NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Kamkin A, Kiseleva I, editors. Mechanosensitivity in Cells and Tissues. Moscow: Academia; 2005.
Prolactin (PRL) is a fundamental hormone for freshwater adaptation in a variety of teleost fish. Consistent with its osmoregulatory role, PRL release from the pituitary of the Mozambique tilapia, a euryhaline teleost, is stimulated as extracellular osmolality is reduced, both in vitro and in vivo. The tilapia PRL cell model allows for the simultaneous measurement of PRL release and cell volume or intracellular Ca2+ concentration from an osmoreceptive cell of known identity. Recent evidence supports the hypothesis that the signal transduction that links a reduction in extracellular osmolality to an increase in PRL levels is mediated by mechanosensitive channels that respond to cell swelling and allow Ca2+ into the PRL cell. The process of hyposmotically-induced PRL release does not depend on voltage-gated calcium channels or IP3-sensitive intracellular calcium stores. The aim of this review is to summarize the basis for stimulus-secretion coupling and recent developments from the tilapia PRL cell model, in the light of other cell types and model systems.
Introduction
Prolactin (PRL) is a versatile hypophysial hormone with over 300 distinct actions reported in vertebrates [5]. As early as 1956, the first indication of a possible osmoregulatory role for (PRL) in fish arose from the work of Burden [11]. He showed that hypophysectomized killifish (Fundulus heteroclitus), a euryhaline teleost, failed to survive in fresh water. Further work on this issue demonstrated that injections of ovine PRL would allow the hypophysectomized fish to survive in fresh water [45]. Later reports showing that PRL was capable of preventing osmotic failure in several other species of teleost fish, including the tilapia, Oreochromis mossambicus, quickly ensued. Prolactin effectively restores salt and water balance after a drop in extracellular osmolality by acting on all osmoregulatory surfaces, mainly gills, kidney and intestine, to reduce ion and water permeability, and to stimulate ion influx [10, 29, 34]. Today, the notion that PRL is the freshwater adapting hormone for euryhaline teleosts is well accepted.
Given that PRL is the main hormone with the ability for restoring hydromineral balance in fresh water, it is not unexpected that PRL cells are directly sensitive to alterations in extracellular osmolality. However, in addition to direct osmotic modulation, there are several factors known to alter PRL release, including steroids and hypothalamic peptides [1, 6, 13, 37, 55, 64]. It has been hypothesized that the direct osmotic control of PRL release may stem from the ability of certain endocrine cells to sense environmental stimuli directly as a consequence of their having been exposed to the environment during their early evolutionary history [2, 3]. The anatomical location of the pituitary gland, adjacent to the oral cavity, supports this view. Layered upon this direct environmental control of hormone release, more recently derived modes of control, such as hypothalamic control, would then have evolved to fine-tune the regulation of PRL release in the context of novel biological activities affected by this hormone, such as reproductive behavior [15, 16]. Nevertheless, this primordial environmental control of PRL release has been shown to be fundamental to freshwater adaptation, as evidenced by the sustained osmoregulatory abilities of tilapia whose pituitaries were transplanted to an ectopic site [58]. The absence of hypothalamic control did not prevent the elevation of PRL gene expression in transplanted pituitaries and the elevation in circulating PRL levels after fish were transferred to fresh water. This intrinsic osmosensitivity is corroborated by numerous studies that have examined both the pituitary and dispersed PRL cells in vitro [17, 37, 54, 57, 65]. Thus, the ability of PRL cells to directly sense an osmotic gradient through mechanosensitive channels, combined with the physiological context of PRL's actions, characterizes the PRL cell as an osmoreceptor.
In this article we will review the basis for stimulus-secretion coupling and mechanosesnsitivity in tilapia PRL cells as well as some of the recent findings on the signal transduction of the osmotic stimulus. We will also discuss the osmoreceptive PRL cell model in light of the mammalian model for osmoreception and the closely related growth hormone cell.
The prolactin cell as a model to study osmoreception
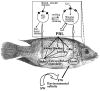
At first, it may seem intriguing that a fundamental sensory modality such as osmoreception has few study models and relatively scarce fundamental information available, compared with other modalities such as vision and olfaction. After close examination, however, the main impediments for fully elucidating this process, at least in mammals, become clear. Not only has the definition of osmoreceptor been the subject of debate and careful definition in terms of its systemic implications [9], but the anatomical arrangement of cells that can be considered osmoreceptors has proven to be a major obstacle for the direct and clear elucidation of how osmotic stimuli are transduced into physiologically relevant osmoregulatory responses. Presently, the mechanism for osmoreception in the rat hypothalamus, the best studied mammalian model in this field, is believed to comprise two main osmosensitive regions, the magnocellular neurons of the supraoptic nucleus (SON) and the AV3V region, which consists mainly of the organum vasculosum of the lamina terminalis (OVLT) and the median preoptic nucleus (MnPO). These areas are interconnected by excitatory neurons and together form an osmoreceptor complex that appears to be essential for the osmotically-induced release of vasopressin and oxytocin, neurohypophysial hormones directly involved in mammalian hydromineral balance [26]. The complex pattern of neural pathways and diversity of interspersed cell types observed in the rat, however, is not shared by the anatomically simpler, albeit no less fundamental for the osmoregulatory reflex, PRL cells of the euryhaline tilapia. Prolactin cells are of similar size and shape and are almost exclusively segregated into one anatomically distinct region of the pituitary gland, the rostral pars distalis (Fig. 1).
