Table
In vitro Rodents
Background
[PubMed]
One of the mechanisms of tumor cells to escape the cytotoxic effects of chemotherapeutic agents, such as adriamycin, vinca alkaloids, epipodophyllotoxins, actinomycin D, and paclitaxel, is to limit their presence inside the cells by a multidrug resistance (MDR-1) gene protein (1, 2). The MDR-1 gene encodes a transmembrane P-glycoprotein (P-gp) as an ATP-dependent multidrug transporter that is capable of actively pumping a variety of agents out of the cells. Injection of unlabeled efflux pump substrates increases the retention of the radioactivity in the tumor rather than less radioactivity as seen with receptor binding radiotracers. Over-expression of P-gp in tumor cells (such as renal carcinoma, hepatoma, pheochromocytoma, and colon carcinoma) leads to resistance to anticancer drugs (3). P-gp is also present in a variety of normal cells, such as intestinal mucosal cells, hepatocytes, renal proximal tubule epithelial cells, and endothelial cells of the blood brain barrier (BBB) (4, 5). Calcium channel blockers, cyclosporin and its non-immunosuppressive analogue PSC 833 are MDR modulators inhibiting transport of P-gp substrates out of the cells (6, 7).
Sestamibi (MIBI) is a substrate for P-gp. 99mTc-MIBI has been approved by the FDA as a myocardial perfusion imaging agent with single photon emission computed tomography (SPECT) to assess the risk of future cardiac events (8). It is also used as a tumor-imaging agent in breast, lung, thyroid, and brain cancers (9-13). Loperamide is an opiate agonist (14) and an avid substrate for P-gp at the BBB (15). [11C]Loperamide ([11C]Lop) has been studied as a positron emission tomography (PET) agent for studying P-gp function and multidrug resistance in tumors and normal tissues non-invasively (16). However, demethylation of [11C]Lop to [N-methyl-11C]N-desmethyl-loperamide ([11C]dLop) precludes quantification of P-gp function with PET if [11C]dLop is also an avid substrate for P-gp. Therefore, [11C]dLop has been studied as a positron emission tomography (PET) agent for studying P-gp function and multidrug resistance in tumors and normal tissues non-invasively (17).
Synthesis
[PubMed]
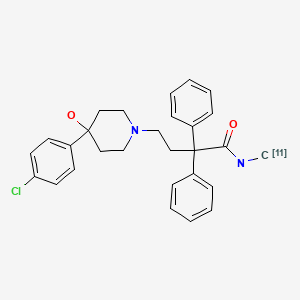
[11C]dLop was synthesized by a reaction of 4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-2,2-diphenylbutanamide with [11C]methyl iodide at 80°C for 6 min (17). The radiochemical purity of purified [11C]dLop was >99% with specific activities of 152 ± 48 GBq/µmol (4.11 ± 1.30 Ci/µmol) at the end of synthesis. The total synthesis time was 40 min with a radiochemical yield of 18% (decay-corrected) based on [11C]CO2.
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
In vitro binding studies using cloned receptors with dLop showed inhibition constant (Ki) values were 0.56, 73 and 328 nM for µ, δ and κ opiate receptor, respectively (16). The measured Log D7.4 of [11C]dLop was 2.60.
Animal Studies
Rodents
[PubMed]
PET imaging studies with [11C]dLop PET imaging in three normal and three P-gp knockout mice showed a higher accumulation of radioactivity in the forebrain in the knockout mice than the wide type mice by 3.6 fold (17). The maximal brain accumulation in the knockout mice occurred at 8-20 min after injection. Three hydrophilic radiometabolites were detected in the plasma and brains with high-performance liquid chromatography (HPLC). In the knockout mice, the fraction of unchanged [11C]dLop in the plasma and brain determined with HPLC was 7.7% and 92% at 60 min after injection, respectively. In the wide type mice, the fraction of unchanged [11C]dLop in the plasma and brain was 11.1% and 42.6% at 60 min after injection, respectively. The ex vivo brain concentrations of [11C]dLop were ~16-fold greater in the knockout mice than in wild-type mice, whereas little differences were observed in the plasma of these mice. There was a 7-fold increase in total ([11C]dLop plus its radiometabolites) radioactivity in the forebrain in the knockout mice as compared with the wide type mice.
