Table
In vitro Rodents
Background
[PubMed]
Cell proliferation and cell growth in various biological systems in an in vitro setting are routinely measured by the incorporation of radiolabeled (usually with radioactive C-14 or H-3) thymidine (TdR) because it is a necessary and exclusive component required for the synthesis of DNA in an organism (1). However, TdR was observed to be unsuitable for in vivo studies because it is easily catabolized by enzymes after administration and generated results that were complicated to interpret (2-4). Investigators initially developed a carbon (11C)-labeled TdR to study tumor growth with positron emission tomography (PET), but realized that the catabolic products of the labeled compound yielded low tumor/normal tissue contrast ratios (4). Therefore, to circumvent the catabolite issue observed with TdR, some investigators developed TdR analogs that were labeled with either radioactive fluorine (18F) or 11C, were not catabolized in vivo by virtue of the 2’-fluoro substitution, and could be used to monitor or image tumors or other rapidly proliferating tissue (3, 5, 6). Among the various TdR analogs, [18F]-3’-deoxy-3’-fluorothymidine ([18F]FLT) was used to study different cancers in the clinic. This compound is converted into a triphosphate by cytosolic thymidine kinase (TK1), is not incorporated into the DNA strand, and terminates elongation of the DNA chain (7). Because [18F]FLT is only phosphorylated by TK1 and is not incorporated into the DNA, the uptake of this radiochemical is considered only as an indicator of the TK1 enzyme activity. Moreover, the use of TK1 activity as a measure of DNA synthesis is not well established (8). In addition, [18F]FLT is known to produce high background in the liver, bone marrow, and the pelvis, so it is not considered to be a suitable agent to detect malignancies in these tissues or in this region of the body (9).
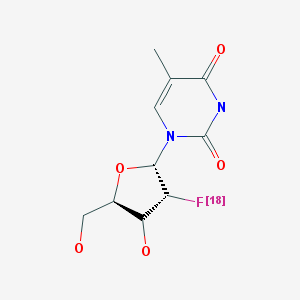
1-(2’-Deoxy-2’-fluoro-β-D-arabinofuranosyl)thymidine (FMAU) is another analog of TdR that was labeled with either 18F or 11C and used in vivo to investigate cell proliferation and tumor imaging (2, 10, 11). FMAU differs from TdR in that a hydrogen atom at the 2’ position of the deoxyarabinose sugar moiety in the molecule is substituted with fluorine, but both molecules possess the same biological properties (e.g., transport across the cell membrane, phosphorylation, and incorporation into cellular DNA) (2, 12). Also, compared to TdR, the clearance of [11C]FMAU from the blood was observed to be slower and showed a higher incorporation in tumors (12). However, the use of [11C]FMAU was limited by the short half-life of 11C, so an 18F-labeled version was produced to image cell proliferation and tumors in patients (10).
Because FMAU is a DNA synthesis inhibitor, it has been used as an antiviral agent and also as an antineoplastic agent for the treatment of cancers (13, 14). FMAU triphosphate was shown to inhibit the hepatitis B virus polymerase enzyme in a concentration-dependent manner, and it has been approved by the United States Food and Drug Administration for evaluation in clinical trials to treat this infection (14).
This chapter details the preclinical and clinical studies performed with [18F]FMAU. Studies performed with [11C]FMAU are presented in a separate chapter in MICAD (www.micad.nih.gov).
Synthesis
[PubMed]
The synthesis of [18F]FMAU was described by Alauddin et al. (15). The initial compound, 2-deoxy-2-[18F]fluoro-1,3,5-tri-O-benzoyl-a-D-arabinofuranose-2 was prepared by the modification of a previously published method (16). Briefly, aqueous [18F]-fluoride was added to a solution of tetrabutylammonium bicarbonate and evaporated azeotropically to dryness with acetonitrile at 80°C under a stream of argon. Subsequently, tetrabutylammonium [18F]-fluoride in dry acetonitrile was added to the dry residue, and the reaction mixture was heated at 80–82°C for 30 min. The mixture was then cooled to room temperature, passed through a Sep-Pak silica gel cartridge, and eluted with ethyl acetate. The solvent was then evaporated at 80°C under a stream of argon, and the product, a radiolabeled fluorosugar, was used for the next step without further purification.
The radiolabeled fluorosugar was dissolved in 1,2-dichloroethane under argon, and hydrogen bromide (HBr) in acetic acid (AcOH) was added to it. The reaction mixture was then heated for 10 min at 80–82°C and diluted with toluene. The HBr/AcOH solvent was evaporated under a stream of argon to obtain a dry, crude product, 1-bromo-2-deoxy-2-[18F]fluoro-3,5-di-O-benzoylarabinofuranose, which was used for the coupling experiment without purification.
