NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Committee on the Review and Assessment of the NIH’s Strategic Research Plan and Budget to Reduce and Ultimately Eliminate Health Disparities; Thomson GE, Mitchell F, Williams MB, editors. Examining the Health Disparities Research Plan of the National Institutes of Health: Unfinished Business. Washington (DC): National Academies Press (US); 2006.

Examining the Health Disparities Research Plan of the National Institutes of Health: Unfinished Business.
Show detailsTo achieve the Strategic Plan’s goals and objectives, activities must be coordinated among the Institutes and Centers (ICs) and Offices within the National Institutes of Health (NIH). Effective coordination presents a major challenge, first because of the research’s scope and complexity and second because of the NIH organizational and functional setting.
THE CHALLENGE OF STRUCTURING A TRANS-NIH HEALTH DISPARITIES RESEARCH PROGRAM AND STRATEGIC PLAN
Biomedical research on health disparities spans almost every research discipline and area, as reflected by the participation of almost all NIH ICs in the Strategic Plan (the National Library of Medicine and the National Center for Research Resources are not involved). Beyond the research itself, another challenge is providing adequate attention to the infrastructure and the capacity for extramural health disparities research, including the enhancement of research facilities and development of scientists involved in health disparities research. Finally, communication of research findings and best practices to providers, the medical education system, patients, and communities represent important research-to-care translation components.
These biomedical research, research capacity, and communication factors should be addressed by the programs of the ICs involved in the Strategic Plan. Indeed, many of these factors were already addressed within the programs of the ICs. But the Strategic Plan should have generated a coordinated organization with more creativity, collaboration, effectiveness, and productivity across the entire minority health and health disparities program.
NIH’s organization and function is a major factor in the success of the Strategic Plan and the minority health and health disparities research program. Increases in the number of ICs and their organizational relationships to NIH as an entity have increased this complexity in recent decades (Committee on the Organizational Structure of the National Institutes of Health, 2003). Emerging fields of biomedical information, technology, and research have been accompanied by a parallel multiplication of medical specialties and academic departments, and increases in the number of NIH ICs. From 1986 through 2000, 8 new Institutes and 4 new Centers were established, many as a result of congressional action. NIH currently includes 19 Institutes, 7 Centers, and the National Library of Medicine.
The NIH Institutes (Figure 6-1) have been described (McGeary and Smith, 2002) as organized in relation to five categories: diseases (cancer, mental health, diabetes, digestive and kidney disease, drug and alcohol abuse); organ systems (heart, lung, and blood; the eye); stage of life (child and human development, aging); scientific field (general medical sciences, environmental health services, the human genome); and profession or technology (nursing, dental, imaging, bioengineering). Some Centers have missions that are supported throughout the NIH, while others conduct and support intramural and extramural research. Within the Office of the Director, four Offices have specific coordinating and support functions: the Office of AIDS Research (OAR), the Office of Research on Women’s Health (ORWH), the Office of Behavioral and Social Sciences Research, and the Office of Disease Prevention.
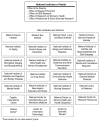
FIGURE 6-1
Current organization of the National Institutes of Health Institutes and Centers. SOURCE: Committee on the Organizational Structure of the National Institutes of Health, 2003.
NIH’s success and immense contribution to progress in biomedical science and health care can partly be attributed to: (a) its adaptation to the need for specialized centers of research capable of focusing highly specialized expertise and (b) the articulation of the ICs with similar concentrations of science and scientists in medicine and academia. At the same time, some have been concerned about whether the growth of ICs has been entirely necessary and how much this proliferation may contribute to increased difficulty in managing NIH as a cohesive agency. This is particularly germane when NIH addresses an extensive, cross-cutting research endeavor that requires coordination among several ICs, as well as collaborations with other government agencies.
Although the number of ICs has been the subject of continued analysis—and even recommendations that there be some consolidation (Committee on the Organizational Structure of the National Institutes of Health, National Research Council, 2003)—there has been even more concern about the organization of the ICs across NIH and their functional relationships with NIH as an entity. Those relation-ships have been described as a loose confederacy of somewhat independent entities with decentralized control. The ICs have considerable independence and autonomy with respect to research programs and a lesser degree of independence regarding budgeting.
Trans-NIH Initiatives
Although much collaborative research is conducted between and among the ICs, broader NIH-wide initiatives have been organized in a number of ways to achieve important centralized, trans-NIH organization and coordination. The Committee reviewed examples and experiences as it assessed the organization of the health disparities research program and the Strategic Plan. Some examples follow.1
OAR. NIH AIDS research, involving several institutes, is overseen by the OAR, which coordinates scientific, budgetary, legislative, and policy elements of the NIH AIDS research program. OAR was established in 1988 as an office within the Office of the NIH Director. Its role and responsibilities were set forth in P.L. 103-43, the National Institutes of Health Revitalization Act of 1993.
