NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Oxcarbazepine is a keto analogue of carbamazepine and, like the parent drug, is a potent anticonvulsant used alone or in combination with other agents in the therapy of partial seizures. Oxcarbazepine has been linked to rare instances of clinically apparent acute drug induced liver injury which resembles carbamazepine hepatotoxicity.
Background
Oxcarbazepine (ox" kar baz' e peen) is a keto analog of carbamazepine and functions as a prodrug being rapidly converted to 10-hydroxycarbazepine. Oxcarbazepine and carbamazepine are iminostilbenes related chemically to the tricyclic antidepressants and unrelated in structure to most other anticonvulsants. They appear to act by suppression of spread of seizure activity by reduction in the posttetanic potentiation of synaptic transmission. Oxcarbazepine was approved for use in epilepsy in the United States in 2000 and remains in common use. Oxcarbazepine is indicated for prevention and management of partial, complex, mixed and generalized seizures and is commonly used alone or in combination with other anticonvulsants. It is used off label to treat bipolar disorder. Oxcarbazepine is available as tablets of 150, 300 and 600 mg generically and under the commercial name of Trileptal and as an extended release form under the name Oxtellar XR. Oral formulations for use in children are also available. The recommended starting dose in adults is 300 mg twice daily followed by increases at weekly intervals based upon clinical response, the usual final dose being 600 mg twice daily. Frequent side effects include drowsiness, sedation, ataxia, blurred vision, nausea, vomiting, and skin rash. Uncommon but potentially severe adverse events include hyponatremia, suicidal ideation and behaviors, anaphylaxis and hypersensitivity reactions, serious cutaneous adverse events including Stevens-Johnson syndrome and toxic epidermal necrolysis.
Hepatotoxicity
Chronic therapy with oxcarbazepine is associated with elevations in serum aminotransferase levels in a small proportion of patients. These elevations are rarely clinically significant and do not usually require dose modification. Clinically apparent hepatotoxicity from oxcarbazepine is uncommon but described, and is less common than occurs with carbamazepine. Oxcarbazepine hepatotoxicity usually arises in the setting of anticonvulsant hypersensitivity syndrome with onset of fever, followed by rash, facial edema, lymphadenopathy, elevations in white count and eosinophilia 2 to 8 weeks after starting therapy. The liver involvement ranges from a mild and transient elevation in serum enzymes to abrupt onset of an acute hepatitis-like syndrome, that can be severe and even fatal. The typical enzyme elevations are usually mixed, but can be either hepatocellular or cholestatic. Liver biopsy shows mixed necroinflammatory-cholestatic injury with prominence of eosinophils and occasionally granulomas.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of oxcarbazepine hepatotoxicity appears to be hypersensitivity or an immunological response to a metabolically generated drug-protein complex. Serious cutaneous reactions due to oxcarbazepine as with carbamazepine has been linked to the HLA-B*15.01 allele, particularly in Han Chinese subjects. Similar HLA-associations have not been linked to liver injury but the numbers of cases described have been few. Oxcarbazepine like other aromatic anticonvulsants may also trigger acute porphyria by inducing delta-aminolevulinic acid (ALA) synthetase activity. It also induces CYP 3A4 and inhibits CYP 2C19 and can cause drug-drug interactions particularly with other drugs used for epilepsy that induce CYP 3A4 such as phenytoin and phenobarbital.
Outcome and Management
Oxcarbazepine and carbamazepine hepatotoxicity is usually rapidly reversible with stopping therapy, improvements beginning within days. In cases of severe injury, progression to acute liver failure and death can occur. Corticosteroids have been used but with uncertain effectiveness. Cross reactivity with other aromatic anticonvulsants (carbamazepine, phenytoin, phenobarbital, primidone, and lamotrigine) is common, but not invariable. Patients with severe hypersensitivity to oxcarbazepine should avoid exposure to other aromatic anticonvulsants and be switched instead to agents such as a benzodiazepine, valproate, levetiracetam, gabapentin or pregabalin.
Drug Class: Anticonvulsants; see also Carbamazepine
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Oxcarbazepine – Generic, Trileptal®
DRUG CLASS
Anticonvulsants
Product labeling at DailyMed, National Library of Medicine, NIH
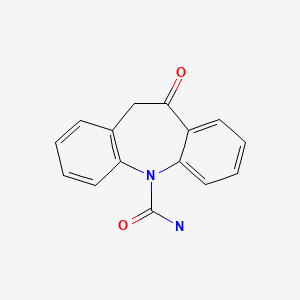
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Oxcarbazepine | 28721-07-5 | C15-H12-N2-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 30 July 2020
See also references for Carbamazepine.
