NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Breman JG, Alilio MS, White NJ, editors. Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives: Supplement to Volume 77(6) of American Journal of Tropical Medicine and Hygiene. Northbrook (IL): American Society of Tropical Medicine and Hygiene; 2007 Dec.

Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives: Supplement to Volume 77(6) of American Journal of Tropical Medicine and Hygiene.
Show detailsAt six sites in western Kenya, we explored the presence of Anopheles immature stages in treeholes. An. gambiae larvae were found in 19 species, 13 of which are exotic. The most common exotic species were Delonix regia, Jacaranda mimosipholia, and Eucalyptus citrodora. In Kisumu city, longitudinal assessments of 10 flamboyant trees showed repeated presence of An. gambiae s.s. in treeholes with water. Production of Anopheles larvae did not correlate with habitat volume but with habitat height, showing a strong but statistically insignificant negative correlation. During a dry season, eggs recovered by rinsing dry treeholes hatched into 2.5 ± 3.06 An. gambiae and 7.9 ± 8.2 Aedes larvae. In cage experiments, An. gambiae s.s. laid more eggs in water originating from treeholes than in distilled or lake water, implying preference for ovipositing in this habitat. Our findings indicate that treeholes represent a hitherto unrecognized habitat for malaria vectors, which needs further studies.
Introduction
Worldwide, ecosystems have been disrupted both by the reduction of native species populations1 and by the introduction of foreign species.2,3 Invasive species can be deliberately or unintentionally introduced, with potentially negative effects on human inhabitants.4 In particular, invasive mosquito species have aided the spread of diseases. Invasive Aedes mosquitoes from Africa and Asia have spread yellow fever and dengue worldwide.5 Anopheles gambiae from Africa became an established vector of malaria in Brazil before a successful eradication campaign in the 1940s.6 In addition to the negative impact of unwanted plants on food production by competing with food crops,7 some species have promoted invasive or indigenous mosquito larvae. Surveys of treeholes in urban areas have shown that non-native, ornamental trees contain larvae of a variety of opportunistic and treehole-adapted mosquito species. Exotic, ornamental “Lucky bamboo” (Dracaena sanderiana) from Asia were directly linked with the introduction of Aedes (Stegomyia) albopictus in southern California.8
Mosquito control programs are most effective when larval habitats are identified for elimination or treatment with insecticides as part of an integrated vector management effort. Members of the An. gambiae complex of sub-Saharan Africa characteristically breed in temporary ground-pools close to human habitations or in more extensive water surfaces such as rice fields, river edges, or swamp margins.9 During a comprehensive survey of treeholes in Kenya in 1981, Lounibos10 found no Anopheles mosquito larvae. However, the types of trees surveyed during that study consisted of native species, which have been reduced by deforestation, especially in the densely populated regions of Kenya, and replaced with cultivated species. In his surveys, larvae of Aedes (Stegomyia) species were most commonly found in treeholes. These species are adapted to treeholes through possession of desiccation-resistant eggs laid on the bark of phytotelemata when drying.11 The return of rain leads to hatching of the embryonated eggs. In contrast, other species use treeholes opportunistically, including the pantropical Southern House Mosquito, Culex quinquefasciatus.12 A preliminary survey of aquatic habitats in western Kenya suggested the presence of An. gambiae larvae in several previously unreported habitat types, including treeholes.13 In view of the significance of this finding, a more detailed survey of exotic and indigenous plants with treeholes in western Kenya was undertaken. Here we detail the results of our findings.
Materials and Methods
Study Area
A map of the Republic of Kenya with an inset of Nyanza province (Figure 1) shows the six study locations. Two study locations are rural (Rusinga Island, Suba Distict and Mosocho, Kisii District) and four are urban (Kisumu City, Kisumu District; Homabay Town, Homabay District; Mbita Town, Suba District; and Kisii Town, Kisii District). Altitudes of the study locations (in meters above sea level) were as follows: Mbita, 1,252; Rusinga Island, 1,274; Homabay, 1,260; Kisumu, 1,277; Kisii Town, 1,851; Mosocho, 1593. The distance from the extreme north of Kisumu to the extreme south of Kisii Town is ~80 km and that from the extreme west of Rusinga Island to the extreme east of Kisumu is ~100 km. The sites are geographically confined to the Lake Victoria Basin, a malaria mesoendemic to holoendemic area, and the highlands of western Kenya, a malaria epidemic area, respectively.
