NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
| Chemical name: | [99mTc-N40-1-bzlg0,D-Phe6,Leu-NHEt13,des-Met14]Bombesin(6–14) |
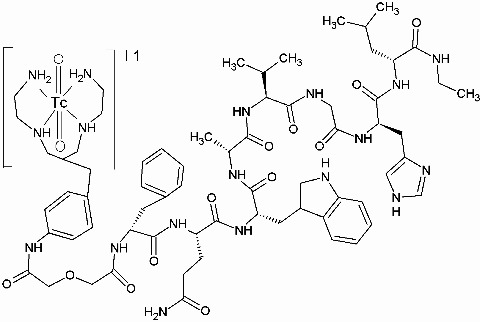
|
| Abbreviated name: | [99mTc]Demobesin 1 | |
| Synonym: | ||
| Agent Category: | Peptide | |
| Target: | Gastrin-releasing peptide receptor (GRPR) | |
| Target Category: | Receptor | |
| Method of detection: | Single-photon emission computed tomography (SPECT); gamma planar imaging | |
| Source of signal / contrast: | 99mTc | |
| Activation: | No | |
| Studies: |
| Structure of [99mTc]Demobesin 1. Click on protein, nucleotide (RefSeq), and gene for more information about gastrin-releasing peptide receptor. |
Background
[PubMed]
[99mTc-N40-1-bzlg0,D-Phe6,Leu-NHEt13,des-Met14]Bombesin (6-14) ([99mTc]Demobesin 1) is a bombesin (BN) derivative that was synthesized and labeled with 99mTc by Nock et al. for molecular imaging of tumors expressing gastrin-releasing peptide receptor (GRPR) (1).
BN is an amphibian neuropeptide consisting of 14 amino acids (pGlu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2) (23), which was first isolated from frog skin in 1970 (4). The search for its mammalian counterpart led to the discovery of gastrin-releasing peptide (GRP), which consists of 27 amino acids. GRP and BN share an identical C-terminal region (-Trp-Ala-Val-Gly-His-Leu-Met-NH2) which is necessary for receptor binding and signal transduction (56). In addition to the release of gastrin, GRP and BN-like peptides also produce a wide range of other biological responses in diverse tissues, such as secretion of endocrine and exocrine glands, maintenance of circadian rhythm, regulation of satiety, and contraction of smooth muscles (7). They also act as potential growth factors for both normal and cancer cells (3, 5, 6). There are four members of the BN receptor family, including three mammalian receptors: GRPR (BB2 or BRS2; 384 amino acids), neuromedin B receptor (NMBR, BB1, or BRS1; 390 amino acids), and BN-like receptor 3 (BB3, BRS3, or orphan; 399 amino acids) (5, 7, 8); the fourth receptor (BB4) has only been found in amphibians. GRPR is the only well characterized receptor of this family. GRPR is a glycosylated, 7-transmembrane, G-protein–coupled receptor that, upon binding with its ligands, gives rise to a complex cascade of intracellular reactions. It is normally found in non-neuroendocrine tissues of the breast and pancreas and in neuroendocrine cells of the brain, gastrointestinal tract, lung, and prostate (9). Interestingly, GRPR is overexpressed in prostate cancer as well as in tumors of the breast, lung, pancreas, ovary, kidney, and gastrointestinal tract. It has been reported that GRPR is expressed at a high density in the intraepithelial neoplasia and primary carcinoma of the prostate, whereas normal prostate tissue and, in most cases, benign prostate hyperplasia are predominantly negative for GRPR (10-13).
GRPR has attracted significant interest as a target for tumor detection, tumor staging, and evaluation of tumor response to therapy (5, 6, 8, 11). A large number of BN derivatives have been developed, and they have been labeled with 99mTc, 177Lu, 67Ga, and 111In for single-photon emission computed tomography and with 64Cu, 68Ga, and 18F for positron emission tomography. The published BN derivatives can be generally classified as truncated BN (6–14 or 7–14) or full-length BN (1-14) analogs (10, 12-18). The truncated BN analogs seem to be favorable for in vivo applications because they are usually more stable than the full-length tetradecapeptides and still bind to the GRPR adequately. However, the full-length peptides offer different labeling methods by attachment of functional groups to the amino acid 1 to 6. In many cases, the amino acids on positions 13 (Leu) and 14 (Met) have been replaced by unnatural amino acids (cyclohexylalanine (CyHAla) and norleucine (Nle)), and Lys has been placed on position 3 for attachment of radiolabels with reactive esters. Spacers, chelators, or radiometals have also been widely used for conjugation and for favorable kinetics (19). The BN derivatives can also be divided into agonists and antagonists (1, 20, 21). By far, most BN derivatives are agonists. Agonists are internalized into and accumulate within cells, and it has been assumed that they exhibit higher uptake by cancer cells than antagonists. However, some reports have shown that uptake of antagonists is much higher than that of agonists because antagonists may have stronger binding for the GRPR than agonists (1, 20).