A solution of freshly prepared 2,4-bis-O-(trimethylsilyl)thymine in 1,2-dichloroethane was added to 1-bromo-2-deoxy-2-[18F]fluoro-3,5-di-O-benzoylarabinofuranose, and the solution was heated at 97–100°C for 60 min. The reaction mixture was then cooled to room temperature and passed through a Sep-Pak silica gel cartridge. The column was eluted with 10% methanol in dichloromethane, and the solvent was evaporated at 100°C under a stream of argon to recover the crude product, 2’-deoxy-2’-[18F]fluoro-3’,5’-di-O-benzoyl-5-methyl-1-β/β-D-arabinofuranosyluracil.
The crude mixture of 2’-deoxy-2’-[18F]fluoro-3’,5’-di-O-benzoyl-5-methyl-1-β/β-D-arabinofuranosyluracil was dissolved in methanol, and a 1 M sodium methoxide solution in methanol was added. The mixture was then heated for 5 min at reflux, cooled, and neutralized with 2 N hydrochloric acid in methanol. The methanol was evaporated, and the remaining contents were diluted with acetonitrile/water and purified with high-performance liquid chromatography (HPLC) on a C18 reverse-phase column. The appropriate fractions were collected and evaporated to dryness. The pure product, [18F]FMAU, was dissolved again in saline, filter-sterilized, and analyzed with analytical HPLC.
The average radiochemical yield from this synthesis was reported to be 25% (n = 4) with a radiochemical purity of >99%. The synthesis time was 3.5–4.0 h from the end of bombardment, and the average specific activity of the product was reported to be 2,300 mCi/μmol (85 TBq/μmol) (15).
Mangner et al. (17) published another multi-step procedure for the synthesis of [18F]FMAU based on the procedure of Howell and colleagues (18, 19). Mangner et al. reported a decay-corrected radiochemical yield of 35–45%, and the radiochemical purity was routinely >98% (17). The synthesis time required was ~3 h, and the specific activity was >3 Ci/μmol (110 MBq/μmol).
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
Using [14C]FMAU (available commercially) as a surrogate molecule, Alauddin et al. performed in vitro uptake studies of the radiochemical in herpes simplex virus 1 thymidine kinase (tk) gene–transduced MDA-MB-468 cells (tk-positive), a human breast cancer cell line (20). For this study, wild-type cells and vector-only–transduced cells (tk-negative) were used as controls. The tk-transduced cells consistently showed a 14–16-fold (P < 0.001) and a 146–233-fold higher (P < 0.001) incorporation of FMAU compared to the tk-negative and the wild-type cells, respectively. The tk-positive cells had a 2.7–7.1-fold higher and the tk-negative cells had a 1.5–3.0-fold higher incorporation of labeled FMAU compared to the wild-type cells. In all three cell types, ~95% of the total radioactivity was detected in the DNA (20). No competition binding studies were reported.
Animal Studies
Rodents
[PubMed]
The uptake of [14C]FMAU was also investigated in nude mice (n = 5) bearing tk-positive and tk-negative tumors derived from the MDA-MB-468 cells described above (20). The ratio of [14C]FMAU uptake between the tk-positive and the tk-negative tumors 1 h after administration was 3.7 (P < 0.001), and the tk-positive tumor/blood ratio was 4.3 (P < 0.001). At 2 h after administration, the tk-positive/tk-negative tumor cell ratio was 5.5 (P < 0.001), and the tk-positive tumor/blood ratio was 13.4 (P < 0.001). The [14C]FMAU uptake ratio between the tk-positive tumor and other organs was reported to be in the range of 5.5–15.6.
Imaging studies with [18F]FMAU in the five nude, tumor-bearing mice tumors described above showed that the label accumulated primarily in the tk-positive tumors and had a biodistribution that was similar to the 14C homolog (20). However, with this compound a high accumulation was noted in the intestine because it obscured the visibility of a tk-positive tumor on the right flank of the animal. No competition binding studies were reported with these animals.
Other Non-Primate Mammals
[PubMed]
Sun et al. investigated the biodistribution of [18F]FMAU in dogs (n = 5) (21).The animals were injected with the tracer, and imaging studies were performed up to 2.5 h after administration. At the end of each study, select tissue was removed to determine the radioactivity that accumulated. Incorporated radioactivity in DNA was also determined after extraction of the macromolecule. On imaging and tissue analysis, an increased accumulation of the label was apparent in the lymph nodes, stomach, small intestines, and the bone marrow, which corresponded with the degree of cell proliferation observed in these tissues. The highly proliferative tissues, the intestines and the bone marrow, were reported to have an accumulation of ~88% and ~65% of the total activity in DNA, respectively (21). The tissue/muscle ratio in the non-proliferative organs such as the lungs, heart, liver, and the kidneys was 1.0, and only ~10% of the total activity was detected in the DNA of these organs.
