Table
In vitro Rodents
Background
[PubMed]
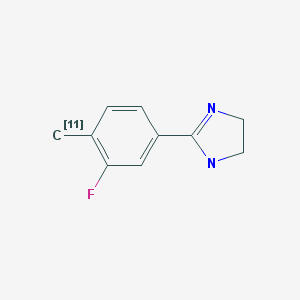
Two major imidazoline binding sites (I1R and I2R) have been identified (1, 2). The I1R and I2R exhibit high affinities for clonidine and idazoxan, respectively. Clonidine and its analogs mediate effects independent of α2-adrenoceptor at the IR receptors. IR receptors are widely distributed in the central and peripheral nervous systems and in various organs such as the pancreas, liver, kidney, lung, and heart (3-6). I1R is associated with hypertension (7), whereas I2R is associated with depression (8), Alzheimer’s disease (9), Parkinson’s disease (10), Huntington’s disease (10), and glial cell tumors (11). High densities of I2R have been observed in the arcuate nucleus, interpeduncular nucleus, pineal gland, and ventricles in human brain (12). The I2R gene has not been identified. Tesson et al. (13) showed that I2R is localized to the mitochondrial outer membrane of the human and rabbit liver. Anastassiadou et al. (14) showed that 2-(3-fluoro-4-tolyl)-4,5-dihydro-1H-imidazole (FTIMD) has a high and selective affinity for I2R. Kawamura et al. (15) evaluated [11C]FTIMD as a positron emission tomography (PET) agent for the noninvasive study of I2R in the brain and peripheral organs.
Related Resource Links:
- Chapters in MICAD (Imidazoline receptors)
- Gene information in NCBI (I1R, α2-adrenoceptors)
- Articles in Online Mendelian Inheritance in Man (OMIM) (I1R, α2-adrenoceptors)
- Clinical trials (Clonidine)
- Drug information in FDA (Clonidine)
Synthesis
[PubMed]
[11C]FTIMD was synthesized remotely via a reaction of [11C]methyl iodine (produced from [11C]CO2) with the tributylstannyl precursor in the presence of tris(dibenzylideneacetone)dipalladium(0) and tri(O-tolyl)phosphine in dimethylformamide for 5 min at 130°C (15). [11C]FTIMD was purified with high-performance liquid chromatography, with a radiochemical yield of 5.4 ± 2.0% (n = 7) from [11C]CO2 at end of bombardment (EOB). The radiochemical purity was >95%, and the specific activity was 108 ± 33 GBq/μmol (2.9 ± 0.9 Ci/μmol) at end of synthesis (EOS). Total time of synthesis was 30 min from EOB. To prepare [11C]FTIMD with ultra-high specific activity (4,470 ± 1,660 GBq/μmol (120 ± 45 Ci/μmol) at EOS), [11C]methyl iodine (produced from iodination of [11C]methane) was used with (n = 11) (16). The cLogD (pH 7.4) value for FTIMD was 1.42.
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
On the basis of in vitro competition binding studies, Anastassiadou et al. (14) reported inhibition constant (Ki) values for FTIMD of 3.0 nM for I2R, >10,000 nM for I1R, >10,000 nM for α1-adrenoceptor, and >10,000 nM for α2-adrenoceptor.
In an in vitro autoradiography study, the distribution pattern of [¹¹C]FTIMD (200 nM) in the monkey brain was studied (17). The rank order of radioactivity was caudate > hippocampus > globus pallidus > putamen > amygdala > thalamus > cortex > cerebellum, which was similar to that of [³H]idazoxan binging to I₂R sites in the human brain (12). The specific binding of [¹¹C]FTIMD accounted for >97% of total binding in the brain regions expressing I₂R using 10,000 nM FTIMD and BU224 as I2R inhibitor ligands.
