Table
In vitro Rodents
Background
[PubMed]
Bisphosphonates (BPs; also known as idphosphonates) labeled with technetium ([99mTc]-BP) are often used with bone scintigraphy to detect osteoporosis and other skeletal-related events (SREs), including bone metastases (2). These chemicals are known to promote osteoclast apoptosis and have a strong affinity for hydroxyapatite, a component of the bone matrix. The mechanism of action of these bone-seeking compounds is described in detail elsewhere (3-5). Bone scintigraphy is usually performed 2–6 h after intravenous injection of a [99mTc]-BP, resulting in exposure of the patient to radiation for an extended time. To develop BPs that are more efficient and require only a short waiting time before bone scintigraphy, investigators generated various BPs, such as zoledronic acid (ZL), which contains an imidazole group in its structure, and evaluated their efficacy for the treatment of various bone-related diseases (6, 7). ZL was determined to be the most potent BP available for treating bone resorption and is approved by the United States Food and Drug Administration for the treatment of various tumor-induced SREs (8).
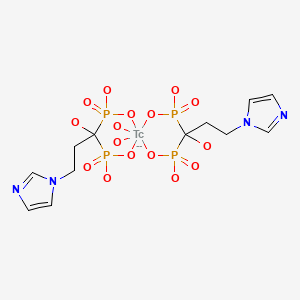
In an earlier study with a goal to further improve the potency of ZL, the imidazole group of the compound was modified to obtain 1-hydroxy-2-(2-ethyl-4-methyl-1H-imidazol-1-yl)ethane-1,1-diyldiphosphonic acid (EMIDP), and the chemical was labeled with 99mTc to produce [99mTc]-EMIDP (2). The biodistribution of [99mTc]-EMIDP was investigated in normal mice, and the radiochemical was evaluated as a bone-imaging agent in rabbits. From these studies, the investigators concluded that [99mTc]-EMIDP bound selectively to the skeletal tissue in rabbits, which is superior to the selectivity of 99mTc-labeled methylene diphosphonate ([99mTc]-MDP), another BP that is commonly used in the clinic. In a continued effort to explore the possibility of generating a high-potency ZL derivative similar to EMIDP, Lin et al. investigated optimization of the linker chain between the imidazolyl and the germinal BP group in the structure of ZL (1). For this, a ZL derivative, 1-hydroxy-3-(1H-imidazol-1-yl)propane-1,1-diyldiphosphonic acid (IPrDP) was synthesized, labeled with 99mTc (to obtain [99mTc]-IPrDP), and investigated for its biodistribution in normal mice. In addition, the bone-imaging properties of [99mTc]-IPrDP with single-photon emission computed tomography (SPECT) imaging were compared with those of [99mTc]-ZL in rabbits.
Related Resource Links
Related chapters in MICAD
Protein and mRNA sequence of human farnesyl diphosphate synthase
Gene information regarding human farnesyl diphosphate synthase (GeneID: 2224)
Farnesyl diphosphate synthase in Online Mendelian Inheritance in Man (OMIM)
Structure of farnesyl diphosphate synthase complexed with a bisphosphonate
Farnesyl diphosphate synthase in Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathways
Clinical trials with bisphosphonates (or diphosphonates)
Synthesis
[PubMed]
The synthesis and labeling of IPrDP with 99m Tc has been described by Lin et al. (1). Both the radiochemical purity and radiochemical yield of [99mTc]-IPrDP were reported to be 97.2%, as determined with thin-layer chromatography. The specific activity (n = 3 determinations) of the radiochemical was 185 ± 17 MBq/nmol (5 ± 0.46 mCi/nmol).
For use as a control, [99mTc]-ZL was also prepared with the same method as described for the production of [99mTc]-IPrDP (1). The radiochemical purity, radiochemical yield, and specific activity of [99mTc]-ZL were not reported.
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
The in vitro stability of [99mTc]-IPrDP was determined by measuring the radiochemical purity of the radiolabeled compound after incubating a fresh preparation of the tracer (buffer not described) for up to 6 h at room temperature (1). The labeled chemical was reported to maintain a radiochemical purity of >95% for at least 6 h at room temperature.
Animal Studies
Rodents
[PubMed]
The biodistribution of [99mTc]-IPrDP (1.85 MBq (0.05 mCi)) was studied in normal Institute of Cancer Research mice (1). The animals (n = 5 mice/group) were injected with the radioligand through the tail vein and euthanized at predetermined time points ranging from 5 min postinjection (p.i.) to 240 min p.i. to determine the amount of radioactivity accumulated in all the major organs, including bone. Data obtained from this study was expressed as a percent of injected dose per gram tissue (% ID/g).
A control set of mice (n = 5 animals/group) were injected with [99mTc]-ZL (1.85 MBq (0.05 mCi)) and treated as described above.
With [99mTc]-IPrDP, the amount of radioactivity accumulated in the bone was 5.37 ± 0.36% ID/g at 5 min p.i., peaked at 11.14 ± 1.85% ID/g at 60 min p.i., and decreased to 8.46 ± 0.44 at 240 min p.i. With [99mTc]-ZL, uptake in the bone was 3.19 ± 0.45% ID/g at 5 min p.i., peaked at 11.7 ± 1.54% ID/g at 120 min p.i., and decreased to 9.95 ± 0.43% ID/g at 240 min p.i. The accumulation of radiolabel in the liver with [99mTc]-IPrDP increased from ~3.5% ID/g to ~5.5% ID/g from 5 min p.i. to 240 min p.i., whereas with [99mTc]-ZL the uptake was >30% ID/g at all the time points. A similar trend was observed in the spleen (<4% ID/g for [99mTc]-IPrDP and >15% ID/g with [99mTc]-ZL at all time points). In the kidney, the uptake of radioactivity from [99mTc]-IPrDP decreased from 13.80 ± 1.34% ID/g at 5 min p.i. to 4.54 ± 0.26% ID/g at 240 min p.i., and with [99mTc]-ZL the amount of accumulated tracer decreased from 13.10 ± 0.74% ID/g to 2.13 ± 0.47% ID/g during the same period. The bone/blood ratio with [99mTc]-IPrDP increased from 0.76 at 5 min p.i. to 16.1 at 60 min p.i., but with [99mTc]-ZL this ratio was ~1 and ~3.3 at 5 min p.i. and 60 min p.i., respectively.