OAR reviews and approves all NIH-conducted and NIH-supported AIDS research, as well as the related budgets. It also produces a comprehensive trans-NIH annual strategic plan (the Plan for HIV-Related Research) and evaluates all AIDS activities of NIH ICs. The Plan for HIV-Related Research is developed with a consensus on scientific priorities set with the assistance of several planning groups, including IC directors and staff, researchers from academia and industry, foundations, community representatives, representatives from other government agencies, and the OAR Advisory Council. Each involved IC comments on the final plan. The ICs’ budgets for AIDS-related research are submitted to OAR and reviewed in relation to the overall Plan for HIV-Related Research, OAR priorities, and the plans of other ICs. The NIH director and the OAR determine the overall NIH budgetary allocation for AIDS research, and the OAR then allocates research budgets to each IC.
As an office within the Office of the Director, OAR does not have grant-making authority but does exercise control and coordination over all NIH AIDS research. OAR is responsible for representing, implementing, coordinating, and monitoring NIH AIDS research. Keys to OAR’s ability to coordinate and manage the trans-NIH AIDS program effectively include clear authority over the budgets for AIDS research, presence in the Office of the NIH director, and extensive use of trans-NIH coordinating committees and advisory groups as resources of scientific expertise.
ORWH. The ORWH was established in 1990. Its responsibilities were described in the NIH Revitalization Act of 1993. While serving to advise the NIH director on women’s health issues, ORWH has a great deal of influence over NIH research in this area. ORWH does not have budgetary authority over such research, but it does provide funding to ICs for projects on women’s health while working across NIH to ensure the development of opportunities for women in biomedical careers and women’s health research. ORWH is also responsible for ensuring the inclusion of women and minorities as subjects in biomedical research including Phase III clinical trials—a role that it manages with great attention to detail and completeness. ORWH issues comprehensive reports that track the participation of individuals as subjects in clinical research (U.S. DHHS, 2005). This central function of ORWH has been a factor in its trans-NIH influence. Reportedly, from its beginning ORWH leadership has been seen as scientifically credible and well integrated into the fabric of NIH activities. Success in ORWH’s trans-NIH roles is attributed to its establishment by legislation, strong support from the Office of the Director of the NIH, responsibility for a trans-NIH reporting function, and the scientific credibility of, and respect for, its leadership.
NIH Obesity Research Task Force. The NIH director established the NIH Obesity Research Task Force in April 2003 “to accelerate progress in obesity research across NIH.” It is co-chaired by the Director of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the Director of the National Heart, Lung, and Blood Institute (NHLBI). The Task Force, which includes representatives from the NIDDK, the NHLBI, and other NIH ICs, was charged with the development of the Strategic Plan for NIH Obesity Research, published in August 2004, with the purpose of providing “a guide for coordinating obesity research activities across NIH and for enhancing the development of new research efforts based on identification of areas of greatest scientific opportunity and challenge.” The planning process involved contributions from external experts at scientific and other meetings, interactions with scientific and advocacy organizations, and review of the draft document by selected individuals.
The Strategic Plan for NIH Obesity Research includes theme areas that are analogous to the goal areas of the health disparities Strategic Plan. Implementation of these theme areas will involve interdisciplinary research teams, a focus on children and racial and ethnic minorities, special attention to translational research, and the dissemination of research results to the public. Beyond development of the Strategic Plan for NIH Obesity Research, the Task Force’s responsibilities and its involvement in coordination are unclear. The success of this trans-NIH planning effort reflects its establishment and support by the NIH director, the leadership of the Task Force, the effective involvement of the ICs, and the extensive involvement of experts from NIH and from the extramural scientific community.
NIH Neuroscience Blueprint. The NIH Neuroscience Blueprint, announced in October 2004, is a new interagency partnership intended to reinforce ongoing NIH efforts to increase collaborative research and information sharing among 14 ICs that conduct or support research on the brain and nervous system. The ICs will carry out independent research but collaborate and share resources on research challenges and training that can be addressed collectively. The Neuroscience Blueprint builds on an existing cooperative relationship established through initiatives and working groups on specific disorders. It will target neuroscience challenges that will benefit from a collaborative approach beginning with three unifying themes: development of the nervous system throughout the life span, neurodegeneration from disease and aging, and nervous system plasticity (changes in response to the environment, experience, injury, and disease). How the Neuroscience Blueprint will be managed and coordinated is not specified.
Two additional trans-NIH initiatives instituted by the director of NIH are aimed at facilitating cohesive, integrated NIH core efforts: the NIH Roadmap and the Office of Portfolio Analysis and Strategic Initiatives (OPASI).