An optimal BN-like radiotracer needs to meet several requirements: high affinity for GRPR, with rapid and specific tumor uptake; high hydrophilicity, with preferred renal excretion and low hepatobiliary excretion; and high stability but relatively rapid clearance from blood (6). Despite a large number of published derivatives, a valid comparison among them for the feasibility of tumor imaging is difficult because standardization between studies is lacking. Generally speaking, the majority of the radiotracers have relatively high renal and hepatic uptake, resulting in low tumor/liver and tumor/kidney ratios. Nock et al. developed a GRPR antagonist, [99mTc]Demobesin 1, and further tested its biodistribution and feasibility of imaging GRPR-expressing tumors (1).
Synthesis
[PubMed]
Demobesin 1 peptide was synthesized in two consecutive steps: coupling and deprotection (1). The tetraamine chelator precursor N,N’,N’’,N’’’-tetrakis-(tert-butoxycarbonyl)-6-{p-[(carboxymethoxy)acetyl]aminobenzyl}-1,4,8,11-tetraazaundecane (N4(Boc)4-bzlg) was first coupled to the N-terminal of the BN antagonist [D-Phe6,Leu-NHEt13,des-Met14]BN(6–14) through the coupling agent O-(7-azabenzotriazolyl-1,1,3,3-tetramethylammonium hexafluorophosphate. The Boc groups were then removed with trifluoroacetic acid treatment. The peptide conjugate, Demobesin 1, was purified with semipreparative reverse-phase high-performance liquid chromatography (RP-HPLC). The overall yield of Demobesin 1 was ~70%. For radiolabeling, the lyophilized peptide was dissolved in acetic acid/EtOH (8/2 v/v), followed by addition of sodium citrate, pertechnetate generator eluate, and freshly prepared ethanolic SnCl2 solution. The mixture was left to react for 30 min at ambient temperature and was then brought to pH 7 by addition of HCl before RP-HPLC purification of the [99mTc]Demobesin 1. Radiochemical analysis showed that the specific activity and radiochemical yield of [99mTc]Demobesin 1 were 37 GBq/μmol (1 Ci/μmol) and >96%, respectively. [99mTc]Demobesin 1 was stable in the open reaction vial for at least 6 h after labeling. The [99gTc]Demobesin 1 was prepared with similar protocol to that for its [99mTc]Demobesin 1 counterpart at tracer level.
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
The metabolic stability of [99mTc]Demobesin 1 was evaluated in mouse plasma and kidney homogenates (1). After incubation in mouse plasma at 37°C, >85% of the [99mTc]Demobesin 1 remained intact after 1 h. During the first 15 min of incubation, the intact peptide fraction exceeded 90%. Rapid degradation was observed in the kidney preparation, with <5% intact peptide detected after just 5 min of incubation. This result was consistent with the analysis of urine collected 30 min after injection of [99mTc]Demobesin 1 in mice, which showed complete degradation of the original peptide. Two major hydrophilic metabolites were detected in the urine; these were, however, unrelated to 99mTcO4-.