Survey of Trees
Trees at five locations (Kisii, Kisumu, Mbita, Rusinga, and Homabay) were examined for treeholes. Holes were categorized as pan holes (having unbroken bark lining) or rot holes (cavities penetrating into the heart wood) as defined by Ketching.14 Trees with holes containing Anopheles mosquito larvae were identified to species.15,16 Local common names and uses of the tree were determined with the assistance of local community members. Anopheles larvae were taken to the laboratory and identified morphologically. Subsamples of the cryptic species complex An. gambiae were identified by polymerase chain reaction (PCR) assay.17
Longitudinal Assessments
To evaluate the consistency of treehole use by mosquitoes for oviposition over time, more detailed studies were made of 10 Flamboyant trees (Delonix regia) on the grounds of the Lumumba Health Clinic in the city of Kisumu, Kenya (Figure 2). Each tree contained a single treehole with the exception of one, which contained three. The treeholes were evaluated during 21 visits (6 December 2003 to 20 March 2004) no more than 1 week apart. Collections of larvae and pupae were made with as little disturbance to the habitats as possible using plastic pipettes. Subsequent to these assessments, the maximum potential volume of each habitat and height of treehole above the ground were determined for purposes of correlating mosquito productivity with these habitat characteristics.
Dry Habitat Assessments
These 12 treeholes were also evaluated five times over the course of 1 month during a dry season (19 February to 16 March 2003) when all treeholes were dry to see if desiccation-resistant mosquito eggs were present. During each of the evaluations, treeholes were rinsed with tap water, which was re-collected (leaving no standing water) and brought back to the laboratory. Hatched larvae were reared to adult or late-instar larvae, identified, and enumerated.
Oviposition Choice Experiments
Cage bioassays were used to determine water source preferences for gravid An. gambiae. Experiments were conducted at the Mbita Point Field Station, from 1 January to 15 April 2003, using human-fed An. gambiae s.s. from a colony maintained at the ICIPE Research Station at Mbita Point. Water samples were collected from Flamboyant and Leucaena treeholes located in Mosocho and Mbita. Alternate substrates consisted of water obtained from Lake Victoria and distilled water. Experiments took place in a screen house insectary, where temperature, humidity, and diurnal variation were the same as that of the ambient conditions in Mbita. Thirty × 30 × 30-cm mesh-netted cages were used for the experiments. Thirty-milliliter cups were used to hold 20 mL of the different water substrates, which were placed at opposite corners of the mesh cages. A single blood-fed gravid female An. gambiae mosquito was placed into each cage at 1700 hours, and the mosquitoes were allowed to oviposit for 24 hours. The following day, cups were removed and checked for presence/absence of eggs under a dissecting microscope. Concurrent with all choice experiments, cages were prepared containing one of the two control substrates with single gravid blood-fed females to establish if the mosquito would oviposit on the competing substrate when given no alternative.
Results
Survey of Trees
A total of 19 species of trees were found to harbor Anopheles larvae (Table 1). According to local inhabitants, 18 of these were important for economic, medical, or ornamental purposes and were deliberately cultivated by inhabitants. Thirteen of these cultivated tree species were not native to Kenya. Of the species likely to be cultivated in large numbers, inhabitants identified the Flamboyant tree (Delonix regia), Jacaranda (Jacaranda mimosifolia), and the Java fig tree (Ficus benjamina) as trees used for ornamental shade in parks, public grounds, or forming an alee along the road side. All habitats were pans (without penetration through the tree bark) except for one rot hole in a fig tree. The rot hole was formed after pruning of the tree.