- Abbreviations used: DRESS, drug rash with eosinophilia and systemic symptoms; SJS/TEN, Stevens-Johnson syndrome and toxic epidermal necrolysis.
- Zimmerman HJ. Anticonvulsants. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott,1999: pp. 498-516.(Expert review of anticonvulsants and liver injury published in 1999; oxcarbazepine is not discussed).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-41.(Review of anticonvulsant induced liver injury mentions that oxcarbazepine undergoes less oxidative metabolism than carbamazepine and is a less potent P450 enzyme inducer; cross reactivity between the 2 drugs is estimated to be 25%).
- Smith MD, Metcalf CS, Wilcox KS. Pharmacology of the epilepsies. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 303-26.(Textbook of pharmacology and therapeutics).
- Mitchell MC, Boitnott JK, Arregui A, Maddrey WC. Granulomatous hepatitis associated with carbamazepine therapy. Am J Med. 1981;71:733–5. [PubMed: 7282758](Two men, ages 52 and 54 years, developed dark urine 3 weeks after starting carbamazepine, both with fever but no rash or eosinophilia [bilirubin 1.5 and 3.4 mg/dL, ALT 61 and 145 U/L, Alk P 80 and 236 U/L], biopsies showed granulomatous inflammation; both recovered within 4 weeks).
- Levy M, Goodman MW, Van Dyne BJ, Sumner HW. Granulomatous hepatitis secondary to carbamazepine. Ann Intern Med. 1981;95:64–5. [PubMed: 7247130](Three patients developed fever, fatigue and abdominal pain 3-4 weeks after starting carbamazepine [bilirubin normal to 3.2 mg/dL, AST 20, 95 and 38 U/L, Alk P 1.5-2x ULN, no eosinophilia], biopsies showed granulomas; rechallenge in one led to immediate appearance of fever and rash; rapid recovery).
- Hopen G, Nesthus I, Laerum OD. Fatal carbamazepine-associated hepatitis. Report of two cases. Acta Med Scand. 1981;210:333–5. [PubMed: 7315533](Two women, ages 23 and 37 years, developed rash and fever followed by jaundice 8 days after starting carbamazepine [peak bilirubin 7.9 and 14.0 mg/dL GGT 5350 and 205 U/L, ALT unknown and 1325 U/L], both had progressive liver failure and died; autopsies showed massive necrosis).
- Yeung Laiwah AA, Rapeport WG, Thompson GG, et al. Carbamazepine-induced non-hereditary acute porphyria. Lancet. 1983;1:790–2. [PubMed: 6132132](Two patients on high doses of carbamazepine had decreased uroporphyrinogen I synthase [URO-S] and elevated δ aminolevulinic acid levels, and 1 developed symptoms of acute intermittent porphyria; cohort of patients on carbamazepine had slightly reduced levels of URO-S).
- Perucca E, Hedges A, Makki KA, Ruprah M, Wilson JF, Richens A. A comparative study on the relative enzyme inducing properties of anticonvulsant drugs in epileptic patients. Br J Clin Pharmacol. 1984;18:401–10. [PMC free article: PMC1463658] [PubMed: 6435654](Cross sectional study of antipyrine clearance in 122 patients with epilepsy, found dose dependent increase in clearance in those on phenytoin, phenobarbital and carbamazepine, but not on valproate).
- Davion T, Capron-Chivrac D, Andrejak M, Capron JP. Gastroenterol Clin Biol. 1985;9:117–26. [Hepatitis due to antiepileptic agents] French. [PubMed: 3920108](Review of 15 cases of carbamazepine hepatotoxicity in the literature; onset in 3-4 weeks in most cases, but occasionally longer; granulomatous hepatitis in 8 of 14 cases with biopsy, these cases being invariably benign; three reports of acute liver failure).
- Larrey D, Hadengue A, Pessayre D, Choudat L, Degott C, Benhamou J-P. Carbamazepine-induced acute cholangitis. Dig Dis Sci. 1987;32:554–7. [PubMed: 3568943](79 year old woman developed itching 4 weeks and fever and jaundice [no rash] 7 weeks after starting clonazepam and carbamazepine [bilirubin 6.6 mg/dL, ALT 91 U/L, Alk P 5 times ULN, 54% eosinophils], liver biopsy showed cholestasis and “cholangitis”, resolving within 3 months of stopping carbamazepine, clonazepam being restarted).