The binding capability and internalization of [99mTc]Demobesin 1 for GRPR were investigated with PC-3 cell membrane homogenates, human tumor biopsy specimens, and PC-3 culture cells (1). [Tyr4]BN peptide served as the positive control and [125I-Tyr4]BN served as the specific radioligand, where [Tyr4]BN was Pyr-Gln-Arg-Tyr-Gly-Asn-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2. Competitive binding assays in PC-3 membrane preparations demonstrated that [125I-Tyr4]BN was displaced by Demobesin 1 from BN binding sites in a monophasic and dose-dependent manner. The 50% inhibitory concentration (IC50) value was 0.7 ± 0.08 nmol, comparable to the IC50 value of [Tyr4]BN (1.5 ± 0.20 nmol). The equilibrium dissociation constant and maximal binding value of [99mTc/99gTc]Demobesin 1 were 0.67 ± 0.10 nmol and 262 ± 13 fmol/mg, respectively. Competition binding experiments in human tumor biopsy specimens showed that Demobesin 1 had high binding affinity in the GRPR-expressing prostate cancer, but not in the NMBR-expressing (gut carcinoid) and BB3-expressing (lung carcinoid) tumors. The IC50 values were 2.6 ± 0.2 nmol in prostate cancer (n = 5) and >1,000 nmol in BB3- or NMBR-expressing tumors (n = 3). Internalization was rapid, but only a small portion (~25% radioactivity) of the [99mTc]Demobesin 1 was detected within the cells after 120 min of incubation with PC-3 culture cells.
Animal Studies
Rodents
[PubMed]
Nock et al. investigated the biodistribution of [99mTc]Demobesin 1 in healthy, male, Swiss albino mice (n = 30) and in female, Swiss, nu/nu mice bearing subcutaneous human PC-3 xenografts (n = 4–6/time point) (1). Each animal was given 148–185 kBq (4–5 μCi) [99mTc]Demobesin 1 (10 pmol of total peptide) via the tail vein. In healthy mice, rapid clearance of the [99mTc]Demobesin 1 was observed via the kidneys, with a small portion of liver uptake. High accumulation in the GRPR-expressing pancreas was detected up to 58.7 ± 6.1% injected dose per gram of tissue (ID/g) at 30 min after injection. The intestine, another GRPR-expressing organ, also had a relatively high accumulation level (7.53 ± 0.85% ID/g at 30 min after injection). Pretreatment of the mice with intraperitoneal administration of 1 mg [Tyr4]BN 35 min before injection strongly blocked uptake of [99mTc]Demobesin 1 in both organs, showing uptake levels of only 2.7 ± 1.1% ID/g in the pancreas and 2.26 ± 0.56% ID/g in the intestine at 30 min after injection (n = 4–6 animals). In mice with PC-3 xenografts (n = 4–6/time point), the uptake of [99mTc]Demobesin 1 was already evident at 1 h after injection in both pancreas (79.5 ± 0.8% ID/g) and tumor (16.2 ± 3.1% ID/g). The high tumor uptake remained at this level (15.61 ± 1.19% ID/g) up to 4 h after injection. Rapid clearance of the [99mTc]Demobesin 1, predominantly via the kidneys, resulted in increased high tumor/background ratios over time. The tumor/nontarget tissue ratios were 12.9 ± 3.3 for the blood, 67.6 ± 13.7 for the muscle, 2.0 ± 0.5 for the liver, and 2.0 ± 0.5 for the kidneys at 1 h after injection, and 23.1 ± 3.6 for the blood, 206.0 ± 21.1 for the muscle, 2.2 ± 0.3 for the liver, and 2.7 ± 0.4 for the kidneys at 4 h after injection.
Blocking studies were performed with intravenous administration of 250 μg [Tyr4]BN along with [99mTc]Demobesin 1 or intraperitoneal administration of 1 mg [Tyr4]BN 35 min before the administration of [99mTc]Demobesin 1 (n = 4–6 mice/group) (1). The uptake of [99mTc]Demobesin 1 in both pancreas and tumor was blocked by the [Tyr4]BN. The uptake level was only 3.26 ± 0.71% ID/g in the pancreas and 2.34 ± 0.33% ID/g in the tumor at 1 h after injection. The tumor/blocked tumor ratios were 6.9 ± 1.3 and 7.7 ± 1.2% ID/g at 1 h and 4 h, respectively.
Static imaging studies were performed in two mice bearing PC-3 tumors with administration of the [99mTc]Demobesin 1 alone in one mouse (unblocked mouse) and together with 250 μg [Tyr4]BN in another mouse (blocked mouse) (1). The images obtained at 1.5 h after injection clearly delineated the tumor in the unblocked mouse. In contrast, the tumor was not visualized in the blocked mouse.
References
- 1.