Besides being morphologically accessible to direct examination, PRL cells operate under a wide range of physiologically relevant osmolalities, in order to respond and adapt to fluctuations in environmental salinity. While steady-state plasma osmolalities depend on past acclimation history and can range from 310 to 320 mOsmolal for tilapia adapted to fresh water and 330 to 350 mOsmolal for those adapted to seawater [52, 57], deviations between 290 to 450 mOsmolal are commonly observed when fish are challenged with acute changes in environmental salinity [57, 69]. On the other hand, mammals are normally bound to a much tighter internal osmotic range, where a deviation in plasma osmolality of 15 % can lead to convulsions and coma in humans [4].
The osmoregulatory reflex exerted by PRL cells also differs from the mammalian model with respect to the nature of osmotic challenge. While in rats the osmoreceptor complex and osmoregulatory output have evolved to be especially responsive to increases in extracellular osmolality, in the euryhaline tilapia, PRL release is stimulated when the environment becomes dilute. Thus, the underlying mechanism for stimulus-secretion coupling for each of the two models operates under opposite ends of the same source of stimulation: while a hyperosmotic stimulus is excitatory in rats and inhibitory in tilapia, the reverse is true when extracellular osmolality decreases.
Signal transduction of an osmotic stimulus is mediated via mechanosensitive Ca2+-permeable channels
Early in vivo and in vitro experiments have clearly established the strong inverse relationship between plasma or extracellular medium osmolality and PRL levels in circulation or released in culture [12, 17, 37, 40, 67–69]. Furthermore, it was established that Ca2+ was an essential component for PRL release, either by deleting it from incubation medium [17], blocking its entry in the cell with CoCl2, [19] or by stimulating its entry in the cell with the Ca2+ ionophore, A23187 [6, 18]. In fact, it has been recently found that Ca2+ from the extracellular milieu triggers PRL release in response to hyposmotic medium without the participation of additional intracellular sources of Ca2+ such as IP3 and ryanodine-sensitive stores [51]. The mechanism by which Ca2+ would link an osmotic stimulus to PRL release had not been experimentally addressed at the time, but the idea that there was a mechanosensitive link among these parameters began to gain strength well before more conclusive evidence had been gathered [14].
Mechanical force is ubiquitous in nature and cells have evolved diverse strategies to convert it into physiologically relevant signals. In fact, it is believed that mechanosensitivity is one of the earliest forms of sensory transduction process, as evidenced by bacterial models [33, 44, 46]. Soon after the first description of mechansensitive channels in embryonic chick skeletal muscle cells [20], several examples of cells containing channels with mechanosensitive properties arose, but very few managed to associate this sensory capability with a clear physiological role [36]. More recently, however, mechanosensitive channels have been implicated in a multitude of physiologically relevant transduction activities, such as sound and pressure [25, 71], and interest in this field has been renewed by cloning and characterization studies of mechanosensitive channels from bacteria to vertebrates [43, 59]. In vertebrates, most mechanosensitive channels link mechanical stimuli to electrical signals, and several models attempting to elucidate the underlying mechanism have emerged [31]. Membrane tensions required for half-activating most mechanosensitive channels are estimated to be equivalent to the force produced by transmembrane osmolality of a few milliOsmols [30]. Based on the patch-clamp technique, two basic classes of mechanosensitive channels had been described: stretch-activated and stretch inactivated channels [35, 48]. While stretch-inactivated channels were found to be directly involved in the hyperosmotically-induced firing of magnocellular neurons in the rat osmoreceptor complex [8], stretch-activated channels quickly became an attractive hypothesis to be tested on the PRL cell model.
While attempting to elucidate the underlying mechanism for stimulus-secretion coupling in PRL cells the first fundamental question we addressed was whether hyposmotically-induced PRL release was directly controlled by a drop in extracellular osmolality per se or by a change in the osmotic gradient across the cell membrane. With the intent of uncoupling osmolality from tonicity, mannitol and urea, membrane-impermeant and permeant molecules, respectively, were added to the incubation medium; while the former contributes to establishing an osmotic gradient, the latter does not, as it freely equilibrates across the cell membrane. Although extracellular osmolality was the same in both groups, 355 mOsmolal, PRL release was lower in PRL cells incubated with mannitol when compared with cells incubated with urea, indicating an association between osmotic gradient and PRL release [65]. This association was strengthened after observing the temporal relationship between cell volume changes and PRL release. Holding medium osmolality at 355 mOsmolal, 55 mOsmolal of NaCl was replaced by 55 mOsmolal of mannitol, but neither cell size nor PRL release changed because mannitol is membrane-impermeant and the transmembranal osmotic gradient was unaltered. By contrast, replacing NaCl (55 mOsmolal) with urea (55 mOsmolal) led to a slow increase in cell size followed closely by an equally slow rise in PRL release even though medium solute concentration remained unchanged [65]. Because urea is cell-permeant, it failed to prevent the osmotically-induced movement of water into the cell and its consequent swelling. The slow rate of increase in cell size derives from the fact that urea penetrates the cell membrane slower than water. This was later demonstrated by gradually changing extracellular osmolality and monitoring cell volume and PRL release every 5 min [52, 53]. The close temporal correspondence between changes in cell size and in PRL release suggested that the two processes were coupled and would therefore provide a good basis for investigating the involvement of mechanosensitive channels.