- Reinikainen KJ, Keranen T, Halonen T, Komulainen H, Riekkinen PJ. Comparison of oxcarbazepine and carbamazepine: a double-blind study. Epilepsy Research. 1987;1:284–9. [PubMed: 3504404](Among 18 patients on carbamazepine and 16 on oxcarbazepine, 2 developed transient ALT and AST elevations neither requiring dose adjustment).
- Pellock JM. Carbamazepine side effects in children and adults. Epilepsia. 1987;28 Suppl 3:S64–70. [PubMed: 2961558](Analysis of side effects among 220 children on carbamazepine; skin rash in 5% and elevated liver tests in 6% but none “were of clinical significance.” Reports to Ciba-Geigy from 1975-86 included 45 with hepatitis and [5-10%] of patients with liver enzyme elevations).
- Shear NH, Spielberg SP. Anticonvulsant hypersensitivity syndrome: in vitro assessment of risk. J Clin Invest. 1988;82:1826–32. [PMC free article: PMC442760] [PubMed: 3198757](PBMC cytotoxicity in response to drug metabolites from 53 patients with hypersensitivity to anticonvulsants including 35/36 to phenytoin, 22/27 to phenobarbital, and 25/27 to carbamazepine; 51% had hepatitis).
- Robbie MJ, Scurry JP, Stevenson P. Carbamazepine-induced severe systemic hypersensitivity reaction with eosinophilia. Drug Intell Clin Pharm. 1988;22:783–4. [PubMed: 3229345](35 year old woman developed rash 3 weeks after starting carbamazepine, followed by fever and dyspnea with eosinophilia, respiratory and renal failure, responding to prednisone but then relapsing, developing jaundice late [bilirubin 8.8 mg/dL, AST 120 U/L, Alk P 2220 U/L], death from multiorgan failure 12 weeks later).
- Aldenhövel HG. The influence of long-term anticonvulsant therapy with diphenylhydantoin and carbamazepine on serum gamma-glutamyltransferase, aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase. Eur Arch Psychiatry Neurol Sci. 1988;237:312–6. [PubMed: 2901959](Among 54 patients on phenytoin and 56 on carbamazepine, elevations in GGT [14-283 U/L] were present in 91% and 64%, ALT [5-81 U/L] in 28% and 9%, and Alk P [71-216 U/L] in 39% vs 14%, most elevations were modest; GGT elevations were dose related, but not the others).
- Dam M, Ekberg R, Løyning Y, Waltimo O, Jakobsen K. A double-blind study comparing oxcarbazepine and carbamazepine in patients with newly diagnosed, previously untreated epilepsy. Epilepsy Res. 1989;3:70–6. [PubMed: 2645120](Controlled trial of oxcarbazepine vs carbamazepine in 235 patients with epilepsy found similar therapeutic effect but lower rate of side effects with oxcarbazepine; liver tests fluctuated during therapy but only one patient [on carbamazepine] required drug discontinuation because of liver injury).
- Durelli L, Massazza U, Cavallo R. Carbamazepine toxicity and poisoning. Incidence, clinical features and management. Med Toxicol Adverse Drug Exp. 1989;4:95–107. [PubMed: 2654545](Review of pharmacology and toxicity of carbamazepine; symptoms of overdose are largely neurological).
- Askmark H, Wiholm B. Epidemiology of adverse reactions to carbamazepine as seen in a spontaneous reporting system. Acta Neurol Scand. 1990;81:131–40. [PubMed: 2327233](Analysis of 505 reports of adverse reaction to carbamazepine during 92 million dose-days from 1965-1987 in Sweden; 71 were liver related [16/100,000 treatment years], 21 with isolated ALT elevation after 4-46 days, 18 with ALT and Alk P elevations [after 16-60 days], and 25 with jaundice after 6 days to 2 years).
- Frey B, Schubiger G, Musy JP. Transient cholestatic hepatitis in a neonate associated with carbamazepine exposure during pregnancy and breast-feeding. Eur J Pediatr. 1990;150:136–8. [PubMed: 2279511](Persistent jaundice at 3 weeks in male infant being breastfed by mother on carbamazepine [direct/total bilirubin 2.4/13.0 mg/dL, ALT 31 U/L, Alk P 402 U/L], liver biopsy showed cholestatic hepatitis, with rapid recovery on stopping).
- Merlob P, Mor N, Litwin A. Transient hepatic dysfunction in an infant of an epileptic mother treated with carbamazepine during pregnancy and breastfeeding. Ann Pharmacother. 1992;26:1563–5. [PubMed: 1362364](Newborn girl being breast fed by a mother taking carbamazepine had persisting jaundice in first 2 weeks of life [direct/total bilirubin 1.4/3.4 mg/dL, ALT 32 U/L, Alk P 293 U/L on day 5], resolving rapidly with stopping breastfeeding).