- Nock B., Nikolopoulou A., Chiotellis E., Loudos G., Maintas D., Reubi J.C., Maina T. [99mTc]Demobesin 1, a novel potent bombesin analogue for GRP receptor-targeted tumour imaging. Eur J Nucl Med Mol Imaging. 2003;30(2):247–58. [PubMed: 12552343]
- 2.
- Maina T., Nock B.A., Zhang H., Nikolopoulou A., Waser B., Reubi J.C., Maecke H.R. Species differences of bombesin analog interactions with GRP-R define the choice of animal models in the development of GRP-R-targeting drugs. J Nucl Med. 2005;46(5):823–30. [PubMed: 15872357]
- 3.
- Smith C.J., Volkert W.A., Hoffman T.J. Gastrin releasing peptide (GRP) receptor targeted radiopharmaceuticals: a concise update. Nucl Med Biol. 2003;30(8):861–8. [PubMed: 14698790]
- 4.
- Erspamer V., Erpamer G.F., Inselvini M. Some pharmacological actions of alytesin and bombesin. J Pharm Pharmacol. 1970;22(11):875–6. [PubMed: 4395815]
- 5.
- Smith C.J., Volkert W.A., Hoffman T.J. Radiolabeled peptide conjugates for targeting of the bombesin receptor superfamily subtypes. Nucl Med Biol. 2005;32(7):733–40. [PubMed: 16243649]
- 6.
- Ananias H.J., de Jong I.J., Dierckx R.A., van de Wiele C., Helfrich W., Elsinga P.H. Nuclear imaging of prostate cancer with gastrin-releasing-peptide-receptor targeted radiopharmaceuticals. Curr Pharm Des. 2008;14(28):3033–47. [PubMed: 18991717]
- 7.
- Ischia J., Patel O., Shulkes A., Baldwin G.S. Gastrin-releasing peptide: different forms, different functions. Biofactors. 2009;35(1):69–75. [PubMed: 19319848]
- 8.
- Maina T., Nock B., Mather S. Targeting prostate cancer with radiolabelled bombesins. Cancer Imaging. 2006;6:153–7. [PMC free article: PMC1693771] [PubMed: 17098646]
- 9.
- Weber H.C. Regulation and signaling of human bombesin receptors and their biological effects. Curr Opin Endocrinol Diabetes Obes. 2009;16(1):66–71. [PubMed: 19115523]
- 10.
- Yang Y.S., Zhang X., Xiong Z., Chen X. Comparative in vitro and in vivo evaluation of two 64Cu-labeled bombesin analogs in a mouse model of human prostate adenocarcinoma. Nucl Med Biol. 2006;33(3):371–80. [PubMed: 16631086]
- 11.
- Liu S., Edwards D.S., Barrett J.A. 99mTc labeling of highly potent small peptides. Bioconjug Chem. 1997;8(5):621–36. [PubMed: 9327124]
- 12.
- Zhang X., Cai W., Cao F., Schreibmann E., Wu Y., Wu J.C., Xing L., Chen X. 18F-labeled bombesin analogs for targeting GRP receptor-expressing prostate cancer. J Nucl Med. 2006;47(3):492–501. [PubMed: 16513619]
- 13.
- Schroeder R.P., van Weerden W.M., Bangma C., Krenning E.P., de Jong M. Peptide receptor imaging of prostate cancer with radiolabelled bombesin analogues. Methods. 2009;48(2):200–4. [PubMed: 19398012]
- 14.
- Chen X., Park R., Hou Y., Tohme M., Shahinian A.H., Bading J.R., Conti P.S. microPET and autoradiographic imaging of GRP receptor expression with 64Cu-DOTA-[Lys3]bombesin in human prostate adenocarcinoma xenografts. J Nucl Med. 2004;45(8):1390–7. [PubMed: 15299066]
- 15.
- Hohne A., Mu L., Honer M., Schubiger P.A., Ametamey S.M., Graham K., Stellfeld T., Borkowski S., Berndorff D., Klar U., Voigtmann U., Cyr J.E., Friebe M., Dinkelborg L., Srinivasan A. Synthesis, 18F-labeling, and in vitro and in vivo studies of bombesin peptides modified with silicon-based building blocks. Bioconjug Chem. 2008;19(9):1871–9. [PubMed: 18754574]
- 16.
- Li Z.B., Wu Z., Chen K., Ryu E.K., Chen X. 18F-labeled BBN-RGD heterodimer for prostate cancer imaging. J Nucl Med. 2008;49(3):453–61. [PubMed: 18287274]
- 17.