Non-Human Primates
[PubMed]
Lazarova et al. (17) showed that DCPQ (P-gp blocker, 8 mg/kg) pretreatment of six rhesus monkeys enhanced brain [11C]dLop PET radioactivity by 7-fold in the frontal cortex and 13-fold in the cerebellum at 30 min after injection. The increase of brain radioactivity was not blocked by administration of naloxone (opiate antagonist) or dLop at 30 min after [11C]dLop injection in the DCPQ-pretreated monkeys. Three hydrophilic radiometabolites were detected in monkey arterial plasma at as early as 10 min after injection. The fraction of unchanged [11C]dLop in the plasma was ~30% at 60 min after injection. DCPQ had little effect on the rate at which each radiometabolite appeared in the plasma. Liow et al. (18) performed similar PET studies showing that the brain (2% ID), liver (39% ID), lung (31% ID) and kidney (11% ID) were the main organs with visually accumulation of radioactivity at 2-30 min after injection. P-gp blockade increased the brain radioactivity to 3% ID, whereas no effects on the other organs. Furthermore, the accumulation of radioactivity among brain regions with P-gp blockade correlated linearly with blood flow, suggesting a high single-pass extraction.
Human Studies
[PubMed]
Seneca et al. (19) performed whole-body scans for 120 min after injection of 744 MBq (20 mCi) [11C]dLop in 8 healthy subjects. The highest absorbed doses were in the kidneys (50.1 ± 6.0 µSv/MBq), spleen (30.5 ± 6.8 µSv/MBq), lungs (27.0 ± 3.4 µSv/MBq), thyroid (14.7 ± 6.2 µSv/MBq), liver (12.9 ± 2.7 µSv/MBq), and urinary bladder wall (10.8 ± 3.3 µSv/MBq). The effective dose (ED) was 7.6 ± 0.6 µSv/MBq. There were minimal brain uptake of [11C]dLop with the rate of brain entry of <0.01 mL.cm-3.min-1. The plasma concentration of [11C]dLop declined rapidly with a biexponential function. The half-lives were 0.4 and 15 min. The fraction of intact [11C]dLop in plasma was 85% at 5 and 120 min after injection. There were 5 hydrophilic radiometabolites.
NIH Support
Intramural research program
References
- 1.
- Endicott J.A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–71. [PubMed: 2570548]
- 2.
- Gottesman M.M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. [PubMed: 8102521]
- 3.
- Fojo A.T., Ueda K., Slamon D.J., Poplack D.G., Gottesman M.M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987;84(1):265–9. [PMC free article: PMC304184] [PubMed: 2432605]
- 4.
- Piwnica-Worms D., Rao V.V., Kronauge J.F., Croop J.M. Characterization of multidrug resistance P-glycoprotein transport function with an organotechnetium cation. Biochemistry. 1995;34(38):12210–20. [PubMed: 7547962]
- 5.
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M.M., Pastan I., Willingham M.C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84(21):7735–8. [PMC free article: PMC299375] [PubMed: 2444983]
- 6.
- Hughes C.S., Vaden S.L., Manaugh C.A., Price G.S., Hudson L.C. Modulation of doxorubicin concentration by cyclosporin A in brain and testicular barrier tissues expressing P-glycoprotein in rats. J Neurooncol. 1998;37(1):45–54. [PubMed: 9525837]
- 7.
- Mayer U., Wagenaar E., Dorobek B., Beijnen J.H., Borst P., Schinkel A.H. Full blockade of intestinal P-glycoprotein and extensive inhibition of blood-brain barrier P-glycoprotein by oral treatment of mice with PSC833. J Clin Invest. 1997;100(10):2430–6. [PMC free article: PMC508442] [PubMed: 9366556]
- 8.
- Piwnica-Worms D., Chiu M.L., Budding M., Kronauge J.F., Kramer R.A., Croop J.M. Functional imaging of multidrug-resistant P-glycoprotein with an organotechnetium complex. Cancer Res. 1993;53(5):977–84. [PubMed: 8094997]
- 9.
