounts of the suspected TLoC (see recommendation 1.1.1.2) to confirm whether or not TLoC has occurred. If this is uncertain it should be assumed that they had TLoC until proven otherwise. But, if the person did not have TLoC, instigate suitable management (for example, if the person is determined to have had a fall, rather than TLoC, refer to ‘Falls: the assessment and prevention of falls in older people’ [NICE clinical guideline 21]150).
1.1.2. Obtaining patient history, physical examination and tests
- 1.1.2.1.
Assess and record:
- details of any previous TLoC, including number and frequency
- the person’s medical history and any family history of cardiac disease (for example, personal history of heart disease and family history of sudden cardiac death)
- current medication that may have contributed to TLoC (for example, diuretics)
- vital signs (for example, pulse rate, respiratory rate and temperature) – repeat if clinically indicated
- lying and standing blood pressure if clinically appropriate
- other cardiovascular and neurological signs.
[Note: The recommendations regarding ECG are repeated for continuity - the evidence is in the following chapter]
- 1.1.2.2.
Record a 12-lead electrocardiogram (ECG) using automated interpretation. Treat as a red flag (see recommendation 1.1.4.2) if any of the following abnormalities are reported on the ECG printout:
- conduction abnormality (for example, complete right or left bundle branch block or any degree of heart block)
- evidence of a long or short QT interval, or
- any ST segment or T wave abnormalities.
- 1.1.2.3.
If a 12-lead ECG with automated interpretation is not available, take a manual 12-lead ECG reading and have this reviewed by a healthcare professional trained and competent in identifying the following abnormalities.
- Inappropriate persistent bradycardia.
- Any ventricular arrhythmia (including ventricular ectopic beats).
- Long QT (corrected QT > 450 ms) and short QT (corrected QT < 350 ms) intervals.
- Ventricular pre-excitation (part of Wolff-Parkinson-White syndrome).
- Left or right ventricular hypertrophy.
- Abnormal T wave inversion.
- Pathological Q waves.
- Atrial arrhythmia (sustained).
- Paced rhythm.
- 1.1.2.4.
If during the initial assessment, there is suspicion of an underlying problem causing TLoC, or additional to TLoC, carry out relevant examinations and investigations (for example, check blood glucose levels if diabetic hypoglycaemia is suspected, or haemoglobin levels if anaemia or bleeding is suspected; see also recommendation 1.2.2.1 for information about the use of electroencephalogram [EEG]).
1.1.3. Recording the event information and transfer of records
1.1.4. Making a judgement based on initial assessment
Red flags: people requiring urgent assessment and treatment
- 1.1.4.1.
If TLoC is secondary to a condition that requires immediate action, use clinical judgement to determine appropriate management and the urgency of treatment.
- 1.1.4.2.
Refer within 24 hours for specialist cardiovascular assessment by the most appropriate local service, anyone with TLoC who also has any of the following.
- An ECG abnormality (see recommendations 1.1.2.2 and 1.1.2.3).
- Heart failure (history or physical signs).
- TLoC during exertion.
- Family history of sudden cardiac death in people aged younger than 40 years and/or an inherited cardiac condition.
- New or unexplained breathlessness.
- A heart murmur.
Consider referring within 24 hours for cardiovascular assessment, as above, anyone aged older than 65 years who has experienced TLoC without prodromal symptoms.
No further immediate management required
- 1.1.4.3.
Diagnose uncomplicated faint (uncomplicated vasovagal syncope) on the basis of the initial assessment when:
- 1.1.4.4.
Diagnose situational syncope on the basis of the initial assessment when:
- there are no features from the initial assessment that suggest an alternative diagnosis and
- syncope is clearly and consistently provoked by straining during micturition (usually while standing) or by coughing or swallowing.
- 1.1.4.5.
If a diagnosis of uncomplicated faint or situational syncope is made, and there is nothing in the initial assessment to raise clinical or social concern, no further immediate management is required. If the presentation is not to the GP, the healthcare professional should:
Further immediate management required
- 1.1.4.6.
If the person presents to the ambulance service, take them to the Emergency Department unless a diagnosis of an uncomplicated faint or situational syncope is clear.
1.2. Further assessment and referral
1.2.1. Suspected orthostatic hypotension
- 1.2.1.1.
Suspect orthostatic hypotension on the basis of the initial assessment when:
- there are no features suggesting an alternative diagnosis and
- the history is typical.
If these criteria are met, measure lying and standing blood pressure (with repeated measurements while standing for 3 minutes). If clinical measurements do not confirm orthostatic hypotension despite a suggestive history, refer the person for further specialist cardiovascular assessment.
If orthostatic hypotension is confirmed, consider likely causes, including drug therapy, and manage appropriately (for example, see ‘Falls: the assessment and prevention of falls in older people’ [NICE clinical guideline 21])150.
1.2.2. Suspected epilepsy
- 1.2.2.1.
Refer people who present with one or more of the following features (that is, features that are strongly suggestive of epileptic seizures) for an assessment by a specialist in epilepsy; the person should be seen by the specialist within 2 weeks (see ‘The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care [NICE clinical guideline 20]150).
- A bitten tongue.
- Head-turning to one side during TLoC.
- No memory of abnormal behaviour witnessed by someone else before, during or after TLoC.
- Unusual posturing.
- Prolonged limb-jerking (note that brief seizure-like activity can often occur during uncomplicated faints).
- Confusion following the event.
- Prodromal déjà vu, or jamais vu (see glossary).
Consider that the episode may not be related to epilepsy if any of the following features are present.
- Prodromal symptoms that on other occasions have been abolished by sitting or lying down.
- Sweating before the episode.
- Prolonged standing that appeared to precipitate the TLoC.
- Pallor during the episode.
Do not routinely use electroencephalogram (EEG) in the investigation of TLoC (see ‘The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care’ [NICE clinical guideline 20]151).
Footnotes
- ‡
Osservatorio Epidemiologico sulla Sincope nel Lazio
- ‡‡
The cause of the inconsistency may have been differences in methodological quality between the two studies or possibly different definitions of the predictor (‘bedwetting’ versus ‘urinary incontinence’)
- Transient loss of consciousness ('blackouts') in over 16s
- Transient loss of consciousness: Evidence Update March 2012: A summary of selected new evidence relevant to NICE clinical guideline 109 'Transient loss of consciousness ('blackouts') management in adults and young people' (2010)
- 2019 surveillance of transient loss of consciousness ('blackouts') in over 16s (NICE guideline CG109)
- Initial assessment and diagnosis of people who had TLoC - Transient Loss of Cons...Initial assessment and diagnosis of people who had TLoC - Transient Loss of Consciousness (‘Blackouts’) Management in Adults and Young People
Your browsing activity is empty.
Activity recording is turned off.
See more...