- Prasanphanich A.F., Retzloff L., Lane S.R., Nanda P.K., Sieckman G.L., Rold T.L., Ma L., Figueroa S.D., Sublett S.V., Hoffman T.J., Smith C.J. In vitro and in vivo analysis of [(64)Cu-NO2A-8-Aoc-BBN(7-14)NH(2)]: a site-directed radiopharmaceutical for positron-emission tomography imaging of T-47D human breast cancer tumors. Nucl Med Biol. 2009;36(2):171–81. [PMC free article: PMC2756974] [PubMed: 19217529]
- 18.
- Santos-Cuevas C.L., Ferro-Flores G., Arteaga de Murphy C., Ramirez Fde M., Luna-Gutierrez M.A., Pedraza-Lopez M., Garcia-Becerra R., Ordaz-Rosado D. Design, preparation, in vitro and in vivo evaluation of (99m)Tc-N2S2-Tat(49-57)-bombesin: a target-specific hybrid radiopharmaceutical. Int J Pharm. 2009;375(1-2):75–83. [PubMed: 19393305]
- 19.
- Fragogeorgi, E.A., C. Zikos, E. Gourni, P. Bouziotis, M. Paravatou-Petsotas, G. Loudos, N. Mitsokapas, S. Xanthopoulos, M. Mavri-Vavayanni, E. Livaniou, A.D. Varvarigou, and S.C. Archimandritis, Spacer Site Modifications for the Improvement of the in Vitro and in Vivo Binding Properties of (99m)Tc-N(3)S-X-Bombesin[2-14] Derivatives. Bioconjug Chem, 2009. [PubMed: 19344122]
- 20.
- Cescato R., Maina T., Nock B., Nikolopoulou A., Charalambidis D., Piccand V., Reubi J.C. Bombesin receptor antagonists may be preferable to agonists for tumor targeting. J Nucl Med. 2008;49(2):318–26. [PubMed: 18199616]
- 21.
- Nock B.A., Nikolopoulou A., Galanis A., Cordopatis P., Waser B., Reubi J.C., Maina T. Potent bombesin-like peptides for GRP-receptor targeting of tumors with 99mTc: a preclinical study. J Med Chem. 2005;48(1):100–10. [PubMed: 15634004]
- Review (99m)Tc-labeled [N(4)(0),Pro(1),Tyr(4)]bombesin.[Molecular Imaging and Contrast...]Review (99m)Tc-labeled [N(4)(0),Pro(1),Tyr(4)]bombesin.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (99m)Tc((v))O-Gly-Gly-Cys-Orn-Orn-Orn-Bombesin[2-14].[Molecular Imaging and Contrast...]Review (99m)Tc((v))O-Gly-Gly-Cys-Orn-Orn-Orn-Bombesin[2-14].Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (99m)Tc-N(2)S(2)-Tat(49-57)-Lys(3)-bombesin.[Molecular Imaging and Contrast...]Review (99m)Tc-N(2)S(2)-Tat(49-57)-Lys(3)-bombesin.Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review 3-Cyano-4-[(18)F]fluoro-benzoyl-Ala(SO(3)H)-Ava-Gln-Trp-Ala-Val-NMeGly-His-Sta-Leu-NH(2).[Molecular Imaging and Contrast...]Review 3-Cyano-4-[(18)F]fluoro-benzoyl-Ala(SO(3)H)-Ava-Gln-Trp-Ala-Val-NMeGly-His-Sta-Leu-NH(2).Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (99m)Tc-Gly-Gly-Cys-(Arg)(3)-bombesin(2-14)-NH(2).[Molecular Imaging and Contrast...]Review (99m)Tc-Gly-Gly-Cys-(Arg)(3)-bombesin(2-14)-NH(2).Shan L. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- [99mTc-N40-1-bzlg0,D-Phe6,Leu-NHEt13,des-Met14]Bombesin(6–14) - Molecular Imagin...[99mTc-N40-1-bzlg0,D-Phe6,Leu-NHEt13,des-Met14]Bombesin(6–14) - Molecular Imaging and Contrast Agent Database (MICAD)
Your browsing activity is empty.
Activity recording is turned off.
See more...

 In vitro
In vitro