Animal Studies
Rodents
[PubMed]
Ex vivo biodistribution of 11–20 MBq (0.30–0.54 mCi) [11C]FTIMD was studied in normal rats (n = 4/group) at 5, 15, and 30 min after injection (15). The tissue with the highest accumulation (standard uptake value) at 5 min after injection was the kidney (12.20), followed by the lung (4.35), brain (2.51), liver (2.49), small intestine (2.29), spleen (2.12), pancreas (2.08), heart (1.22), muscle (1.17), and blood (0.38). Most tissues showed moderate to rapid washout, except for the liver, which showed a gradual increase. The brain/blood ratio at 15 min after injection was 7.0. Co-injection of FTIMD (1 mg/kg) reduced the brain accumulation and brain/blood ratio by 22% and 32% (P < 0.05), respectively. Co-injection of another I2R inhibitor BU224 (1 mg/kg) reduced the brain accumulation and brain/blood ratio by 21% and 33% (P < 0.05), respectively. On the other hand, co-injection of I1R/α2-adrenoceptor ligands moxonidine (1 mg/kg) and efaroxan (1 mg/kg) showed little effect on the brain accumulation and brain/blood ratio. [11C]FTIMD remained >98% intact in the brain at 15 min and 30 min after injection, whereas only 57% and 37% remained intact in the plasma at 15 min and 30 min after injection, respectively. In a separate study in mice, specific accumulation of [11C]FTIMD was observed in the liver and pancreas (18).
Kawamura et al. (15) performed dynamic PET scans in four rats (46–117 MBq, 1.24–3.16 mCi) for 60 min after injection of [11C]FTIMD. High levels of radioactivity were detected in the arcuate nucleus, interpeduncular nucleus, hippocampus, and ependymal cell layer. Pretreatment with BU224 (1 mg/kg, 5 min) reduced radioactivity across the brain regions. Multilinear analysis showed a better fit than one- and two-compartment analysis. Regions of analysis showed the highest distribution volume (VT) values in the ependymal cell layer (15.09), followed by the hippocampus (14.24), cortex (13.85), arcuate nucleus (13.54), interpeduncular nucleus (12.87), and cerebellum (10.90). Pretreatment with BU224 (1 mg/kg, 5 min) reduced the VT values by 17%–34%. Using [11C]FTIMD with ultra-high specific activity, the VT values (13.77–19.02) were higher than those of [11C]FTIMD (10.90-15.09) with normal specific activity (16). Pretreatment with BU224 reduced the VT values by 29%–45%, which were significantly higher than those of [11C]FTIMD with normal specific activity (P < 0.05).
Non-Human Primates
[PubMed]
In PET studies, 160 MBq (4.3 mCi) [11C]FTIMD (1.26 nmol) was intravenously injected into a conscious male monkey (17). The radioactivity was accumulated in the thalamus, arcuate nucleus, cingulate cortex, occipital cortex, hippocampus, striatum, cerebellum, and frontal cortex. Pretreatment with BU224 (5 mg/kg, 5 min) reduced the accumulated radioactivity to approximately 66%–75% of the baseline measurement at 15–45 min after injection of [¹¹C]FTIMD.
References
- 1.
- Michel M.C., Ernsberger P. Keeping an eye on the I site: imidazoline-preferring receptors. Trends Pharmacol Sci. 1992;13(10):369–70. [PubMed: 1413085]
- 2.
- Hamilton C.A. Imidazoline receptors, subclassification, and drug-induced regulation. Ann N Y Acad Sci. 1995;763:57–65. [PubMed: 7677375]
- 3.
- El-Ayoubi R., Gutkowska J., Regunathan S., Mukaddam-Daher S. Imidazoline receptors in the heart: characterization, distribution, and regulation. J Cardiovasc Pharmacol. 2002;39(6):875–83. [PubMed: 12021582]
- 4.
- Ernsberger P., Graves M.E., Graff L.M., Zakieh N., Nguyen P., Collins L.A., Westbrooks K.L., Johnson G.G. I1-imidazoline receptors. Definition, characterization, distribution, and transmembrane signaling. Ann N Y Acad Sci. 1995;763:22–42. [PubMed: 7677333]
- 5.
- Ruggiero D.A., Regunathan S., Wang H., Milner T.A., Reis D.J. Distribution of imidazoline receptor binding protein in the central nervous system. Ann N Y Acad Sci. 1995;763:208–21. [PubMed: 7677332]
- 6.
- Lanier S.M., Ivkovic B., Singh I., Neumeyer J.L., Bakthavachalam V. Visualization of multiple imidazoline/guanidinium-receptive sites. J Biol Chem. 1993;268(21):16047–51. [PubMed: 8340426]
- 7.
- Nikolic, K. and D. Agbaba, Imidazoline Antihypertensive Drugs: Selective I(1) -Imidazoline Receptors Activation. Cardiovasc Ther, 2011. [PubMed: 21884004]
- 8.