In order to accurately track changes in cell volume and PRL release we developed a system in which PRL cell images could be recorded from the same preparation of dispersed cells and time course used for quantifying PRL release [54]. From these data it was clear that cell volume preceded an abrupt increase in PRL release. Using the same experimental conditions, intracellular Ca2+ concentration ([Ca2+]i) was also shown to rise rapidly in dispersed cells after exposure to hyposmotic medium. When juxtaposed in the same temporal scale, these parameters help elucidate the sequence and duration of events that underlie stimulus-secretion coupling in tilapia (Fig. 2). When Ca2+ was deleted from the incubation media, however, both hyposmotically-induced [Ca2+]i and PRL release, but not a rise in cell volume, were inhibited [54]. The normal responses of [Ca2+]i and PRL release to hyposmotic medium could be recovered when Ca2+ was present in the incubation medium. Therefore, it was concluded that while cell volume increase is a trigger for hyposmotically-induced PRL release, a rise in [Ca2+]i is essential for this endocrine reflex to take place.
The tilapia PRL cell is highly sensitive to depolarizing conditions such as high [K+], and responds rapidly but briefly by increasing [Ca2+]i and PRL release, indicating that these cells are excitable and possess voltage-gated Ca2+ channels (VGCCs) [19, 27, 47]. It was found that PRL cells remain unresponsive to further stimulation by high [K+] until they are allowed to repolarize in normal medium [47], suggesting that these channels become inactivated after depolarization. Thus, if hyposmotically-induced PRL release were dependent on the same VGCCs required for high [K+]-induced PRL release, then pre-existing depolarizing conditions would prevent any further increase in PRL as a consequence of reducing extracellular osmolality. In a series of experiments employing hyposmotic medium (300 mOsmolal), depolarizing conditions (56 mM KCl) or both simultaneously, while analyzing changes in PRL cell volume, [Ca2+]i and PRL release, it was shown that the stimulation of both [Ca2+]i and PRL release by hyposmotic medium was unaffected by previous exposure to depolarizing conditions [56]. Cell volume always correlated with a decrease in osmolality, and did not change with high [K+] treatment alone. Furthermore, nifedipine, a blocker of L-type VGCCs, inhibited high [K+]-induced PRL release but not hyposmotically-induced PRL release [56]. Taken together, these experiments suggested that during hyposmotically-induced PRL release, channels mediating Ca2+ entry into the cell were not voltage-gated, supporting the hypothesis that this stimulus-secretion coupling occurs via stretch-activated Ca2+ channels.
Further evidence supporting the role of mechanosensitive channels during osmoreception was provided by experiments employing the lanthanide, gadolinium (Gd3+). In the presence of this element, hyposmotically-induced rise in [Ca2+]i and PRL release were inhibited and therefore uncoupled from the rise in cell volume [56]. Deleting Ca2+ from the incubation medium similarly blocked hyposmotically-induced PRL release and [Ca2+]i without affecting cell swelling [54]. Interestingly, in the mammalian osmoreceptor complex, Gd3+ was effective in inhibiting the hyperosmotically-induced firing of magnocellular neurons, which are believed to transduce osmotic signals via stretch-inactivated channels [41]. While Gd3+ had been previously regarded as the best available pharmacological tool to block stretch-activated channels [21, 22], recent evidence indicates it can act indirectly on these channels by altering the nature of the lipid bilayer [60]. In an attempt at stretching the lipid bilayer by non-osmotic means, PRL cells were perfused with isosmotic medium (330 mOsmolal) containing chlorpromazine, a cationic amphipath, which has been shown to increase the open probability of mechanosensitive channels in E. coli by modifying the lipid bilayer [32]. In accordance with the time course previously reported for the effects of this compound [32], PRL release gradually increased after a delay of several minutes, while cell volume did not dramatically change (unpublished observations). Despite the diversity of described actions for any single pharmacological agent, the interpretation of the data derived from the use of several approaches strongly supports the role of mechanosensitive channels in the tilapia osmoreception. Based on these approaches, we have described a model for stimulus-secretion coupling under hyposmotic conditions [53], in which the rapid (minutes) increase in PRL release is dependent on the entry of extracellular Ca2+ through stretch-activated ion channels following cell swelling.