Other Non-Primate Mammals
[PubMed]
The bone-imaging properties of [99mTc]-IPrDP were investigated in rabbits (n = 1 animal/tracer) and compared with those of [99mTc]-ZL in the same animals (1). The rabbits were anesthetized with a mixture of 0.2 g ketamine and 10 mg diazepam and injected with either [99mTc]-IPrDP or [99mTc]-ZL (155 MBq (4.2 mCi)) through the marginal ear vein; the mice were then scanned to acquire SPECT images at various time points. From the images, it was clear that both radiocompounds accumulated mainly in the skeleton, kidneys, and the urinary bladder of the animals. The rabbit skeleton was clearly visible with [99mTc]-IPrDP at 1 h p.i., indicating a selective binding of the tracer to the bone and rapid clearance from the soft tissues as observed during the biodistribution studies with mice (see above). The bone/soft tissue uptake (B/T) ratios were calculated from the various images as detailed by Lin et al. (1). The B/T ratios with [99mTc]-IPrDP were observed to be higher than those of [99mTc]-ZL at all time points. This indicated that, compared to [99mTc]-ZL, the radioactivity from [99mTc]-IPrDP was rapidly cleared from the soft tissue of these animals.
In another study, rabbits were injected with [99mTc]-IPrDP, [99mTc]-ZL, and [99mTc]-MDP (n = 1 animal/tracer), and static images were acquired from the animals at different time points ranging from 1 h p.i. to 4 h p.i (1). From the images, it was evident that the skeletal profile of the animals generated with [99mTc]-IPrDP was superior to skeletal profiles obtained with either [99mTc]-ZL or [99mTc]-MDP from 2 h p.i. onward.
From these studies, the investigators concluded that [99mTc]-IPrDP had superior biodistribution (in mice) and bone-imaging properties (in rabbits) compared to either [99mTc]-ZL or [99mTc]-MDP (1).
References
- 1.
- Lin J., Qiu L., Cheng W., Luo S., Ye W. Preparation and in vivo biological investigations on a novel radioligand for bone scanning: technetium-99m-labeled zoledronic acid derivative. Nucl Med Biol. 2011;38(5):619–29. [PubMed: 21718936]
- 2.
- Lin J., Luo S., Chen C., Qiu L., Wang Y., Cheng W., Ye W., Xia Y. Preparation and preclinical pharmacological study on a novel bone imaging agent (99m)Tc-EMIDP. Appl Radiat Isot. 2010;68(9):1616–22. [PubMed: 20363146]
- 3.
- Kimmel D.B. Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J Dent Res. 2007;86(11):1022–33. [PubMed: 17959891]
- 4.
- Zhang Y., Cao R., Yin F., Hudock M.P., Guo R.T., Krysiak K., Mukherjee S., Gao Y.G., Robinson H., Song Y., No J.H., Bergan K., Leon A., Cass L., Goddard A., Chang T.K., Lin F.Y., Van Beek E., Papapoulos S., Wang A.H., Kubo T., Ochi M., Mukkamala D., Oldfield E. Lipophilic bisphosphonates as dual farnesyl/geranylgeranyl diphosphate synthase inhibitors: an X-ray and NMR investigation. J Am Chem Soc. 2009;131(14):5153–62. [PMC free article: PMC2753403] [PubMed: 19309137]
- 5.
- Mitterhauser M., Toegel S. Radiopharmaceutical considerations on bone seeker uptake: should we learn from therapeutical targets of bisphosphonates? Nucl Med Biol. 2011;38(5):617–8. [PubMed: 21718935]
- 6.
- Widler L., Jaeggi K.A., Glatt M., Muller K., Bachmann R., Bisping M., Born A.R., Cortesi R., Guiglia G., Jeker H., Klein R., Ramseier U., Schmid J., Schreiber G., Seltenmeyer Y., Green J.R. Highly potent geminal bisphosphonates. From pamidronate disodium (Aredia) to zoledronic acid (Zometa). J Med Chem. 2002;45(17):3721–38. [PubMed: 12166945]
- 7.
- Asikoglu M., Durak F.G. The rabbit biodistribution of a therapeutic dose of zoledronic acid labeled with Tc-99m. Appl Radiat Isot. 2009;67(9):1616–21. [PubMed: 19457677]
- 8.
- Smith M.R. Osteoclast targeted therapy for prostate cancer: bisphosphonates and beyond. Urol Oncol. 2008;26(4):420–5. [PMC free article: PMC3090666] [PubMed: 18593621]
Publication Details
Author Information and Affiliations
Publication History
Created: July 7, 2011; Last Update: September 8, 2011.
Copyright
Publisher
National Center for Biotechnology Information (US), Bethesda (MD)
NLM Citation
Chopra A. 99mTc-Labeled 1-hydroxy-3-(1H-imidazol-1-yl)propane-1,1-diyldiphosphonic acid (IPrDP) 2011 Jul 7 [Updated 2011 Sep 8]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
 In vitro
In vitro