- Forbes GM, Jeffrey GP, Shilkin KB, Reed WD. Carbamazepine hepatotoxicity: another cause of the vanishing bile duct syndrome. Gastroenterology. 1992;102:1385–8. [PubMed: 1551543](59 year old man developed fever, rash and jaundice 2 months after starting carbamazepine [bilirubin 12.4 mg/dL, AST 99 U/L, Alk P 1030 U/L], evolving into chronic cholestasis and vanishing bile duct syndrome, which improved clinically, but liver tests were still abnormal 1 year later).
- Friis ML, Kristensen O, Boas J, et al. Therapeutic experiences with 947 epileptic out-patients in oxcarbazepine treatment. Acta Neurol Scand. 1993;87:224–7. [PubMed: 8475694](Retrospective study of 947 patients treated with oxcarbazepine in 8 centers from 1981-90: adverse events in 33%, rash in 6%, elevations in laboratory results [ALT, AST, GGT or WBC] in <2%).
- Wallace SJ. A comparative review of the adverse effects of anticonvulsants in children with epilepsy. Drug Saf. 1996;15:378–93. [PubMed: 8968693](Systematic review; ALT elevations occur in 4% of children on phenytoin, 6% on valproate, 1% on carbamazepine but none reported in children on tiagabine or gabapentin).
- Nathan DL, Belsito DV. Carbamazepine-induced pseudolymphoma with CD-30 positive cells. J Am Acad Dermatol. 1998;38:806–9. [PubMed: 9591791](44 year old woman with a history of phenytoin sensitivity developed fever, lymphadenopathy, pneumonitis, and rash one month after starting carbamazepine [bilirubin not given, AST 282 U/L, Alk P 44 U/L] and atypical CD-30 T cells in blood, resolution in 3 weeks on stopping).
- Schlienger RG, Shear NH. Antiepileptic drug hypersensitivity syndrome. Epilepsia. 1998;39 Suppl 7:S3–7. [PubMed: 9798755](Review of the clinical features of anticonvulsant hypersensitivity syndrome).
- Hamer HM, Morris HH. Hypersensitivity syndrome to antiepileptic drugs: a review including new anticonvulsants. Cleve Clin J Med. 1999;66:239–45. [PubMed: 10199060](Clinical review of anticonvulsant hypersensitivity syndrome, which occurs in 1-5 per 10,000 users, higher risk in African Americans and affected siblings; liver involvement common, but most cases anicteric; other manifestations include facial edema, lymphadenopathy, bone marrow aplasia, pseudolymphoma, thyroiditis, interstitial nephritis).
- Knowles SR, Shapiro LE, Shear NH. Anticonvulsant hypersensitivity syndrome: incidence, prevention and management. Drug Saf. 1999;21:489–501. [PubMed: 10612272](Review of anticonvulsant hypersensitivity syndrome: triad of fever, rash and internal organ injury occurring 1-8 weeks after exposure to anticonvulsant; liver being most common internal organ involved. Occurs in 1:1000-1:10,000 initial exposures to phenytoin, carbamazepine, phenobarbital or lamotrigine, unrelated to dose, perhaps predisposed by valproate; liver injury arises 1-4 weeks after onset of rash and ranges in severity from asymptomatic ALT elevations to icteric hepatitis to acute liver failure. High mortality rate with jaundice; other organs include muscle, kidney, brain, heart and lung. Pseudolymphoma syndrome and serum sickness like syndrome are separate complications of anticonvulsants. Role of corticosteroids uncertain; cross reactivity among the agents should be assumed).
- Hamer HM, Morris HH. Successful treatment with gabapentin in the presence of hypersensitivity syndrome to phenytoin and carbamazepine: a report of three cases. Seizure. 1999;8:190–2. [PubMed: 10356381](3 patients developed rash, fever, lymphadenopathy and eosinophilia 4-6 weeks after starting either phenytoin or carbamazepine [bilirubin 0.5-1.8 mg/dL, ALT 866-1402 U/L, Alk P 69-364 U/L], resolving after stopping and not recurring during gabapentin therapy).
- Descamps V, Valance A, Edlinger C, Fillet AM, Grossin M, Lebrun-Vignes B, Belaich S, et al. Association of human herpesvirus 6 infection with drug reaction with eosinophilia and systemic symptoms. Arch Dermatol. 2001;137:301–4. [PubMed: 11255328](Among 7 patients with DRESS syndrome, all had anti-HHV-6, 2 in rising titers, 4 with IgM, none had HHV-6 DNA; 5 cases due to carbamazepine, 1 sulfasalazine and 1 ibuprofen).