Beyond mechanotransduction
Cells typically regulate their volume when presented with an osmotic challenge [28, 50, 66], but tilapia PRL cells, like osmosensory neurons of the rat supraoptic nucleus, do not regulate their volume, at least under physiological ranges of osmotic change, between 40–60 mOsmolal [54, 72]. Diminished volume regulation may be essential to the functioning of osmoreceptors and may account for sustained changes in PRL release that accompany changes in extracellular osmolality. In fact, continued exposure to a hyposmotic stimulus for hours and days evokes changes in the tilapia PRL cell in vitro that extend beyond rapid rise in PRL release and second messenger metabolism [17, 37, 57]. Since cellular stores of PRL are limited, a sustained elevation in PRL release would appear to require the activation of PRL gene expression and synthesis. In tilapia with their rostral pars distalis, containing exclusively PRL cells, transplanted to the optic nerve, plasma PRL and pituitary PRL mRNA varied inversely with plasma osmolality [58]. These results indicate that the long-term response is the direct effect of osmolality on PRL cell function and that hypothalamic innervation is not required for the PRL cell to respond to extracellular osmolality. Recently it has been shown that PRL mRNA levels were significantly higher in hyposmotic (300 mOsmolal) than in hyperosmotic (350 mOsmolal) medium by 8 h of incubation [61]. Earlier, it was found that PRL synthesis, estimated by 3H-leucine incorporation into the PRL molecule, is elevated after 4–6 h of incubation in hyposmotic medium [70]. Thus, our findings clearly indicate that continued exposure to a chronic osmotic stimulus induces sustained changes in the gene expression, synthesis, and release of PRL.
In earlier studies, we found that an elevation in cAMP turnover rate, which accompanies hyposmotic stimulation, may play a role in sustaining the release of PRL [14, 23]. The exact role played by cAMP or its relationship to elevations in [Ca2+]i during hyposmotic stimulation remain to be clarified. Recent experiments employing agonists of the IP3 pathway as well as inhibitors of IP3 receptors and intracellular Ca2+ stores suggest that the IP3-mediated cascade and intracellular Ca2+ stores do not play a role in the acute hyposmotically-induced [Ca2+]i and PRL release [51]. However, further research on the mechanisms of PRL gene expression and sustained PRL release may reveal the involvement of these and other mediators not required for the acute response such as the cAMP/PKA and CaM-activated kinase II/PKC cascades.
While PRL is the main freshwater-adapting hormone in euryhaline teleosts, growth hormone (GH) has been shown to be involved in SW osmoregulation in certain teleost fish including the tilapia [34, 49]. Pituitary GH content, volume of GH-immunostained region, and the activity of GH cells are higher in seawater tilapia than in freshwater fish [7], and GH release has been shown to increase in response to increases in extracellular osmolality in vitro and in vivo [24, 52, 57, 69]. Growth hormone cells are interspersed with thyrotropin and gonadotropin cells in the proximal pars distalis (PPD) of the pituitary [38, 42, 62, 63], and their close proximity and morphological resemblance to PRL cells has provided, for many studies, a good control cell type for osmoreception experiments.
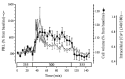
Although recent observations have shown that GH cells can also significantly respond to hyposmotic medium in vitro [52, 57], the extent of GH release from perfused PPD cells was not as pronounced as PRL release (Fig. 3A). Furthermore, reducing medium osmolality did not alter GH mRNA levels, indicating the specific nature of the long-term osmoregulatory reflex of tilapia PRL cells [61]. Most conclusive, however, is the observation that the short-term response of fish transferred from seawater to fresh water, and subsequent reduction in plasma osmolality, significantly increases circulating PRL levels, while GH release remains unchanged (Fig. 3B). It is possible that the transient hyposmotically-induced release in GH seen in vitro is also mediated by mechanosensitive channels as a result of morphological similarities to PRL cells, rather then an acquired physiological adaptation. Although speculative, the possibility that the response of GH cells to hyperomostic medium is mediated by stretch-inactivated ion channels, similar to the ones present in mammalian osmosensitive neurons, may provide an interesting avenue for future research.
Conclusions and perspectives
Prolactin cells are osmoreceptors especially tailored for freshwater adaptation. Recent studies have allowed us to expand our understanding of the fundamental mechanisms underlying this powerful stimulus-secretion reflex and how it is intimately linked to osmotic homeostasis. To strengthen this view, several lines of evidence were presented in the discussion above: 1) PRL has a well established osmoregulatory function in tilapia; 2) PRL is released from the dispersed cell in response to small alterations in extracellular osmolality, well within the range observed in vivo; 3) the PRL response to to hyposmotic medium is strong, having acute and sustained phases, which involves not only immediate release of stored hormone, but also activation of gene expression and maintenance of elevated baseline release; 4) hyposmotically-induced PRL release requires Ca2+, a ubiquitous intracellular mediator involved in many stimulus-secretion reflexes; 5) several lines of evidence suggest the presence of mechanosensitive Ca2+-permeable channels that are activated in response to hyposmotically-induced cell stretch; 6) the magnitude, duration and physiological role of PRL release in response to hyposmotic stimulation is not shared by other pituitary cell types, such as GH.
The stimulus-secretion coupling seen in PRL cells provides an excellent example of a direct and simplified osmoreceptive reflex and possibly represents the only model in which mechanotransduction of a hyposmotic stimulus can restore systemic osmoregulatory balance through endocrine mediation. It is not surprising that such a fundamental homeostatic process is tightly and directly controlled by a source of stimulus (extracellular osmolality) that can regulate and be regulated, forming a negative feedback loop (Fig. 4).
The tilapia PRL cell model may have broad applicability to the understanding of osmoreception. Although recent advances were achieved on understanding the underlying mechanisms involved in stimulus secretion -coupling and acute osmoregulatory reflex, there is much room for further discoveries. Particularly interesting findings may arise from a closer examination of the mechanisms involved in sustained PRL release and the underlying hyperosmotic stimulation of GH cells in the context of seawater adaptation.