NIH Roadmap. The NIH Roadmap is an initiative spearheaded by the director of NIH with the purpose of identifying major opportunities and gaps in biomedical research, in order to enhance the progress of medical research. Development of the Roadmap involved broad consultation with representatives of the scientific community and public constituencies and the extensive participation of NIH working groups, the NIH Council of Public Representatives, and the Advisory Committee to the Director.
From 2004 to 2005, the first Roadmap initiatives were begun. They reflect the themes of: (a) New Pathways, which seeks to advance understanding of biological systems; (b) Research Teams of the Future, which explores new organizational models for team sciences; and (c) Re-engineering the Clinical Research Enterprise, which will develop new approaches to discovery and clinical validation of research results. The Roadmap, initiated and guided by the director of the NIH, forms the basis for the overarching planning of NIH’s strategies for research for the coming years.
OPASI. In FY 2006, the NIH plans to create a new office within the Office of the Director, OPASI, which is intended to provide tools to facilitate the planning and management of trans-NIH initiatives, including an improved process for collecting IC data on expenditures on various diseases, conditions, and research fields, and improvements in data about the burden of disease. OPASI will also develop, with input from the ICs, common processes and formats, where necessary, for the conduct of NIH-wide planning and evaluation. For its trans-NIH planning efforts, OPASI will seek broad public input—from the public, health care providers, policy makers, and scientists—in addition to soliciting advice from within the NIH. The office will also coordinate and make more effective use of the NIH-wide evaluation process (Kington, 2005). The ultimate structure, responsibilities, and authorities of OPASI with respect to the trans-NIH initiatives are not yet clear, but OPASI could exert far-reaching effects on the management and coordination of the minority health and health disparities program and the Strategic Plan.
The Committee’s review of trans-NIH programs and efforts noted that apparent success of trans-NIH coordination and management is related to several factors, including:
- Legislative authority
- Budget authority
- A clear science agenda/focus
- Clear support from the director of the NIH
- Responsibility and accountability within ICs
- Strong, structured articulation of ICs with a central coordinating entity, including trans-NIH committees and other groups led by the coordinating entity
COORDINATION OF THE STRATEGIC PLAN AND MINORITY HEALTH AND HEALTH DISPARITIES RESEARCH
It could be said that there is no trans-NIH research effort more challenging than the health disparities research program and its Strategic Plan. Although most similar, the AIDS research program differs in scope because it is developed around a single disease, complicated and challenging as it is. By contrast, the minority health and health disparities research includes a broader scope of diseases and conditions.
Coordination of the minority health and health disparities program and the Strategic Plan across NIH should address needs for:
- Concerted involvement of ICs and Offices in the development of the Strategic Plan, including continuous review and annual updates, which are a collective result of experiences, assessments, new inclusions, and other changes.
- Ensuring that all ICs and pertinent offices are attentive to the mission, goals, and objectives of the Strategic Plan.
- Avoiding gaps, such as populations, conditions, needs, and approaches, that would otherwise not be identified and addressed by the independent operation of the ICs.
- Bringing the best expertise from across NIH and from the external scientific community as a resource for the program and for strategic planning.
- Avoiding duplication of administrative and research efforts.
- Facilitating collaborative and coordinated approaches to minority and health disparity research areas that affect and involve more that one institute or center.
- Coordinating approaches to those aspects of outreach and communication that, rather than being addressed individually by the ICs, would benefit from collectively planned and coordinated trans-NIH efforts, including evaluations of project results and identification and further trials of promising methods.
- Creating an NIH coordination structure and mechanism that will articulate with other government agencies (e.g., the Centers for Disease Control and Prevention, the Agency for Healthcare Research and Quality, the Department of Health and Human Services) on minority health and health disparities, particularly with respect to the relation between research on disparities in health status and disparities in access to health care and its quality.
- Monitoring such a broad NIH activity. This includes avoiding duplication of efforts and use of resources, ensuring that funds committed in Strategic Plans are expended as described, and regularly assessing progress and outcomes.
- Addressing research and budget priorities.
Particularly important is recognizing, and attending to, the interface between fundamental priorities. IC programs and budgets are the products of commitments, mandates, and priorities resulting from presentations and requests to, and authorizations from, Congress. If there is truly a concerted trans-NIH priority for minority health and health disparities research, that prioritization should be demonstrably active in the program priority decisions of the ICs.
The Committee saw little evidence of integration, coordination, or monitoring of health disparities research and the Strategic Plan across NIH. Several observations, as detailed below, led to this conclusion.