- Sullivan JR, Shear NH. The drug hypersensitivity syndrome. What is the pathogenesis? Arch Dermatol. 2001;137:357–64. [PubMed: 11255340](Review of clinical features, major causes and suspected pathogenesis of DRESS; major causes are aromatic anticonvulsants, abacavir, azathioprine, and sulfonamides).
- Garcia M, Mhanna MJ, Chung-Park MJ, Davis PH, Srivastava MD. Efficacy of early immunosuppressive therapy in a child with carbamazepine-associated vanishing bile duct and Stevens-Johnson syndromes. Dig Dis Sci. 2002;47:177–82. [PubMed: 11837721](4 year old developed necrotizing rash and fever 4 months after starting carbamazepine [bilirubin 4.0 mg/dL, ALT 673 U/L, Alk P 481 U/L, GGT 613 U/L], Stevens-Johnson syndrome; biopsy showing “early” vanishing bile duct syndrome, treated with corticosteroids with rapid response but slow decline in GGT and cholesterol at the time of 6 week follow up).
- Frey B, Braegger CP, Ghelfi D. Neonatal cholestatic hepatitis from carbamazepine exposure during pregnancy and breast feeding. Ann Pharmacother. 2002;36:644–7. [PubMed: 11918515](Newborn boy developed jaundice and mild elevations in GGT, Alk P and ALT 3 to 4 weeks after difficult birth to mother on carbamazepine who breastfed: two previous cases discussed).
- Bosdure E, Cano A, Roquelaure B, Reynaud R, Boyer M, Viard L, Sarles J. Arch Pediatr. 2004;11:1073–7. [Oxcarbazepine and DRESS syndrome: a paediatric cause of acute liver failure] French. [PubMed: 15350998](11 year old girl developed rash, fever and eosinophilia 43 days after starting oxcarbazepine [bilirubin not given, ALT 25 times ULN, Alk P not given, factor V 15%], progressing to hepatic failure, but eventually improving spontaneously and resolving completely within 6 weeks).
- Tohyama M, Hashimoto K, Yasukawa M, Kimura H, Horikawa T, Nakajima K, Urano Y, et al. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol. 2007;157:934–40. [PubMed: 17854362](Anti-HHV-6 testing of 100 patients with drug induced hypersensitivity syndrome [34% with hepatitis] found rise in IgG levels in 62 patients, largely in more severe cases; HHV-6 DNA detected in 18; drugs included carbamazepine, phenobarbital, phenytoin, allopurinol, sulfasalazine and mexiletine).
- Ganeva M, Gancheva T, Lazarova R, Troeva J, Balarenov I, Vassilev I, Hristakieva E, et al. Carbamazepine-induced drug reaction with eosinophilia and systemic symptoms(DRESS) syndrome: report of four cases and brief review. Int J Dermatol. 2008;47:853–60. [PubMed: 18717872](Review of carbamazepine induced hypersensitivity syndromes and 4 case reports; onset after 3-4 weeks of starting carbamazepine with rash, fever and eosinophilia, 2 with liver involvement and 1 with jaundice, all treated with corticosteroids and all resolved without recurrence, 3 taking valproate).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, valproate accounted for 6, lamotrigine 5, phenytoin 5, gabapentin and topiramate 1 each; none were due to oxcarbazepine).
- Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scand. 2008;118:281–90. [PubMed: 18341684](Review of all anticonvulsants including 60 cases of carbamazepine usually part of hypersensitivity syndrome, typically mixed enzyme pattern, 27% with eosinophilia, mean onset at 5 weeks, 17% mortality more common in children, with hepatocellular pattern of enzymes and with longer latency to onset; mentions oxcarbazepine has been linked to a few cases reports showing mild-to-moderate liver test elevations).
- Franzoni E, Gentile V, Pellicciari A, Garone C, Iero L, Gualandi S, Cordelli DM, et al. Prospective study on long-term treatment with oxcarbazepine in pediatric epilepsy. J Neurol. 2009;256:1527–32. [PubMed: 19597919](Among 36 children with epilepsy treated with oxcarbazepine for up to 3 years, “no hepatic dysfunctions were reported”).
- Buggy Y, Layton D, Fogg C, Shakir SA. Safety profile of oxcarbazepine: results from a prescription-event monitoring study. Epilepsia. 2010;51:818–29. [PubMed: 20132298](Results of mail survey of 2243 patients given a prescription for oxcarbazepine by physicians in England between 2000 and 2003, mostly for seizures but also trigeminal neuralgia and neuropathic pain; 40% as monotherapy, most common side effects were sedation, nausea, malaise and dizziness; no mention of liver injury or ALT elevations).