Acknowledgements
This work was funded in part by grants from National Science Foundation (BN 01-33714), United State Department of Agriculture (#983506644), and also from the National Oceanic and Atmospheric Administration, project #R/AQ-62, which is sponsored by the University of Hawaii Sea Grant College Program.
References
- 1.
- Barry TP, Grau EG. Estradiol-17 beta and thyrotropin-releasing hormone stimulate prolactin release from the pituitary gland of a teleost fish in vitro. Gen Comp Endocrinol. (1986);62:306–314. [PubMed: 3096813]
- 2.
- Bern HA (1980) Primitive control of endocrine systems. In "Hormones, Adaptation and Evolution" (Ishii S, Hirano T and Wada M, eds.), pp. 25–33, Japan Sci Soc Press / Springer-Verlag, Tokyo.
- 3.
- Bern HA. Functional evolution of prolactin and growth hormone in lower vertebrates. Am Zool. (1983);23:663–671.
- 4.
- Berne RM, Levy M N, Koeppen BM, Stanton BA (1998) Physiology, Mosby, St. Louis.
- 5.
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endo Rev. (1998);19:225–268. [PubMed: 9626554]
- 6.
- Borski RJ, Helms LMH, Richman NH, Grau EG. Cortisol rapidly reduces prolactin release and cAMP and 45Ca2+ accumulation in the cichlid fish pituitary in vitro. Proc Natl Acad Sci. (1991);88:2558–2762. [PMC free article: PMC51318] [PubMed: 11607172]
- 7.
- Borski RJ, Yoshikawa JSM, Madsen SS, Nishioka RS, Zabetian C, Bern HA, Grau EG. Effects of environmental salinity on pituitary growth hormone content and cell activity in the euryhaline tilapia, Oreochromis mossambicus. Gen Comp Endocrinol. (1994);95:483–494. [PubMed: 7821785]
- 8.
- Bourque CW. Osmoregulation of vasopressin neurons: a synergy of intrinsic and synaptic processes. Prog Brain Res. (1998);119:59–76. [PubMed: 10074781]
- 9.
- Bourque CW, Oliet SH. Osmoreceptors in the central nervous system. Ann Rev Physiol. (1997);59:601–619. [PubMed: 9074779]
- 10.
- Brown PS, Brown SC (1987) Osmoregulatory actions of prolactin and other adenohypophysial hormones. In "Vertebrate Endocrinology: Fundamentals and Biomedical Implications" (Pang PKT, Schreibman MP and Sawyer WH, eds.), pp. 45–84, Academic Press, London.
- 11.
- Burden CE. The failure of hypophysectomized Fundulus heteroclitus to survive in fresh water. Biol Bull. (1956);110:8–28.
- 12.
- Dharmamba M, Nishioka RS. Response of "prolactin-secreting" cells of Tilapia mossambica to environmental salinity. Gen Comp Endocrinol. (1968);10:409–420. [PubMed: 4298161]
- 13.
- Grau EG, Ford CA, Helms LMH, Shimoda SK, Cooke IM. Somatostatin and altered medium osmotic pressure elicit rapid changes in prolactin release from the rostral pars distalis of the tilapia, Oreochromis mossambicus, in vitro. Gen Comp Endocrinol. (1987);65:12–18. [PubMed: 2879767]
- 14.
- Grau EG, Helms LMH. The tilapia prolactin cell: A model for stimulus-secretion coupling. Fish Physiol Biochem. (1989);7:11–19. [PubMed: 24221750]
- 15.
- Grau EG, Helms MH (1990) The tilapia prolactin cell - twenty-five years of investigation. In Progress in Comparative Endocrinology, vol. 342 (Epple A, Scanes CG, Stetson MH, eds.), pp. 534–540, Wiley-Liss, New York. [PubMed: 2200034]
- 16.
- Grau EG, Richman NH III, Borski RJ (1994) Osmoreception and a simple endocrine reflex of the prolactin cell of the tilapia Oreochromis mossambicus. In "Perspectives in Comparative Endocrinology" (Davey KG, Peter RE and Tobe SS, eds.), pp. 251–256, National Research Council of Canada, Ottawa.
- 17.
- Grau EG, Nishioka RS, Bern HA. Effects of osmotic pressure and calcium ion on prolactin release in vitro from the rostral pars distalis of the tilapia Sarotherodon mossambicus. Gen Comp Endocrinol. (1981);45:406–408. [PubMed: 7297849]
- 18.
- Grau EG, Nishioka RS, Bern HA. Effects of somatostatin and urotensin II on tilapia pituitary prolactin release and interactions between somatostatin, osmotic pressure, Ca2+, and adenosine 3′,5′-monophoshate in prolactin release in vitro. Endocrinology. (1982);110:910–915. [PubMed: 6173210]
- 19.
- Grau EG, Shimoda SK, Ford CA, Helms LMH, Cooke IM, Pang PKT. The role of calcium in prolactin release from the pituitary of a teleost fish in vitro. Endocrinology. (1986);119:2848–2855. [PubMed: 2430785]
- 20.
- Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. (1984);352:685–701. [PMC free article: PMC1193237] [PubMed: 6086918]
- 21.
- Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. (2001);81:685–740. [PubMed: 11274342]
- 22.
- Hamill OP, McBride DWJ. The pharmacology of mechanogated membrane ion channels. Pharmacol Rev. (1996);48:231–252. [PubMed: 8804105]
- 23.
- Helms LMH, Grau EG, Borski RJ. Effects of osmotic pressure and somatostatin on the cAMP messenger system of the osmosensitive prolactin cell of a teleost fish, the tilapia Oreochromis mossambicus. Gen Comp Endocrinol. (1991);83:111–117. [PubMed: 1715302]
- 24.
- Helms LMH, Grau EG, Shimoda SK, Nishioka RS, Bern HA. Studies on the regulation of growth hormone release from the proximal pars distalis of the tilapia, Oreochromis mossambicus, in vitro. Gen Comp Endocrinol. (1987);65:48–55. [PubMed: 2879768]
- 25.
- Holt JR, Corey DP. Two mechanisms for transducer adaptation in vertebrate hair cells. Proc Natl Acad Sci USA. (2000);97:11730–11735. [PMC free article: PMC34342] [PubMed: 11050202]
- 26.
- Honda K. Mechanisms controlling neurohypophysial hormone release in the rat. J Reprod Dev. (2003);49:1–11. [PubMed: 14967944]
- 27.
- Hyde GN, Seale AP, Grau EG, Borski RJ. Cortisol rapidly suppresses intracellular calcium and voltage-gated calcium channel activity in prolactin cells of the tilapia (Oreochromis mossambicus). Am J Physiol. (2004);286:E626–E633. [PubMed: 14656715]
- 28.
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. (1998);78:247–306. [PubMed: 9457175]
- 29.
- Manzon LA. The role of prolactin in fish osmoregulation: A review. Gen Comp Endocrinol. (2002);125:291–310. [PubMed: 11884075]
- 30.
- Martinac B (1993) Mechanosensitive ion channels: biophysiscs and physiology. In "Thermodynamics of Membrane Receptors and Channels" (Jackson, M. B., ed.), pp 327–351, CRC Press, Boca Raton.
- 31.
- Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci. (2004);117:2449–2460. [PubMed: 15159450]
- 32.
- Martinac B, Alder J, Kung C. Mechanosensitive ion channels of E. Coli activated by amphipaths. Nature. (1990);348:261–263. [PubMed: 1700306]
- 33.
- Martinac B, Kloda A. Evolutionary origins of mechanosensitive ion channels. Prog Biophys Mol Biol. (2003);82:11–24. [PubMed: 12732265]
- 34.
- McCormick SD. Endocrine control of osmoregulation in teleost fish. Am Zool. (2002);41:781–794.
- 35.
- Morris C, Sigurdson WJ. Stretch-inactivated ion channels coexist with stretch-activated ion channels. Science. (1989);243:807–811. [PubMed: 2536958]
- 36.
- Morris CE. Are stretch-sensitive channels in molluscan cells and elsewhere physiological mechanotransducers? Experientia. (1992);48:852–858. [PubMed: 1383023]
- 37.
- Nagahama Y, Nishioka RS, Bern HA, Gunther RL. Control of prolactin secretion in teleosts, with special reference to Gillichthys mirabilis and Tilapia mossambica. Gen Comp Endocrinol. (1975);25:166–188. [PubMed: 1150073]
- 38.
- Nagahama Y, Olivereau M, Farmer SW, Nishioka RS, Bern HA. Immunocytochemical identification of the prolactin- and growth hormone-secreting cells in the teleost pituitary with antisera to tilapia prolactin and growth hormone. Gen Comp Endocrinol. (1981);44:389–395. [PubMed: 7026355]
- 39.
- Nishioka RS, de Jesus GT, Hyodo S. Localization of mRNAs for a pair of prolactins and growth hormone in the tilapia pituitary using in situ hybridization with oligonucleotide probes. Gen Comp Endocrinol. (1993);89:72–81. [PubMed: 8428650]
- 40.
- Nishioka RS, Kelley KM, Bern HA. Control of prolactin and growth hormone secretion in teleost fishes. Zool Sci. (1988);5:267–280.
- 41.
- Oliet SHR, Bourque CW. Gadolinium uncouples mechanical detection and osmoreceptor potential in supraoptic neurons. Neuron. (1996);16:176–181. [PubMed: 8562082]
- 42.
- Pandolfi M, Paz DA, Maggese C, Ravaglia M, Vissio P. Ontogeny of immunoreactive somatolactin, prolactin and growth hormone secretory cells in the developing pituitary gland of Cichlasoma dimerus (Teleostei, Perciformes). Anat Embryol. (2001);203:461–468. [PubMed: 11453163]
- 43.
- Perozo E, Cortes DM, Sompornpisut P, Kloda A, Martinac B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. (2002);418:942–948. [PubMed: 12198539]
- 44.
- Perozo E, Rees DC. Structure and mechanism in prokaryotic mechano-sensitive channels. Curr Opin Struct Biol. (2003);13:432–442. [PubMed: 12948773]
- 45.
- Pickford, GE, Phillips JG. Prolactin, a factor in promoting survival in hypophy-sectomized killifish in fresh water. Science. (1959);130:454–455. [PubMed: 13675773]
- 46.