Review and revision of the Strategic Plan does not involve the coordinated, concerted, and collective participation of the ICs. There is no ongoing, continuous update process with an established trans-NIH structure involving ICs and others that produces planning improvements and results in periodic, meaningful updates and revisions of the Strategic Plan. There is no evidence of trans-NIH planning of priorities regarding minority health and health disparities research activities and resources for the NIH as a whole or with respect to the ICs. In discussions with the Committee, directors and other leading members of several large ICs with extensive minority health and health disparities programs expressed a very high level of commitment to and enthusiasm for these activities. However, it was evident that there had been little to no contact with the National Center for Minority Health and Health Disparities (NCMHD) during the development or implementation of the projects and programs. Activities and programs were pursued independently of NCMHD, except that some, particularly in the past, had been co-funded or totally funded by NCMHD.
There is no manifest organizational structure for the trans-NIH Strategic Plan and health disparities program. Advisory and coordinating committees are not described or apparent. Experts from scientific, health care, and affected communities are not involved in advising and participating in ongoing planning in established, structured, predictable ways. Thus, there is a great loss of opportunity to properly inform and contribute to the identification of research and related needs, planning, and strategizing.
No results summarizing the monitoring and assessment of minority health and health disparities research and related activities for NIH or the ICs are evident. Annual reports are late, languish incomplete and unapproved, and do not contain evidence of central NIH assessments of research and program activities. Moreover, budget and finance issues are not addressed by a centralized entity responsible for the minority health and health disparities research program and the Strategic Plan.
Recognizing this need for leadership and management, the Committee found it difficult to be certain of an established, clear responsibility and authority for coordinating and monitoring the Strategic Plan and health disparities research. The enabling legislation, P.L. 106-525, expects NCMHD to have and to be involved with such responsibilities, as indicated in Section 485E(e):
The director of the Center shall act as the primary Federal official with responsibility for coordinating all minority health disparities research and other health disparities research conducted or supported by the National Institutes of Health.
Also, Section 485E(f) indicates that “the Director of NIH, the Director of the Center, and the directors of the other agencies of the National Institutes of Health in collaboration (and in consultation with the advisory council for the Center)” together are responsible for establishing the Strategic Plan and budget and reviewing its progress. These responsibilities include ensuring that the Strategic Plan and budget establish priorities, verifying that the amounts appropriated are expended in accord with the Strategic Plan and budget, and reviewing and revising the Strategic Plan and budget annually.
These monitoring responsibilities are described in the legislation as the joint responsibilities of the director of NIH, the director of NCMHD, and the directors of the ICs. To avoid misunderstanding of the authority to manage and monitor the program across NIH, it is important to clarify how such an arrangement of responsibilities is achieved in practice. That is, it must be made clear whether specific authority is delegated to the director of NCMHD by the director of NIH and understood to exist by the directors of the ICs—or, alternatively, whether there are truly joint responsibilities and operational authorities (a situation that would be confusing). When the Committee requested clarification, it was told by the NIH director that the responsibilities and authority were shared between the director of NIH and the director of NCMHD. Moreover, reviews and discussions with leaders and representatives of the ICs and Offices within the Office of the Director suggested that the responsibilities and authorities were not uniformly clear. This situation differs from other trans-NIH initiatives.
The Executive Summaries of the 2002 Strategic Plan and the 2004 draft indicate the following: “Within the NIH, the National Center on Minority Health and Health Disparities (NCMHD) serves as the focal point for planning and coordinating minority health and other health disparities research.” Also, in the approved Strategic Plan for 2002–2006 and the draft of the 2004–2008 Strategic Plan, NCMHD’s responsibility for establishing and updating the Strategic Plan and budget and coordinating health disparities research is set forth. This is appropriate and in accord with the legislation. However, whether NCMHD’s authority is understood and acted upon throughout NIH is unclear.
Finding: The level of trans-NIH coordination needed to effectively implement the Strategic Plan has not been evident. Instead, the Committee concluded that an uncoordinated, unmonitored, loosely administered trans-NIH program existed, with substantial commitments and activities of largely independent ICs, but without the coordinated, concerted program needed. Clarity regarding the responsibilities and authority may be a factor in achieving more effective management. The mandates of the NIH director are key elements in structuring and assuring effective management.
Recommendation 10: The NIH director, through the established authority of the NCMHD director, should ensure continuous, effective coordination of the health disparities research program across NIH, including:
Footnotes
- 1
See the following websites for information on trans-NIH research programs at the NIH: http://www
.obesityresearch .nih.gov/about/about.htm, http://nihroadmap .nih.gov/index.asp, http://www4 .od.nih.gov/orwh/, http://www .nih.gov/od/oar/index.htm, http://www .niddk.nih .gov/fund/diabetesspecialfunds/funding .htm, and http: //neuroscienceblueprint.nih.gov/.
- Management of the Strategic Plan and the Health Disparities Research Program - E...Management of the Strategic Plan and the Health Disparities Research Program - Examining the Health Disparities Research Plan of the National Institutes of Health
Your browsing activity is empty.
Activity recording is turned off.
See more...