NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
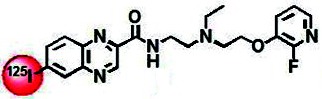
| Chemical name: | [125I]N-[2-[N-Ethyl-N-[2-(2-fluoropyridin-3-yloxy)ethyl]amino]ethyl]-6-iodoquinoxaline-2-carboxamide dihydrochloride salt |
|
| Abbreviated name: | [125I]56 | |
| Synonym: | [131I]56 | |
| Agent Category: | Compounds | |
| Target: | Melanin | |
| Target Category: | Others | |
| Method of detection: | Single-photon emission computed tomography (SPECT), planar imaging | |
| Source of signal / contrast: | 125I | |
| Activation: | No | |
| Studies: |
| Chemical structure of [125I]56 (1). |
Background
[PubMed]
Radioiodinated N-[2-[N-ethyl-N-[2-(2-fluoropyridin-3-yloxy)ethyl]amino]ethyl]-6-iodoquinoxaline-2-carboxamide dihydrochloride salt, abbreviated as [125I]56, is a quinoxaline benzamide (BZA) derivative that was synthesized for melanin-targeted radionuclide imaging and therapy of melanoma (1).
BZA derivatives represent a versatile class of aromatic compounds that possess a common structure element of Ph-CONH(CH2)mNR2 (m = 1, 2) and exhibit comparable properties, including high and specific binding with melanin in melanoma cells and melanocytes (2-4). Some of these compounds, such as N-(2-diethylaminoethyl)-4-[123I]iodobenzamide ([123I]BZA) and [123I]-N-(2-diethylaminoethyl)-2-iodobenzamide ([123I]BZA2), have been successfully evaluated in melanoma patients, showing high sensitivity and selectivity in the detection of melanoma and its metastases (5-8).
The promising results with [123I]BZA have prompted great efforts from several groups in screening BZA analogs (2, 9, 10). Among them is a group of investigators in France who synthesized a series of BZA derivatives via structure-activity studies (11, 12). On the basis of the structure of the lead agent [123I]BZA, they synthesized a group of spermidine BZA derivatives by replacing the diethylaminoethyl moiety in the BZA structure with a triamine (spermidine). Spermidine BZA derivatives exhibit high affinity for melanin comparable to that of [123I]BZA; however, these compounds exhibit less accumulation in tumors than [123I]BZA in animal models of melanoma (13). More recently, the investigators generated a class of heteroaromatic BZA analogs by incorporating the heteroaromatic structure in place of the benzene moiety to take advantage of the polycyclic aromatic compounds that display a strong affinity for melanin while keeping the lipophilic side chain (14). The heteroaromatic analogs, [125I]5a through [125I]5I, showed high specific and long-lasting uptake in the melanoma, which is favorable for combined imaging and therapy. At the same time, the investigators also identified a group of quinoxaline analogs; radioiodinated derivative 3 (ICF01012) is one of these compounds, which show the most favorable pharmacokinetic properties for radionuclide therapy (15, 16). The rapid and specific tumor uptake of quinoxaline compounds also suggests that they are potentially valuable for radionuclide imaging. For radiofluorination, ICF01012 was modified by incorporating 2- or 6-fluoropyridine in the N,N-diethylethylenediamine framework of ICF01012. This strategy allows nucleophilic heteroaromatic radiofluorination of corresponding halogeno- or nitro-precursors without the need for an additional electron-withdrawing substituent in the aromatic ring. Fluoropyridine was introduced on the tertiary amine either directly or in combination with various linkers, which resulted in a group of amide tracers, such as the agents [125I]56 and [18F]44 (compound 56 is the dihydrochloride salt of 44) (1). These derivatives showed favorable properties for combined radionuclide imaging (18F, 125I) and therapy (131I) of melanoma using a single chemical structure. The following is a list of some representative agents that were synthesized and tested by the investigators from the group in France.
BZA derivatives: [125/123I]BZA, [125I]BZA2, [125I]BZ18, and [125I]5a through [125I]5I; and quinoxaline derivatives: [125/131I]ICF01012 (or [125/131I]3), [125I]56, and [18F]44.
This chapter summarizes the data of imaging studies obtained with [125I]56 (1).