Table 1
Tree species with phytotelemata containing Anopheles larvae assessed at five locations in western Kenya
Of the 116 Anopheles mosquitoes collected in treeholes and reared to adult in the insectary, all were morphologically identified as An. gambiae s.l. PCR identification of the samples revealed 109 (94%) An. gambiae s.s. and 7 (6%) unknown.
Longitudinal Assessments
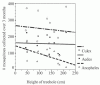
In a series of 21 assessments (December 2003 to March 2004) from Flamboyant trees on the grounds of Lumumba Health Clinic in Kisumu, Anopheles larvae were consistently found at lower densities than Culex or Aedes larvae. Maximum potential habitat volume ranged from 1.0 to 27.3 L (mean = 7.2 L, SD = 7.4 L). Three of the treeholes contained water during every assessment. Seven treeholes were dry for one or two assessments. One treehole was dry six times, and another was dry seven times. With exclusion of these last two treeholes from analysis, there is a strong negative correlation between the log of habitat volume and the number of times it was found to be dry (R = −0.642, P = 0.045). (All correlations reported are Pearson’s R, SPSS 11.0; SPSS, Chicago, IL.) Maximum potential volume data were log-transformed to reduce the skewing effects of a single outlier. Partial correlation coefficients (controlling for drying of habitats) were calculated for comparing larval production with habitat volume. This showed that habitat size was strongly correlated with production of Culex (R = 0.612, P = 0.045) and Aedes (R = 0.515, P = 0.105) larvae but not with that of Anopheles larvae (R = 0.176, P = 0.606). Height of treehole above the ground ranged from 42 to 212 cm (mean = 122.6 cm, SD = 60.0 cm) and showed a strong, but statistically insignificant, negative correlation with Anopheles production (R = −0.554, P = 0.061). Height of the habitat did not correlate with Aedes (R = −0.043, P = 0.895) or Culex (R = −0.097, P = 0.764) larval production (Figure 3).
Dry Habitat Assessments
A mean of 2.5 (SD = 3.06, range = 0–13) An. gambiae larvae and 7.9 (SD = 8.2, range = 0–30) Aedes larvae hatched from egg collections per treehole for all visits. The single tree possessing three treeholes had one hole that contained Anopheles and Aedes larvae in all cases and one that never contained Anopheles but sometimes Aedes larvae. All other treeholes contained both Anopheles and Aedes larvae in some of the samplings.
Total number of Anopheles collected over 1 month (using five assessments) was not significantly correlated with habitat volume (R = 0.127, P = 0.695) and had a strong but not statistically significant negative correlation with height above ground (R = −0.387, P = 0.214). Total number of Aedes strongly correlated with habitat volume (R = 0.497, P = 0.100) but not with height above ground (R = −0.262, P = 0.410). There was a very strong positive correlation between the number of Anopheles and that of Aedes in the habitats (R = 0.853, P < 0.001).
Oviposition Choice Experiments
In oviposition choice experiments, gravid female Anopheles gambiae s.s. laid eggs in only one of the two water substrate choices. A binomial test for significance was calculated using a test proportion of 0.50 for the presence of eggs in the cups. Eggs were found more often in water from Flamboyant and Leucaena treeholes than in either distilled water or lake water (Table 2).