Human Studies
[PubMed]
A pilot study was conducted to evaluate [18F]FMAU for DNA synthesis in tumors and to determine its biodistribution in cancer patients (10). The radiopharmaceutical was used to image patients (n = 14) with either prostate, brain, colorectal, lung, or breast cancer. Dynamic PET images were obtained 60 min after the administration of [18F]FMAU, and metabolite clearance was also determined with HPLC in the blood and urine. The normal bone marrow was reported to have a mean standardized uptake value (SUV) of 0.7, and the tumor SUVs were 2.19, 1.28, 2.21, and 2.27–4.42 for lesions in the breast, brain, lungs, and prostate. High SUVs were observed in the liver (SUV 10.07–20.88) and the kidneys (SUV 7.18–15.66), probably because of metabolism and excretion in these organs. Compared to these organs, the bladder had a low mean SUV of 2.03. The investigators reported that 95% of the activity was cleared from blood circulation within 10 min, and 70% of the activity in the urine was intact [18F]FMAU at 60 min after administration (10).
Tehrani et al. investigated the uptake of [18F]FMAU in patients with brain (n = 4) and prostate (n = 6) cancers to determine the easiest approach for image acquisition and analysis (11). The investigators obtained 60-minute dynamic images and determined the mean and maximum SUVs for the tumors. The mean tumor SUVs obtained between 5 and 11 min were reported to correlate with the values obtained between 30 and 60 min (r2 = 0.92; P < 0.0001), and the maximum SUVs also showed a similar correlation. The investigators cautioned that, although this radiopharmaceutical was suitable to obtain usable images of the brain and prostate tumors at 11 min after administration of [18F]FMAU, the study was performed with a small number of patients which means that it would be necessary to perform this study in a larger patient population with a wide range of tumors to generalize this procedure.
Supplemental Information
NIH Support
Some of the studies presented in this chapter were funded by NCI grants CA 83131, CA 82645, and CA 72896.
References
- 1.
- Shields A.F. Positron emission tomography measurement of tumor metabolism and growth: its expanding role in oncology. Mol Imaging Biol. 2006; 8 (3):141–50. [PubMed: 16534552]
- 2.
- Conti P.S. , Hilton J. , Wong D.F. , Alauddin M.M. , Dannals R.F. , Ravert H.T. , Wilson A.A. , Anderson J.H. High performance liquid chromatography of carbon-11 labeled thymidine and its major catabolites for clinical PET studies. Nucl Med Biol. 1994; 21 (8):1045–51. [PubMed: 9234362]
- 3.
- Lu L. , Samuelsson L. , Bergstrom M. , Sato K. , Fasth K.J. , Langstrom B. Rat studies comparing 11C-FMAU, 18F-FLT, and 76Br-BFU as proliferation markers. J Nucl Med. 2002; 43 (12):1688–98. [PubMed: 12468521]
- 4.
- Mankoff D.A. , Shields A.F. , Link J.M. , Graham M.M. , Muzi M. , Peterson L.M. , Eary J.F. , Krohn K.A. Kinetic analysis of 2-[11C]thymidine PET imaging studies: validation studies. J Nucl Med. 1999; 40 (4):614–24. [PubMed: 10210220]
- 5.
- Grierson J.R. , Shields A.F. , Zheng M. , Kozawa S.M. , Courter J.H. Radiosyntheses of labeled beta-pseudothymidine ([C-11]- and [H-3]methyl) and its biodistribution and metabolism in normal and tumored mice. Nucl Med Biol. 1995; 22 (5):671–8. [PubMed: 7581179]
- 6.
- Krohn K.A. , Mankoff D.A. , Eary J.F. Imaging cellular proliferation as a measure of response to therapy. SupplJ Clin Pharmacol. 2001:96S–103S. [PubMed: 11452736]
- 7.
- Reske S.N. , Deisenhofer S. Is 3'-deoxy-3'-(18)F-fluorothymidine a better marker for tumour response than (18)F-fluorodeoxyglucose? Suppl 1Eur J Nucl Med Mol Imaging. 2006; 33 :38–43. [PubMed: 16721567]
- 8.
- Rasey J.S. , Grierson J.R. , Wiens L.W. , Kolb P.D. , Schwartz J.L. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med. 2002; 43 (9):1210–7. [PubMed: 12215561]
- 9.
- Groves A.M. , Win T. , Haim S.B. , Ell P.J. Non-[18F]FDG PET in clinical oncology. Lancet Oncol. 2007; 8 (9):822–30. [PubMed: 17765191]
- 10.