Synthesis
[PubMed]
Maisonial et al. described the chemical synthesis and characterization of compound 56 in detail (1). Compound 56 was synthesized starting from the corresponding stannane precursor 76 (N-[2-[N-ethyl-N-[2-(2-fluoropyridin-3-yloxy)ethyl]amino]ethyl]-6-tributylstannylquinoxaline-2-carboxamide). The formula of compound 56 was C20H21FIN5O2,2HCl,H2O. The chemical yield was 91%. Radiolabeled versions of compound 56, [125I]56 and [131I]56, were synthesized with a classic electrophilic radioiodo-demetalation reaction, using no-carrier-added [125I]NaI and [131I]NaI, respectively, in acidic medium (citrate buffer) in the presence of chloramine-T monohydrate. The radiochemical yield and purity of [125I]56 were 53% and 99.7%, respectively, and the specific activity was 31.9 GBq/μmol (0.86 Ci/µmol). [131I]56 was obtained with a specific activity of 106.9 GBq/μmol (2.89 Ci/µmol) and a radiochemical purity of 98.5%; the radiochemical yield of [131I]56 was not reported.
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
The in vitro data of the lead compound 3 were summarized in the literature regarding [125I]ICF01012 (or [125I]3) (15, 16). In vitro studies with cells were not performed for the compound 56.
Animal Studies
Rodents
[PubMed]
Maisonial et al. performed a biodistribution study in C57B16 mice (n = 3) bearing melanoma B16F0 tumors after injection of 2.7 MBq (0.073 mCi) [125I]56, with [125I]3 as the reference (1). High tumor accumulation was observed at 1 h, and it increased gradually to a maximum at 3–6 h. The radioactivity continued to be measurable for at least 5 days. The tumor uptake levels were12.7 ± 2.3, 20.2 ± 4.2, 18.8 ± 1.6, 18.2 ± 0.6, 10.3 ± 1.7, 5.2 ± 1.2, 3.2 ± 0.4, 1.7 ± 0.4, 0.6 ± 0.3% injected dose per gram tissue (ID/g) at 1, 3, and 6 h and 1, 3, 5, 7, 10, and 14 days after injection, respectively. In comparison, the tumor uptake levels of [125I]3 were 21.8 ± 6.6, 26.3 ± 6.6, 29.6 ± 8.4, 28.0 ± 8.2, 12.3 ± 3.7, 7.3 ± 3.6, 3.4 ± 0.3, and 1.9 ± 0.4% ID/g 1, 3, and 6 h and 1, 3, 5, 7, and 10 days after injection. [125I]56 showed a rapid excretion (20% remaining after 24 h), similar to that observed with [125I]3. These results suggest that [125I]56 maintains the high tumor affinity, giving rapid and long-lasting tumor radioactivity uptake. The long-lasting tumor radioactivity uptake led to a calculated biological half-life of 38 h for [131I]56 and a tumor-absorbed dose of 1.12 Gy/MBq of injected [131I]56. Although these values were lower than those obtained with [131I]3 (49.3 h and 1.71 Gy/MBq injected), they were in a range compatible with radionuclide therapy for melanoma No blocking studies were performed.
Serial scintigraphic images also illustrated rapid clearance from nontarget organs, allowing well-defined images of the tumor. In nontarget organs of liver, lung, or kidney (n = 2 mice), radioactive uptake decreased to a very low level after 3 h and was undetectable after 24 h. The tumor/muscle ratios were 23 and 37 at 1 h and 3 h after injection, respectively. The pigmented structures of the eyes exhibited a radioactivity uptake profile similar to that of the tumor. High radioactive uptake was also visualized in the throat area, mainly in the salivary glands and thyroid, likely due to a nonspecific concentration. Analyses on collected urine and feces showed an imbalance between urinary and fecal elimination routes, at 38.1% and 36.1%, respectively, for the 0−24 h period and 45.1% and 38.7%, respectively, for the 0−72 h period.
The therapeutic efficacy of [131I]56 was assessed in C57B16 mice bearing subcutaneous B16F0 melanoma (n = 10 mice). Two doses of [131I]56 (2 × 18.5 MBq) were administered at day 6 and day 10, respectively. B16F0 tumors in the untreated group (n = 10 mice) showed exponential growth, with a tumor-doubling time of 2.8 ± 0.3 days, whereas [131I]56 inhibited the in vivo growth of B16F0 tumors (doubling time, 3.8 ± 0.3 days) (P < 0.001). The concentration of [131I]56 used here was much lower than its 50% lethal dose (data not shown).
In summary, Maisonial et al. synthesized 14 new iodinated and fluorinated analogs of the lead compound 3 ([131I]3) (1). All of these tracers contain a 2- or 6-fluoropyridine moiety incorporated in the N,N-diethylethylenediamine scaffold. Most of these novel radioiodinated compounds showed significant tumor retention, especially the derivative [125/131I]56, which presented high and long-lasting tumor uptake combined with a rapid clearance from nontarget organs, offering both diagnostic (125I and 18F) and therapeutic (131I) potentialities (1).