Table 2
Oviposition choice experiments comparing preference of gravid An. gambiae for water from trees vs. control sources
Discussion
This study showed invasion of Anopheles gambiae s.s., the principal malaria vector in sub-Saharan Africa, into the treehole ecosystem. The discovery of An. gambiae in treeholes is in contrast to the detailed studies of Lounibos conducted in 1974–1977.10 In his studies, conducted near the Kenyan coast, only species of Aedes, Culex, Eretmapodites, and Toxorhynchites were found. However, this study focused on a densely populated region where native trees have largely been removed. There has been a significant shift in the fauna of Africa with increases in human population densities, which has led to transformation of land for crops, buildings, and fuel, and resulting deforestation and introduction of exotic trees.18 The shift to cultivating exotic species of trees has led to a substantial change in the kinds of habitats available to mosquito species. Similarly, massive ecological changes in the wetlands of the Kenyan highlands, maintained by strong socioeconomic motivations, have been implicated in the onset of malaria epidemics in that area historically free from tropical diseases.13
In addition to the major ecological changes that have taken place since the comprehensive studies of treehole mosquitoes in Kenya by Lounibos, there is the geographical barrier of the Rift Valley between the areas in his study and the present one. The Rift Valley has been shown to be a significant barrier to gene flow in An. gambiae and may be a second cause for the failure of Lounibos to find Anopheles.10,19
Although altitude has been thought to play an important role in limiting malaria in the tropical highlands by negatively influencing the development of vector mosquitoes, An. gambiae were found in high densities in treeholes in Kisii town, which is at 1,650 m above sea level, and in surrounding areas at altitudes > 2,000 ms (FX Omlin, unpublished data). This finding along with the discovery of high densities of ground-pool–inhabiting An. gambiae in disturbed habitats13 suggests that altitude itself is insufficient to prevent the development of vector mosquitoes.
The large treehole habitats in Flamboyant trees (Figure 3) are similar to the temporary ground pools traditionally used by An. gambiae larvae in their size and temporality. As in previous treehole studies, size of Flamboyant treeholes was found to be linked with longevity of the habitat.10 The smallest habitat in our longitudinal assessments was 1 L and ranged up to 27.3 L. Over 3 months of observation, 3 of the 12 treeholes did not dry up. We found that Anopheles do not discriminate between size of habitats over the range of sizes in this study; however, they may avoid highly temporary, small treeholes. Affinity of An. gambiae to habitats lower to the ground further suggests a link between use of traditional ground pool habitats and the large treehole habitats of this study. Although oviposition experiments in this study show that eggs are laid in lakes or distilled water, water from treeholes is clearly preferred.
Previous research has evaluated the ability of immature Anopheles to resist desiccation for short periods of time. A study by Beier and others20 revealed the ability of both An. gambiae s.s. and An. arabiensis eggs to survive desiccation at the periphery of drying ground pools in the coastal region of Kenya. However, the reported densities were far below those in the treeholes in this study. The ability of both eggs and larvae of An. gambiae s.s. to survive desiccation on soil has also been documented in laboratory experiments, but survival was measured only in days on damp soil.21 In our study, the hatching of larvae from dry treeholes rinsed over the course of a month during the dry season suggests the presence of eggs adapted for resistance to desiccation. Anopheles larvae were found on each of five total washes, suggesting either that Anopheles adults continue to oviposit on the treeholes in a manner similar to Aedes (Stegomyia) treehole mosquitoes or that multiple washes are needed to recover all eggs as also seen with Aedes (Stegomyia) species.10
The non-recovery of Culex species and the correlation of Aedes and Anopheline numbers in dry habitat assessments further support the assumption that eggs (rather than persistence of larvae) were the source of Anopheles produced in these treeholes. The development of eggs with desiccation-resistant traits probably occurred on the ground in drying pools before exploitation of treeholes as oviposition habitats. This would be in contrast to Culex quinquefasciatus, which oviposits in a wide variety of habitats and likely uses treeholes opportunistically.12 Although some species of Anopheles have adapted to treeholes in other parts of the world22 and have been found in low densities in leave axils23 in Kenya, our finding of high densities of An. gambiae is cause for alarm.
The results of this study may be pertinent to urban planners. Although the choices made by individuals with specific socio-economic motivations may be hard to alter, many of the trees bearing large treeholes in this study were on the grounds of governmental or institutional property. For example, on the grounds of the Lumumba Health Clinic in Kisumu, the 13 treeholes described in this study were within a 2,050-m2 area. By planting species of tree with a lower propensity for generating large treeholes at governmental facilities and along public roadways, a significant decrease in larval habitats may be realized.