- Sun H. , Sloan A. , Mangner T.J. , Vaishampayan U. , Muzik O. , Collins J.M. , Douglas K. , Shields A.F. Imaging DNA synthesis with [18F]FMAU and positron emission tomography in patients with cancer. Eur J Nucl Med Mol Imaging. 2005; 32 (1):15–22. [PubMed: 15586282]
- 11.
- Tehrani O.S. , Muzik O. , Heilbrun L.K. , Douglas K.A. , Lawhorn-Crews J.M. , Sun H. , Mangner T.J. , Shields A.F. Tumor imaging using 1-(2'-deoxy-2'-18F-fluoro-beta-D-arabinofuranosyl)thymine and PET. J Nucl Med. 2007; 48 (9):1436–41. [PubMed: 17785728]
- 12.
- Bading J.R. , Shahinian A.H. , Vail A. , Bathija P. , Koszalka G.W. , Koda R.T. , Alauddin M.M. , Fissekis J.D. , Conti P.S. Pharmacokinetics of the thymidine analog 2'-fluoro-5-methyl-1-beta-D-arabinofuranosyluracil (FMAU) in tumor-bearing rats. Nucl Med Biol. 2004; 31 (4):407–18. [PubMed: 15093810]
- 13.
- Fanucchi M.P. , Leyland-Jones B. , Young C.W. , Burchenal J.H. , Watanabe K.A. , Fox J.J. Phase I trial of 1-(2'-deoxy-2'-fluoro-1-beta-D-arabinofuranosyl)-5-methyluracil (FMAU). Cancer Treat Rep. 1985; 69 (1):55–9. [PubMed: 2981621]
- 14.
- Korba B.E. , Furman P.A. , Otto M.J. Clevudine: a potent inhibitor of hepatitis B virus in vitro and in vivo. Expert Rev Anti Infect Ther. 2006; 4 (4):549–61. [PubMed: 17009935]
- 15.
- Alauddin M.M. , Conti P.S. , Fissekis J.D. Synthesis of [18F]-labeled 2'-deoxy-2'-fluoro-5-methyl-1-beta-D-arabinofuranosyluracil. J Label Compd Radiopharm. 2002; 45 (7):583–590.
- 16.
- Alauddin M.M. , Conti P.S. , Mathew T. , Fissekis J.D. , Prakash G.K.S. , Watanabe K.A. Stereospecific fluorination of 1,3,5-tri-O-benzoyl-α--ribofuranose-2-sulfonate esters: preparation of a versatile intermediate for synthesis of 2'-[18F]-fluoro-arabinonucleosides. Journal of Fluorine Chemistry. 2000; 106 (1):87–91.
- 17.
- Mangner T.J. , Klecker R.W. , Anderson L. , Shields A.F. Synthesis of 2'-deoxy-2'-[18F]fluoro-beta-D-arabinofuranosyl nucleosides, [18F]FAU, [18F]FMAU, [18F]FBAU and [18F]FIAU, as potential PET agents for imaging cellular proliferation. Synthesis of [18F]labelled FAU, FMAU, FBAU, FIAU. Nucl Med Biol. 2003; 30 (3):215–24. [PubMed: 12745012]
- 18.
- Howell H.G. , Brodfuehrer P.R. , Brundidge S.P. , Benigni D.A. , Sapino C. Antiviral nucleosides. A stereospecific, total synthesis of 2'-fluoro-2'-deoxy-beta-D-arabinofuranosyl nucleoside. J Org Chem. 1988; 53 :85–88.
- 19.
- Tann C.H. , Brodfuehrer P.R. , Brundidge S.P. , Sapino C. , Howell H.G. Fluorocarbohydrates in synthesis. An efficient synthesis of 1-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)-5-iodouracil (b-FIAU) and 1-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)thymine (b-FMAU). J Org Chem. 1985; 50 :3644–3647.
- 20.
- Alauddin M.M. , Shahinian A. , Gordon E.M. , Conti P.S. Direct comparison of radiolabeled probes FMAU, FHBG, and FHPG as PET imaging agents for HSV1-tk expression in a human breast cancer model. Mol Imaging. 2004; 3 (2):76–84. [PubMed: 15296672]
- 21.
- Sun H. , Mangner T.J. , Collins J.M. , Muzik O. , Douglas K. , Shields A.F. Imaging DNA synthesis in vivo with 18F-FMAU and PET. J Nucl Med. 2005; 46 (2):292–6. [PubMed: 15695789]
Publication Details
Author Information and Affiliations
Publication History
Created: January 9, 2008; Last Update: February 14, 2008.
Copyright
Publisher
National Center for Biotechnology Information (US), Bethesda (MD)
NLM Citation
Chopra A. 1-(2’-Deoxy-2’-[18F]-fluoro-β-D-arabinofuranosyl)thymine. 2008 Jan 9 [Updated 2008 Feb 14]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
 In vitro
In vitro