References
- 1.
- Maisonial A., Kuhnast B., Papon J., Boisgard R., Bayle M., Vidal A., Auzeloux P., Rbah L., Bonnet-Duquennoy M., Miot-Noirault E., Galmier M.J., Borel M., Askienazy S., Dolle F., Tavitian B., Madelmont J.C., Moins N., Chezal J.M. Single photon emission computed tomography/positron emission tomography imaging and targeted radionuclide therapy of melanoma: new multimodal fluorinated and iodinated radiotracers. J Med Chem. 2011;54(8):2745–66. [PubMed: 21417462]
- 2.
- Oltmanns D., Eisenhut M., Mier W., Haberkorn U. Benzamides as melanotropic carriers for radioisotopes, metals, cytotoxic agents and as enzyme inhibitors. Curr Med Chem. 2009;16(17):2086–94. [PubMed: 19519383]
- 3.
- Chehade F., De Labriolle-Vaylet C., Michelot J., Moins N., Moreau M.F., Hindie E., Papon J., Escaig F., Galle P., Veyre A. Distribution of I-BZA (N-2-diethylaminoethyl-4-iodobenzamide) in grafted melanoma and normal skin: a study by secondary ion mass spectroscopy. Cell Mol Biol (Noisy-le-grand). 2001;47(3):529–34. [PubMed: 11441960]
- 4.
- Greguric I., Taylor S.R., Denoyer D., Ballantyne P., Berghofer P., Roselt P., Pham T.Q., Mattner F., Bourdier T., Neels O.C., Dorow D.S., Loc'h C., Hicks R.J., Katsifis A. Discovery of [18F]N-(2-(diethylamino)ethyl)-6-fluoronicotinamide: a melanoma positron emission tomography imaging radiotracer with high tumor to body contrast ratio and rapid renal clearance. J Med Chem. 2009;52(17):5299–302. [PubMed: 19691348]
- 5.
- Bacin F., Michelot J., Bonafous J., Veyre A., Moreau M.F., Kemeny J.L., Chossat F., Bekhechi D. Clinical study of [123I] N-(2-diethylaminoethyl)-4-iodobenzamide in the diagnosis of primary and metastatic ocular melanoma. Acta Ophthalmol Scand. 1998;76(1):56–61. [PubMed: 9541435]
- 6.
- Michelot J.M., Moreau M.F., Veyre A.J., Bonafous J.F., Bacin F.J., Madelmont J.C., Bussiere F., Souteyrand P.A., Mauclaire L.P., Chossat F.M. et al. Phase II scintigraphic clinical trial of malignant melanoma and metastases with iodine-123-N-(2-diethylaminoethyl 4-iodobenzamide). J Nucl Med. 1993;34(8):1260–6. [PubMed: 8326382]
- 7.
- Mansard S., Papon J., Moreau M.F., Miot-Noirault E., Labarre P., Bayle M., Veyre A., Madelmont J.C., Moins N. Uptake in melanoma cells of N-(2-diethylaminoethyl)-2-iodobenzamide (BZA2), an imaging agent for melanoma staging: relation to pigmentation. Nucl Med Biol. 2005;32(5):451–8. [PubMed: 15982575]
- 8.
- Moins N., D'Incan M., Bonafous J., Bacin F., Labarre P., Moreau M.F., Mestas D., Noirault E., Chossat F., Berthommier E., Papon J., Bayle M., Souteyrand P., Madelmont J.C., Veyre A. 123I-N-(2-diethylaminoethyl)-2-iodobenzamide: a potential imaging agent for cutaneous melanoma staging. Eur J Nucl Med Mol Imaging. 2002;29(11):1478–84. [PubMed: 12397467]
- 9.
- Labarre P., Papon J., Moreau M.F., Moins N., Bayle M., Veyre A., Madelmont J.C. Melanin affinity of N-(2-diethylaminoethyl)-4-iodobenzamide, an effective melanoma imaging agent. Melanoma Res. 2002;12(2):115–21. [PubMed: 11930107]
- 10.
- Moins N., Papon J., Seguin H., Gardette D., Moreau M.F., Labarre P., Bayle M., Michelot J., Gramain J.C., Madelmont J.C., Veyre A. Synthesis, characterization and comparative biodistribution study of a new series of p-iodine-125 benzamides as potential melanoma imaging agents. Nucl Med Biol. 2001;28(7):799–808. [PubMed: 11578901]
- 11.