- Hsu HF, Huang SY. Severe hepatitis associated with administration of oxcarbazepine. Pediatr Int. 2010;52:677–8. [PubMed: 20958883](8 year old girl with partial seizures developed malaise, fever and pharyngitis without rash 12 days after starting oxcarbazepine [bilirubin 0.6 mg/dL, ALT 1258 U/L, Alk P 128 U/L], resolving rapidly upon stopping and then tolerating levetiracetam).
- Drugs for epilepsy. Treat Guidel Med Lett. 2013;11:9–18. Erratum in Treat Guidel Med Lett 2013; 11: 112. [PubMed: 23348233](Concise review of drugs of choice for epilepsy; oxcarbazepine alone or in combination is useful for treatment of partial seizures; adverse events include somnolence, dizziness, diplopia, ataxia, nausea and vomiting; liver injury is not mentioned).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 1 attributed to phenytoin among only 410 persons receiving the drug in Iceland; no case was attributed to oxcarbazepine).
- Planjar-Prvan M, Bielen A, Sruk A, Marusić M, Bielen I. Acute oxcarbazepine-induced hepatotoxicity in a patient susceptible to developing drug-induced liver injury. Coll Antropol. 2013;37:281–4. [PubMed: 23697284](23 year old woman developed fatigue, anorexia, and abnormal liver tests without fever or rash 3 weeks after starting oxcarbazepine [bilirubin normal, peak ALT 17 times ULN, Alk P 2.2 times ULN], falling into the normal range 4 months after switching to gabapentin; two years later she developed abnormal enzymes during therapy with amoxicillin/clavulanate).
- Kaniwa N, Saito Y. The risk of cutaneous adverse reactions among patients with the HLA-A* 31:01 allele who are given carbamazepine, oxcarbazepine or eslicarbazepine: a perspective review. Ther Adv Drug Saf. 2013;4:246–53. [PMC free article: PMC4125310] [PubMed: 25114785](Review of HLA-A*31:01 and cutaneous reactions to carbamazepine found a significant association with the spectrum of cutaneous reactions [maculopapular, erythema multiforme, SJS and TEN] in both Asian and European populations, unlike HLA-B*15:02 which is closely linked to SJS/TEN but largely among Han Chinese populations; associations of A*31:01 with oxcarbazepine have not been made and links of cutaneous reactions with B*15:02 appear to be weaker than with carbamazepine).
- French JA, Baroldi P, Brittain ST, Johnson JK., PROSPER Investigators Study Group. Efficacy and safety of extended-release oxcarbazepine (Oxtellar XR™) as adjunctive therapy in patients with refractory partial-onset seizures: a randomized controlled trial. Acta Neurol Scand. 2014;129:143–53. [PMC free article: PMC4033571] [PubMed: 24359313](Among 366 patients with partial onset seizures treated with adjunctive extended release oxcarbazepine [1200 or 2400 mg] or placebo for 16 weeks, seizure frequency decreased with the higher dose of oxcarbazepine, while adverse events were also more frequent particularly dizziness, asthenia and fatigue; no mention of ALT elevations or hepatotoxicity).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, 7 [4%] of which were attributed to anticonvulsants including 3 due to phenytoin, 3 to valproate and 1 to carbamazepine; none were attributed to oxcarbazepine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 [4.5%] were due to anticonvulsants including 12 due to phenytoin, 9 lamotrigine, 7 valproate, 4 carbamazepine, 3 gabapentin, 2 topiramate and 1 each for ethosuximide, fosphenytoin, and pregabalin; none were attributed to oxcarbazepine).
- Chung SS, Johnson JK, Brittain ST, Baroldi P. Long-term efficacy and safety of adjunctive extended-release oxcarbazepine (Oxtellar XR®) in adults with partial-onset seizures. Acta Neurol Scand. 2016;133:124–30. [PMC free article: PMC5042072] [PubMed: 26248506](Among 214 patients with refractory partial onset seizures participating in a controlled trial [French 2014] who were then treated with open label oxcarbazepine [1200 mg] for 48 weeks, adverse events included dizziness, headache, diplopia, nausea and asthenia and led to early discontinuation in 5% of patients; no mention of ALT elevations or cutaneous reactions).
- Trivedi BS, Darji NH, Malhotra SD, Patel PR. Antiepileptic drugs-induced Stevens-Johnson syndrome: a case series. J Basic Clin Pharm. 2016;8:42–44. [PMC free article: PMC5201065] [PubMed: 28104975](Among 9 cases of Stevens-Johnson syndrome seen at a large referral center in India, 5 were due to phenytoin, 2 carbamazepine and 2 oxcarbazepine: 18 year old female and 58 year old man who developed rash within 4 and 7 days of starting oxcarbazepine, both had rapid improvement on stopping; no mention of hepatic involvement).