- Poolman B, Spitzer JJ, Wood JM. Bacterial osmosensing: roles of membrane structure and electrostatics in lipid-protein and protein-protein interactions. Biochim Biophys Acta. (2004);1666:88–104. [PubMed: 15519310]
- 47.
- Richman NH, Helms LM, Ford CA, Benishin C, Pang PK, Cooke IM, Grau EG. Effects of depolarizing concentrations of K+ and reduced osmotic pressure on 45Ca2+ accumulation by the rostral pars distalis of the tilapia (Oreochromis mossambicus). Gen Comp Endocrinol. (1990);77:292–297. [PubMed: 2307348]
- 48.
- Sachs F, Morris CE. Mechanosensitive ion channels in nonspecialized cells. Rev Physiol Biochem Pharmacol. (1998);132:1–77. [PubMed: 9558913]
- 49.
- Sakamoto T, Shepherd BS, Madsen SS, Nishioka RS, Siharath K, Richman NH, Bern HA, Grau EG. Osmoregulatory actions of growth hormone and prolactin in an advanced teleost. Gen Comp Endocrinol. (1997);106:95–101. [PubMed: 9126469]
- 50.
- Sardini A, Amey JS, Weylandt KH, Nobles M, Valverde MA, Higgins CF. Cell volume regulation and swelling-activated chloride channels. Biochim Biophys Acta. (2003);1618:153–162. [PubMed: 14729152]
- 51.
- Seale A, Cooke I, Hirano T, Grau G. Evidence that IP3 and ryanodine-sensitive intra-cellular Ca2+ stores are not involved in acute hyposmotically-induced prolactin release in tilapia. Cell Physiol Biochem. (2004);14:155–166. [PubMed: 15107592]
- 52.
- Seale AP, Fiess J, Hirano T, Grau EG (2005) Disparate release of prolactin and growth hormone from the tilapia pituitary in response to osmotic stimulation and acclimation salinity. Gen Comp Endocrinol (in press). [PubMed: 16242686]
- 53.
- Seale AP, Hirano T, Grau EG (2005) Osmoreception: a fish model for a fundamental sensory modality. In Fish Endocrinology (Zaccone G and Reinecke M, eds.), Oxford & IBH Publishing Company (in press).
- 54.
- Seale AP, Richman NH III,, Hirano T, Cooke I, Grau EG. Cell volume increase and extracellular Ca2+ are needed for hyposmotically induced prolactin release in tilapia. Am J Physiol. (2003);284:C1280–C1289. [PubMed: 12540379]
- 55.
- Seale AP, Itoh T, Moriyama S, Takahashi A, Kawauchi H, Sakamoto T, Fujimoto M, Riley LG, Hirano T, Grau EG. Isolation and characterization of a homologue of mammalian prolactin-releasing peptide from the tilapia brain and its effect on prolactin release from the tilapia pituitary. Gen Comp Endocrinol. (2002);125:328–339. [PubMed: 11884078]
- 56.
- Seale AP, Richman NH, Hirano, T., Cooke, I. M, Grau, E. G. Evidence that signal transduction for osmoreception is mediated by stretch-activated ion channels in tilapia. Am J Physiol. (2003);284:C1290–C1296. [PubMed: 12540380]
- 57.
- Seale AP, Riley LG, Leedom TA, Kajimura S, Dores RM, Hirano T, Grau EG. Effects of environmental osmolality on release of prolactin, growth hormone and ACTH from the tilapia pituitary. Gen Comp Endocrinol. (2002);128:91–101. [PubMed: 12392682]
- 58.
- Shepherd BS, Sakamoto T, Hyodo S, Nishioka RS, Ball C, Bern HA, Grau EG. Is the primitive regulation of pituitary prolactin (tPRL177 and tPRL188) secretion and gene expression in the euryhaline tilapia (Oreochromis mossambicus) hypothalamic or environmental? J Endocrinol. (1999);161:121–129. [PubMed: 10194536]
- 59.
- Sidi S, Friedrich RW, Nicolson T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science. (2003);301:96–99. [PubMed: 12805553]
- 60.
- Tanaka T, Tamba Y, Masum SM, Yamashita Y, Yamazaki M. La3+ and Gd3+ induce shape change of giant unilamellar vesicles of phosphatidylcholine. Biochim Biophys Acta. (2002);1564:173–82. [PubMed: 12101010]
- 61.
- Uchida K, Yoshikawa-Ebesu JS, Kajimura S, Yada T, Hirano T, Grau EG. In vitro effects of cortisol on the release and gene expression of prolactin and growth hormone in the tilapia, Oreochromis mossambicus. Gen Comp Endocrinol. (2004);135:116–125. [PubMed: 14644651]
- 62.
- Ueda H, Kagawa H, Fujimoto S. Immunoelectron microscopic localization of growth hormone in the pituitary glands of two teleosts, tilapia (Sarotherodon mossambicus) and amago salmon (Oncorhynchus rhodurus). Gen Com Endocrin. (1985);59:149–154. [PubMed: 4018550]
- 63.
- Villaplana M, Ayala AG, Agulleiro PB. Identification of mammosomatotropes, growth hormone cells and prolactin cells in the pituitary gland of the gilthead sea bream (Sparus aurata L., Teleostei) using light immunocytochemical methods: an ontogenetic study. Anat Embryol. (2000);202:421–429. [PubMed: 11089933]
- 64.