Treeholes in highly populated areas may constitute a significant source of malaria-vectoring mosquitoes near human habitations. Malaria control programs use multiple techniques concurrently to reduce transmission, including indoor residual sprayings, insecticide treated nets, drug distribution programs, and larval habitat treatments. Modeling of the potential impact of interventions in larval habitats suggests that this is an important component of malaria vector and malaria control programs.24 To be effective, treatment of larval habitats with insecticides requires comprehensive knowledge of habitat distributions. In light of the number of Anopheles produced in the treeholes of exotic tree species, it will be important to establish the distribution of these trees.
Studies over a greater geographical scale and comparing the current distribution of exotic and indigenous trees with the patterns 20 years ago could provide insight into the evolving use of these habitats by malaria vectors. Changes in tree patterns over Africa (in particular, in sub-Saharan Africa) may have also altered the patterns of malaria transmission. The scope of the malaria public health threat caused by treeholes in Africa needs to be addressed.
Acknowledgments: The authors dedicate this study to both the former Director General of ICIPE, H. R. Herren and his successor, C. Borgemeister, for consistent interest, support, and encouragement. The authors thank Pamela Seda of ICIPE Malaria Research Laboratory for the PCR analyses. Figure 1 was prepared by Ronald Osano. The biology teacher, Father Macarios, of Cardinal Otunga High-School (Mosocho, Kisii District) helped in species identification of the treehole-bearing trees. Morphologic mosquito species identification was carried out at Kenya Medical Research Institute (KEMRI), Kisumu. ICIPE’s field project staff made valuable contributions to this study
Financial support: This study was supported by the Government of Finland trough Grant 24811201 and BioVision, Switzerland to ICIPE (Francois X. Omlin). Travel expenses for John C. Carlson were provided through NIH ICIDR Grant U19 A145511 and personal financial support provided through CDC fellowship Training Grant CCT 622308-02.
Disclaimer: The opinions or assertions contained in this manuscript are the private ones of the authors and are not to be construed as official or reflecting the views of the US Public Health Service or Department of Health and Human Services. Use of trade names is for identification only and does not imply endorsement by the US Public Health Service or Department of Health and Human Services.
References
- 1.
- Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth’s ecosystems. Science. 1997;277:494–499.
- 2.
- Vitousek PM, D’Antonio CM, Loope LL, Westbrooks R. Biological invasions as global environmental change. Am Sci. 1996;84:468–478.
- 3.
- Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, A. BF. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl. 2000;10:689–710.
- 4.
- Corvalan CF, Hales S, McMichael AJ, Butler C, Campbell-Lendrum D, Confalonieri U, Leitner K, Lewis N, Patz J. Ecosystems and Human Well-Being: Health Synthesis. Geneva: World Health Organization; 2005.
- 5.
- Lounibos LP. Invasions by insect vectors of human disease. Annu Rev Entomol. 2002;47:233–266. [PubMed: 11729075]
- 6.
- Soper FL, Wilson DB. Anopheles gambiae in Brazil: 1930 to 1940. New York: The Rockefeller Foundation; 1943.
- 7.
- Vilà M, Williamson M, Lonsdale M. Competition experiments on alien weeds with crops: lessons for measuring plant invasion impact? Biol Invasions. 2004;6:59–69.
- 8.
- Madon MB, Mulla MS, Shaw MW, Kluh S, Hazelrigg JE. Introduction of Aedes albopictus (Skuse) in southern California and potential for its establishment. J Vector Ecol. 2002;27:149–154. [PubMed: 12125866]
- 9.
- Minakawa N, Sonye G, Mogi M, Yan G. Habitat characteristics of Anopheles gambiae s.s larvae in a Kenyan highland. Med Vet Entomol. 2004;18:301–305. [PubMed: 15347399]
- 10.
- Lounibos LP. Habitat segregation among African treehole mosquitoes. Ecol Entomol. 1981;6:129–154.
- 11.
- Sota T, Mogi M. Interspecific variation in desiccation survival time of Aedes (Stegomyia) mosquito eggs is correlated with habitat and egg size. Oecologia. 1992;90:353–358. [PubMed: 28313521]
- 12.
- Jenkins DW, Carpenter SJ. Ecology of the tree hole breeding mosquitoes of nearctic North America. Ecol Monogr. 1946;16:31–47.