- Berman D.S., Kiat H., Van Train K., Garcia E., Friedman J., Maddahi J. Technetium 99m sestamibi in the assessment of chronic coronary artery disease. Semin Nucl Med. 1991;21(3):190–212. [PubMed: 1835137]
- 10.
- George E.F., Komisar A., Scharf S.C., Ferracci A., Blaugrund S. Diagnostic value of the preoperative sestamibi scan in intraoperative localization of parathyroid adenomas: a case study. Laryngoscope. 1998;108(5):627–9. [PubMed: 9591536]
- 11.
- Mansoor M.R., Heller G.V. Recent developments in the prognostic use of myocardial perfusion imaging. Curr Opin Cardiol. 1997;12(6):571–80. [PubMed: 9429830]
- 12.
- Maublant J. Scintigraphic imaging of breast tumors. Eur J Radiol. 1997;24(1):2–10. [PubMed: 9056144]
- 13.
- Vermeersch H., Loose D., Ham H., Otte A., Van de Wiele C. Nuclear medicine imaging for the assessment of primary and recurrent head and neck carcinoma using routinely available tracers. Eur J Nucl Med Mol Imaging. 2003;30(12):1689–700. [PubMed: 14574516]
- 14.
- Awouters F., Megens A., Verlinden M., Schuurkes J., Niemegeers C., Janssen P.A. Loperamide. Survey of studies on mechanism of its antidiarrheal activity. Dig Dis Sci. 1993;38(6):977–95. [PubMed: 8508715]
- 15.
- Sadeque A.J., Wandel C., He H., Shah S., Wood A.J. Increased drug delivery to the brain by P-glycoprotein inhibition. Clin Pharmacol Ther. 2000;68(3):231–7. [PubMed: 11014404]
- 16.
- Zoghbi S.S., Liow J.S., Yasuno F., Hong J., Tuan E., Lazarova N., Gladding R.L., Pike V.W., Innis R.B. 11C-loperamide and its N-desmethyl radiometabolite are avid substrates for brain permeability-glycoprotein efflux. J Nucl Med. 2008;49(4):649–56. [PubMed: 18344435]
- 17.
- Lazarova N., Zoghbi S.S., Hong J., Seneca N., Tuan E., Gladding R.L., Liow J.S., Taku A., Innis R.B., Pike V.W. Synthesis and evaluation of [N-methyl-11C]N-desmethyl-loperamide as a new and improved PET radiotracer for imaging P-gp function. J Med Chem. 2008;51(19):6034–43. [PMC free article: PMC2646255] [PubMed: 18783208]
- 18.
- Liow J.S., Kreisl W., Zoghbi S.S., Lazarova N., Seneca N., Gladding R.L., Taku A., Herscovitch P., Pike V.W., Innis R.B. P-glycoprotein function at the blood-brain barrier imaged using 11C-N-desmethyl-loperamide in monkeys. J Nucl Med. 2009;50(1):108–15. [PMC free article: PMC2652692] [PubMed: 19091890]
- 19.
- Seneca N., Zoghbi S.S., Liow J.S., Kreisl W., Herscovitch P., Jenko K., Gladding R.L., Taku A., Pike V.W., Innis R.B. Human Brain Imaging and Radiation Dosimetry of 11C-N-Desmethyl-Loperamide, a PET Radiotracer to Measure the Function of P-Glycoprotein. J Nucl Med. 2009;50(5):807–13. [PMC free article: PMC2792991] [PubMed: 19372478]
Publication Details
Author Information and Affiliations
Publication History
Created: May 2, 2009; Last Update: December 3, 2009.
Copyright
Publisher
National Center for Biotechnology Information (US), Bethesda (MD)
NLM Citation
Leung K. [N-methyl-11C]4-(4-(4-Chlorophenyl)-4-hydroxypiperidin-1-yl)-2,2-diphenyl-N-methyl-butanamide. 2009 May 2 [Updated 2009 Dec 3]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
 In vitro
In vitro