- Chait Mermelstein A, Mermelstein J, Adam T, Brody BD, Dubin MJ. Oxcarbazepine-induced liver injury after sensitization by valproic acid: a case report. Bipolar Disord. 2016;18:307–9. [PubMed: 27041538](46 year old woman with bipolar disorder treated with valproate for 3 years was found to have liver enzyme elevations [ALT 163 U/L, Alk P 180 UL, bilirubin normal], which improved on stopping valproate but ALT rose again when she was started on oxcarbazepine which resolved on stopping and recurred on restarting).
- Yip VL, Pirmohamed M. The HLA-A*31:01 allele: influence on carbamazepine treatment. Pharmgenomics Pers Med. 2017;10:29–38. [PMC free article: PMC5293506] [PubMed: 28203102](Review of HLA-A*31:01 and risk of cutaneous adverse events due to carbamazepine mentions that the spectrum of associations is wider than with HLA-B*15:02 and is a risk factor in both Asian and European populations; in contrast there is little evidence of linkage of A*31:01 or B*15:02 with oxcarbazepine cutaneous reactions, although few cases have been studied).
- Kim JH, Lee SK, Loesch C, Namgoong K, Lee HW, Hong SB. Korean N01367 Study Group. Comparison of levetiracetam and oxcarbazepine monotherapy among Korean patients with newly diagnosed focal epilepsy: A long-term, randomized, open-label trial. Epilepsia. 2017;58:e70–e74. [PubMed: 28395124](Among 347 adults with new onset focal epilepsy treated with levetiracetam or oxcarbazepine for 48 weeks, seizure free rates were similar in the 2 groups as were adverse event rates; no mention of severe cutaneous reactions or hepatotoxicity).
- Vidaurre J, Gedela S, Yarosz S. Antiepileptic drugs and liver disease. Pediatr Neurol. 2017;77:23–36. [PubMed: 29097018](Review of the use of anticonvulsants in patients with liver disease recommends use of agents that have little hepatic metabolism such as levetiracetam, lacosamide, topiramate, gabapentin and pregabalin, levetiracetam being an "ideal" first line therapy for patients with liver disease because of its safety and lack of pharmacokinetic interactions).
- Drugs for epilepsy. Med Lett Drugs Ther. 2017;59(1526):121–30. [PubMed: 28746301](Concise review of the drugs available for therapy of epilepsy list oxcarbazepine as similar chemically to carbamazepine, but as being better tolerated and with fewer drug-drug interactions and lower rates of serious adverse events including Stevens-Johnson syndrome and toxic epidermal necrolysis; no mention of ALT elevations or hepatotoxicity).
- Borrelli EP, Lee EY, Descoteaux AM, Kogut SJ, Caffrey AR. Stevens-Johnson syndrome and toxic epidermal necrolysis with antiepileptic drugs: An analysis of the US Food and Drug Administration Adverse Event Reporting System. Epilepsia. 2018;59:2318–24. [PMC free article: PMC6420776] [PubMed: 30395352](Review of adverse event reports to the FDA between 2014 and 2018 identified ~2.9 million reports, 1034 for SJS/TEN, the most common class of drugs being anticonvulsants with 17 of 34 currently available anticonvulsants having at least one report, those most frequently linked being lamotrigine [n=106], carbamazepine [22], levetiracetam [14], phenytoin [14], valproate [9], clonazepam [8], zonisamide [7]; pregabalin [4], gabapentin [4] and oxcarbazepine [3]; no mention of accompanying liver injury or whether attribution was as a single agent or one of several).
- Tangamornsuksan W, Scholfield N, Lohitnavy M. Association between HLA genotypes and oxcarbazepine-induced cutaneous adverse drug reactions: a systematic review and meta-analysis. J Pharm Pharm Sci. 2018;21:1–18. [PubMed: 29370880](Metaanalysis of six studies of HLA associations with oxcarbazepine induced cutaneous reactions identified 229 cases, 251 drug-tolerant controls and 2358 population controls [Asians] with a significant association of SJS/TEN with HLA-B*15:02 and of maculopapular rash with HLA-A*31:01:).