- 13.
- Carlson JC, Byrd BD, Omlin FX. Field assessments in western Kenya link malaria vectors to environmentally disturbed habitats during the dry season. BMC Public Health. 2004;4:33. [PMC free article: PMC512294] [PubMed: 15296512]
- 14.
- Kitching RL. An ecological study of water-filled treeholes and their position in the woodland ecosystem. J Anim Ecol. 1971;40:281–302.
- 15.
- Dale IR, Greenway PJ. Kenya Trees and Shrubs. Nairobi: Buchanan’s Kenya Estates; 1961.
- 16.
- Dharani N. Field Guide to Common Trees and Shrubs of East Africa. Cape Town: Struik Publishers; 2002.
- 17.
- Lanzaro GC, Touré YT, Carnahan J, Zheng L, Dolo G, Traoré S, Petrarca V, Vernick KD, Taylor CE. Complexities in the genetic structure of Anopheles gambiae populations in west Africa as revealed by microsatellite DNA analysis. Proc Natl Acad Sci USA. 1998;95:14260–14265. [PMC free article: PMC24361] [PubMed: 9826688]
- 18.
- Geist HJ, Lambin EF. Proximate causes and underlying driving forces of tropical deforestation. Bioscience. 2002;52:143–150.
- 19.
- Lehmann T, Blackston CR, Besansky NJ, Escalante AA, Collins FH, Hawley WA. The Rift Valley complex as a barrier to gene flow for Anopheles gambiae in Kenya: the mtDNA perspective. J Hered. 2000;91:165–168. [PubMed: 10768135]
- 20.
- Beier JC, Copeland R, Oyaro C, Masinya A, Odago WO, Oduor S, Koech DK, Roberts CR. Anopheles gambiae complex egg-stage survival in dry soil from larval development sites in western Kenya. J Am Mosq Control Assoc. 1990;6:105–109. [PubMed: 2324714]
- 21.
- Shililu JI, Grueber WB, Mbogo CM, Githure JI, Riddiford LM, Beier JC. Development and survival of Anopheles gambiae eggs in drying soil: influence of the rate of drying, egg age, and soil type. J Am Mosq Control Assoc. 2004;20:243–247. [PubMed: 15532921]
- 22.
- Zavortink TJ. The treehole Anopheles of the New World. Contributions of the American Entomological Institute. 1970;5:1–35.
- 23.
- Lounibos LP. Mosquitoes occurring in the axils of Pandanus rabaiensis Rendle on the Kenya toast. Cah ORSTOM Ser Entomol Med Parasitol. 1979;17:25–29.
- 24.
- Gu W, Novak RJ. Habitat-based modeling of impacts of mosquito larval interventions on entomological inoculation rates, incidence, and prevalence of malaria. Am J Trop Med Hyg. 2005;73:546–552. [PubMed: 16172479]
Authors’ addresses: Francois X. Omlin, International Centre of Insect Physiology and Ecology (ICIPE), PO Box 35 Kisii, Kenya, Telephone: 254-727-801284, Fax: 254-20-8632001/2, E-mail: gro.epici@nilmof and ten.mocmim.naisik@nilmof. John C. Carlson, Tulane University, Department of Pediatrics, New Orleans, LA 70112, E-mail: ude.enalut@oslracj. C. Brandon Ogbunugafor, Yale University, School of Medicine, New Haven, CT 06510, E-mail: ude.elay@nodnarb.ekeihc. Ahmed Hassanali, International Centre of Insect Physiology and Ecology (ICIPE), PO Box 30772 Nairobi, Kenya, E-mail: gro.epici@ilanassaha.
- Anopheles Gambiae Exploits the Treehole Ecosystem in Western Kenya: A New Urban ...Anopheles Gambiae Exploits the Treehole Ecosystem in Western Kenya: A New Urban Malaria Risk? - Defining and Defeating the Intolerable Burden of Malaria III: Progress and Perspectives
Your browsing activity is empty.
Activity recording is turned off.
See more...