- Weber GM, Powell JFF, Park M, Fischer WH, Craig AG, Rivier JE, Nanakorn U, Parhar IS, Ngamvongchon S, Grau EG, Sherwood NM. Evidence that gonadotropin-releasing hormone (GnRH) functions as a prolactin-releasing factor in a teleost fish (Oreochromis mossambicus) and primary structures for three native GnRH molecules. J Endocrinol. (1997);155:121–132. [PubMed: 9390014]
- 65.
- Weber GM, Seale AP, Richman NH, Stetson M, Grau EG. Hormone release is tied to changes in cell size in the osmoreceptive prolactin cell of a euryhaline teleost fish, the tilapia, Oreochromis mossambicus. Gen Comp Endocrinol. (2004);138:8–13. [PubMed: 15242746]
- 66.
- Wehner F, Olsen H, Tinel H, Kinne-Saffran E, Kinne RK. Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev Physiol Biochem Pharmacol. (2003);148:1–80. [PubMed: 12687402]
- 67.
- Wigham T, Nishioka RS, Bern HA. Factors affecting in vitro activity of prolac-tin cells in the euryhaline teleost Sarotherodon mossambicus (Tilapia mossambica). Gen Comp Endocrinol. (1977);32:120–131. [PubMed: 408224]
- 68.
- Yada T, Hirano T. Influence of seawater adaptation on prolactin and growth hormone release from organ-cultured pituitary of rainbow trout. Zool Sci. (1992);9:143–148.
- 69.
- Yada T, Hirano T, Grau EG. Changes in plasma levels of the two prolactins and growth hormone during adaptation to different salinities in the euryhaline tilapia, Oreochromis mossambicus. Gen Comp Endocrinol. (1994);93:214–223. [PubMed: 8174927]
- 70.
- Yoshikawa-Ebesu JSM, Borski RJ, Richman NH, Grau EG. Effects of acclimation salinity and in vitro medium osmotic pressure on the incorporation of 3H-Leucine into the two prolactins of the tilapia, Oreochromis mossambicus. J Exp Zool. (1995);271:331–339.
- 71.
- Zagorodnyuk VP, Brookes SJ. Transduction sites of vagal mechano-receptors in the guinea pig esophagus. J Neurosci. (2000);20:6249–6255. [PMC free article: PMC6772604] [PubMed: 10934275]
- 72.
- Zhang Z, Bourque CW. Osmometry in osmosensory neurons. Nat Neurosci. (2003);6:1021–1022. [PubMed: 12973356]
- Evidence that IP3 and ryanodine-sensitive intra-cellular Ca2+ stores are not involved in acute hyposmotically-induced prolactin release in Tilapia.[Cell Physiol Biochem. 2004]Evidence that IP3 and ryanodine-sensitive intra-cellular Ca2+ stores are not involved in acute hyposmotically-induced prolactin release in Tilapia.Seale AP, Cooke IM, Hirano T, Grau GE. Cell Physiol Biochem. 2004; 14(3):155-66.
- Cell volume increase and extracellular Ca2+ are needed for hyposmotically induced prolactin release in tilapia.[Am J Physiol Cell Physiol. 2003]Cell volume increase and extracellular Ca2+ are needed for hyposmotically induced prolactin release in tilapia.Seale AP, Richman NH 3rd, Hirano T, Cooke I, Grau EG. Am J Physiol Cell Physiol. 2003 May; 284(5):C1280-9. Epub 2003 Jan 22.
- Involvement of the cAMP messenger system and extracellular Ca(2+) during hyposmotically-induced prolactin release in the Mozambique tilapia.[Gen Comp Endocrinol. 2011]Involvement of the cAMP messenger system and extracellular Ca(2+) during hyposmotically-induced prolactin release in the Mozambique tilapia.Seale AP, Mita M, Hirano T, Gordon Grau E. Gen Comp Endocrinol. 2011 Jan 15; 170(2):401-7. Epub 2010 Nov 2.
- Review Osmoreception: perspectives on signal transduction and environmental modulation.[Gen Comp Endocrinol. 2012]Review Osmoreception: perspectives on signal transduction and environmental modulation.Seale AP, Watanabe S, Grau EG. Gen Comp Endocrinol. 2012 May 1; 176(3):354-60. Epub 2011 Oct 20.
- Review Endocrine regulation of prolactin cell function and modulation of osmoreception in the Mozambique tilapia.[Gen Comp Endocrinol. 2013]Review Endocrine regulation of prolactin cell function and modulation of osmoreception in the Mozambique tilapia.Seale AP, Yamaguchi Y, Johnstone WM 3rd, Borski RJ, Lerner DT, Grau EG. Gen Comp Endocrinol. 2013 Oct 1; 192:191-203. Epub 2013 May 28.
- Stimulus–Secretion Coupling in the Osmoreceptive Prolactin Cell of the Tilapia -...Stimulus–Secretion Coupling in the Osmoreceptive Prolactin Cell of the Tilapia - Mechanosensitivity in Cells and Tissues
Your browsing activity is empty.
Activity recording is turned off.
See more...
 .
.