- Piletz J.E., Zhu H., Ordway G., Stockmeier C., Dilly G., Reis D., Halaris A. Imidazoline receptor proteins are decreased in the hippocampus of individuals with major depression. Biol Psychiatry. 2000;48(9):910–9. [PubMed: 11074229]
- 9.
- Garcia-Sevilla J.A., Escriba P.V., Walzer C., Bouras C., Guimon J. Imidazoline receptor proteins in brains of patients with Alzheimer's disease. Neurosci Lett. 1998;247(2-3):95–8. [PubMed: 9655601]
- 10.
- Reynolds G.P., Boulton R.M., Pearson S.J., Hudson A.L., Nutt D.J. Imidazoline binding sites in Huntington's and Parkinson's disease putamen. Eur J Pharmacol. 1996;301(1-3):R19–21. [PubMed: 8773473]
- 11.
- Martin-Gomez J.I., Ruiz J., Callado L.F., Garibi J.M., Aguinaco L., Barturen F., Javier Meana J. Increased density of I2-imidazoline receptors in human glioblastomas. Neuroreport. 1996;7(8):1393–6. [PubMed: 8856683]
- 12.
- De Vos H., Bricca G., De Keyser J., De Backer J.P., Bousquet P., Vauquelin G. Imidazoline receptors, non-adrenergic idazoxan binding sites and alpha 2-adrenoceptors in the human central nervous system. Neuroscience. 1994;59(3):589–98. [PubMed: 8008210]
- 13.
- Tesson F., Prip-Buus C., Lemoine A., Pegorier J.P., Parini A. Subcellular distribution of imidazoline-guanidinium-receptive sites in human and rabbit liver. Major localization to the mitochondrial outer membrane. J Biol Chem. 1991;266(1):155–60. [PubMed: 1845963]
- 14.
- Anastassiadou M., Danoun S., Crane L., Baziard-Mouysset G., Payard M., Caignard D.H., Rettori M.C., Renard P. Synthesis and pharmacological evaluation of imidazoline sites I1 and I2 selective ligands. Bioorg Med Chem. 2001;9(3):585–92. [PubMed: 11310592]
- 15.
- Kawamura K., Naganawa M., Konno F., Yui J., Wakizaka H., Yamasaki T., Yanamoto K., Hatori A., Takei M., Yoshida Y., Sakaguchi K., Fukumura T., Kimura Y., Zhang M.R. Imaging of I2-imidazoline receptors by small-animal PET using 2-(3-fluoro-[4-11C]tolyl)-4,5-dihydro-1H-imidazole ([11C]FTIMD). Nucl Med Biol. 2010;37(5):625–35. [PubMed: 20610167]
- 16.
- Kawamura K., Kimura Y., Yui J., Wakizaka H., Yamasaki T., Hatori A., Kumata K., Fujinaga M., Yoshida Y., Ogawa M., Nengaki N., Fukumura T., Zhang M.R. PET study using [(11)C]FTIMD with ultra-high specific activity to evaluate I(2)-imidazoline receptors binding in rat brains. Nucl Med Biol. 2012;39(2):199–206. [PubMed: 21958848]
- 17.
- Kawamura K., Maeda J., Hatori A., Okauchi T., Nagai Y., Higuchi M., Suhara T., Fukumura T., Zhang M.R. In vivo and in vitro imaging of I imidazoline receptors in the monkey brain. Synapse. 2011;65(5):452–5. [PubMed: 21370281]
- 18.
- Kawamura K., Yui J., Konno F., Yamasaki T., Hatori A., Wakizaka H., Fujinaga M., Kumata K., Yoshida Y., Ogawa M., Nengaki N., Yanamoto K., Fukumura T., Zhang M.R. Synthesis and evaluation of PET probes for the imaging of I2 imidazoline receptors in peripheral tissues. Nucl Med Biol. 2012;39(1):89–99. [PubMed: 21831654]
Publication Details
Author Information and Affiliations
Publication History
Created: February 19, 2012; Last Update: May 30, 2012.
Copyright
Publisher
National Center for Biotechnology Information (US), Bethesda (MD)
NLM Citation
Leung K. 2-(3-Fluoro-[4-11C]tolyl)-4,5-dihydro-1H-imidazole. 2012 Feb 19 [Updated 2012 May 30]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
 In vitro
In vitro