- Han XD, Koh MJ, Wong SMY. Drug reaction with eosinophilia and systemic symptoms in a cohort of Asian children. Pediatr Dermatol. 2019;36:324–9. [PubMed: 30920020](Among 10 children with DRESS syndrome seen at a single, Singapore referral center between 2006 and 2016, 3 cases were attributed to SMZ/TMP, 2 to carbamazepine, 1 sulfasalazine, 2 phenobarbital and 1 levetiracetam, but none to oxcarbazepine; all had ALT elevations [88 to 1172 U/L], bilirubin was elevated in 7, but none had acute liver failure and none were fatal).
- Cano-Paniagua A, Amariles P, Angulo N, Restrepo-Garay M. Epidemiology of drug-induced liver injury in a University Hospital from Colombia: Updated RUCAM being used for prospective causality assessment. Ann Hepatol. 2019;18:501–7. [PubMed: 31053545](Among 286 patients with liver test abnormalities seen in a single hospital in Colombia over a 1 year period, 17 were diagnosed with drug induced liver injury, the most common cause being antituberculosis therapy [n=6] followed by anticonvulsants [n=3, 1 each due to phenytoin, gabapentin and valproate]).
- Wang YH, Chen CB, Tassaneeyakul W, Saito Y, Aihara M, Choon SE, Lee HY, et al. Asian Severe Cutaneous Adverse Reaction Consortium. The medication risk of Stevens-Johnson Syndrome and toxic epidermal necrolysis in Asians: the major drug causality and comparison with the US FDA label. Clin Pharmacol Ther. 2019;105:112–20. [PubMed: 29569740](Among 1028 cases of SJS/TEN reported to registries in 8 Asian countries, the most frequently implicated class of drugs was anticonvulsants including carbamazepine [26%], phenytoin [13%], lamotrigine [10%], phenobarbital [2%] and oxcarbazepine [1.7%]; non-anticonvulsant causes included allopurinol [20%] and sulfamethoxazole [8%]).
- Kim HK, Kim DY, Bae EK, Kim DW. Adverse skin reactions with antiepileptic drugs using Korea adverse event reporting system database, 2008-2017. J Korean Med Sci. 2020;35:e17. [PMC free article: PMC6995813] [PubMed: 31997613](Among 2942 reports of cutaneous drug reactions made to a Korean pharmacovigilance registry between 2008 and 2017, 241 [8%] were for severe cutaneous reactions [DRESS in 109, SJS in 106 and TEN in 25] the most common causes of which were carbamazepine [24%], lamotrigine [24%], valproate [8%], phenytoin [6%] and oxcarbazepine [5%]; no mention of accompanying ALT elevations or hepatotoxicity ).
- Li R, Zhou Q, Ou S, Wang Y, Li Y, Xia L, Pan S. Comparison of long-term efficacy, tolerability, and safety of oxcarbazepine, lamotrigine, and levetiracetam in patients with newly diagnosed focal epilepsy: an observational study in the real world. Epilepsy Res. 2020;166:106408. [PubMed: 32679487](Among 388 patients with new onset focal epilepsy, seizure free rates at three years were lower for oxcarbazepine [26%], than lamotrigine [40%] or levetiracetam [40%], while adverse events leading to discontinuation were higher 9% vs 9% vs 2%], 14% of discontinuations were attributed to abnormal liver tests; no mention of clinically apparent hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Oxcarbazepine: an update of its efficacy in the management of epilepsy.[CNS Drugs. 2001]Review Oxcarbazepine: an update of its efficacy in the management of epilepsy.Wellington K, Goa KL. CNS Drugs. 2001; 15(2):137-63.
- Review Oxcarbazepine, an antiepileptic agent.[Clin Ther. 2001]Review Oxcarbazepine, an antiepileptic agent.Kalis MM, Huff NA. Clin Ther. 2001 May; 23(5):680-700; discussion 645.
- Spotlight on oxcarbazepine in epilepsy.[CNS Drugs. 2004]Spotlight on oxcarbazepine in epilepsy.Bang LM, Goa KL. CNS Drugs. 2004; 18(1):57-61.
- Oxcarbazepine: new preparation. An alternative to carbamazepine in partial epilepsy.[Prescrire Int. 2001]Oxcarbazepine: new preparation. An alternative to carbamazepine in partial epilepsy.. Prescrire Int. 2001 Dec; 10(56):170-4.
- Review Oxcarbazepine. A review of its pharmacology and therapeutic potential in epilepsy, trigeminal neuralgia and affective disorders.[Drugs. 1992]Review Oxcarbazepine. A review of its pharmacology and therapeutic potential in epilepsy, trigeminal neuralgia and affective disorders.Grant SM, Faulds D. Drugs. 1992 Jun; 43(6):873-88.
- Oxcarbazepine - LiverToxOxcarbazepine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...