- Desbois N., Gardette M., Papon J., Labarre P., Maisonial A., Auzeloux P., Lartigue C., Bouchon B., Debiton E., Blache Y., Chavignon O., Teulade J.C., Maublant J., Madelmont J.C., Moins N., Chezal J.M. Design, synthesis and preliminary biological evaluation of acridine compounds as potential agents for a combined targeted chemo-radionuclide therapy approach to melanoma. Bioorg Med Chem. 2008;16(16):7671–90. [PubMed: 18656367]
- 12.
- Labarre P., Papon J., Rose A.H., Guerquin-Kern J.L., Morandeau L., Wu T.D., Moreau M.F., Bayle M., Chezal J.M., Croisy A., Madelmont J.C., Turner H., Moins N. Melanoma affinity in mice and immunosuppressed sheep of [(125)I]N-(4-dipropylaminobutyl)-4-iodobenzamide, a new targeting agent. Nucl Med Biol. 2008;35(7):783–91. [PubMed: 18848663]
- 13.
- Moreau M.F., Papon J., Labarre P., Moins N., Borel M., Bayle M., Bouchon B., Madelmont J.C. Synthesis, in vitro binding and biodistribution in B16 melanoma-bearing mice of new iodine-125 spermidine benzamide derivatives. Nucl Med Biol. 2005;32(4):377–84. [PubMed: 15878507]
- 14.
- Chezal J.M., Papon J., Labarre P., Lartigue C., Galmier M.J., Decombat C., Chavignon O., Maublant J., Teulade J.C., Madelmont J.C., Moins N. Evaluation of radiolabeled (hetero)aromatic analogues of N-(2-diethylaminoethyl)-4-iodobenzamide for imaging and targeted radionuclide therapy of melanoma. J Med Chem. 2008;51(11):3133–44. [PubMed: 18481842]
- 15.
- Bonnet M., Mishellany F., Papon J., Cayre A., Penault-Llorca F., Madelmont J.C., Miot-Noirault E., Chezal J.M., Moins N. Anti-melanoma efficacy of internal radionuclide therapy in relation to melanin target distribution. Pigment Cell Melanoma Res. 2010;23(5):e1–11. [PubMed: 20444199]
- 16.
- Bonnet-Duquennoy M., Papon J., Mishellany F., Labarre P., Guerquin-Kern J.L., Wu T.D., Gardette M., Maublant J., Penault-Llorca F., Miot-Noirault E., Cayre A., Madelmont J.C., Chezal J.M., Moins N. Targeted radionuclide therapy of melanoma: anti-tumoural efficacy studies of a new 131I labelled potential agent. Int J Cancer. 2009;125(3):708–16. [PubMed: 19437532]
- PubMedLinks to PubMed
- Review N-[2-[N-Ethyl-N-[2-(2-[(18)F]fluoropyridin-3-yloxy)ethyl]amino]ethyl]-6-iodoquinoxaline-2-carboxamide.[Molecular Imaging and Contrast...]Review N-[2-[N-Ethyl-N-[2-(2-[(18)F]fluoropyridin-3-yloxy)ethyl]amino]ethyl]-6-iodoquinoxaline-2-carboxamide.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review Radioiodinated N-(2-diethylaminoethyl)-6-iodoquinoxaline-2-carboxamide dihydrochloride salt.[Molecular Imaging and Contrast...]Review Radioiodinated N-(2-diethylaminoethyl)-6-iodoquinoxaline-2-carboxamide dihydrochloride salt.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review [(125)I]N-(4-Dipropylaminobutyl)-4-iodobenzamide.[Molecular Imaging and Contrast...]Review [(125)I]N-(4-Dipropylaminobutyl)-4-iodobenzamide.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review Radiolabeled (hetero)aromatic analogs of N-(2-diethylaminoethyl)-4-iodobenzamide for imaging and therapy of melanoma.[Molecular Imaging and Contrast...]Review Radiolabeled (hetero)aromatic analogs of N-(2-diethylaminoethyl)-4-iodobenzamide for imaging and therapy of melanoma.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review N-(2-Diethylaminoethyl)-4-[(18)F]fluorobenzamide for imaging melanoma.[Molecular Imaging and Contrast...]Review N-(2-Diethylaminoethyl)-4-[(18)F]fluorobenzamide for imaging melanoma.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- [125I]N-[2-[N-Ethyl-N-[2-(2-fluoropyridin-3-yloxy)ethyl]amino]ethyl]-6-iodoquino...[125I]N-[2-[N-Ethyl-N-[2-(2-fluoropyridin-3-yloxy)ethyl]amino]ethyl]-6-iodoquinoxaline-2-carboxamide dihydrochloride salt - Molecular Imaging and Contrast Agent Database (MICAD)
Your browsing activity is empty.
Activity recording is turned off.
See more...

 In vitro
In vitro