NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Mefloquine is a quinoline derivative used for the prevention and therapy of P. falciparum malaria. Mefloquine therapy is associated with a low rate of transient and asymptomatic serum enzyme elevations and is a rare cause of clinically apparent acute liver injury.
Background
Mefloquine (mef' loe kwin) is a quinoline methanol similar to quinine and is active against the asexual stages of malaria. Its exact mechanism of activity is unknown. Mefloquine is effective as prophylaxis against malaria and is widely used in therapy against chloroquine-resistant P. falciparum infection. Unfortunately, mefloquine resistance is becoming an enlarging problem. Mefloquine was approved for use in the United States in 1989 and is available in tablets of 250 mg in several generic forms and under the brand name Lariam. The recommended dosage for suppressive prophylaxis is 250 mg once weekly for 1 week before to 4 weeks after travel to an endemic area. Specific recommendations on the therapy of malaria including details on diagnosis, drug dosage and safety are available at the CDC website: http://www.cdc.gov/malaria/. Common side effects of mefloquine include headache, fatigue, insomnia, vivid dreams, anorexia, nausea, diarrhea, abdominal discomfort, dizziness, rash and pruritus. Rare side effects include hallucinations, disorientation and seizures.
Hepatotoxicity
Chronic therapy with mefloquine is associated with asymptomatic, transient serum enzyme elevations in up to 18% of patients. These elevations are usually mild and resolve without dose modifications. Despite widespread use, mefloquine has rarely been linked to clinically apparent acute liver injury and too few reports are available to characterize the clinical features of such injury. Instances of acute hepatocellular injury as well as cholestatic hepatitis have been linked to use of mefloquine. Allergic manifestations (rash, fever, eosinophilia) and autoantibody formation are rare.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the hepatic injury from mefloquine is unknown, but may relate to a metabolic intermediate with direct toxicity or ability to induce an immune mediated hepatotoxicity. Mefloquine undergoes extensive hepatic metabolism to an inactive metabolite that is excreted in the urine.
Outcome and Management
There does not seem to be cross reactivity to hepatic injury among the various antimalarial agents and switching to other drug can be done.
Drug Class: Antimalarial Agents
CASE REPORT
Case 1. Acute hepatitis due to mefloquine prophylaxis of malaria.
[Modified from: Bruguera M, Herrera S. [Acute hepatitis associated with mefloquine therapy]. Gastroenterol Hepatol 2007; 30: 102-3. Spanish. PubMed Citation]
A 35 year old woman working in health care in Liberia developed nausea, poor appetite and pruritus 2 months after starting malarial prophylaxis with mefloquine (250 mg weekly). She had no history of liver disease, alcohol abuse or risk factors for viral hepatitis. She denied fever. On examination, she was jaundiced and had a desquamating erythematous rash over the hands and palms. She had stopped the mefloquine when she first began to feel ill. Laboratory testing showed marked elevations in serum aminotransferase and alkaline phosphatase levels (Table) with a total serum bilirubin of 7.1 mg/dL. The prothombin time was normal. Tests for hepatitis A, B and C were negative. Ultrasound of the abdomen showed no evidence of biliary obstruction. Over the next few days she began to improve. In follow up 5 weeks later, serum liver tests had returned to near normal levels.
Key Points
| Medication: | Mefloquine (250 mg once weekly) |
| Pattern: | Hepatocellular (R=10.8) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 2 months |
| Recovery: | 6 weeks |
| Other medications: | None |
Laboratory Values
Comment
Despite the frequency of serum aminotransferase elevations during mefloquine therapy, there have been few reports of clinically apparent liver disease with jaundice. The current report described a self limited acute hepatitis-like syndrome arising 2 months after starting a prophylactic regimen of mefloquine. Although the height of the serum aminotransferase levels were indicative of a hepatocellular pattern of injury, the high alkaline phosphatase levels and early presentation with pruritus suggests that the hepatic injury was at least partially cholestatic. This “mixed” pattern is typical of drug induced liver injury.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Mefloquine – Generic, Lariam®
DRUG CLASS
Antimalarial Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Mefloquine | 51773-92-3 | C17-H16-F6-N2-O.Cl-H |
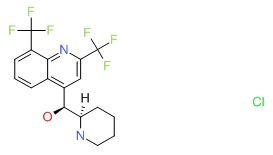 |
ANNOTATED BIBLIOGRAPHY
References updated: 07 February 2017
- Zimmerman HJ. Antiprotozoal agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 623-5.(Expert review of hepatotoxicity published in 1999; mentions that mefloquine can lead to elevated ALT levels).
- Vinetz JM, Clain J, Bounkeua V, Eastman RT, Fidock D. Chemotherapy of malaria.l In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman.s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1383-418.(Textbook of pharmacology and therapeutics).
- Reisinger EC, Horstmann RD, Dietrich M. Tolerance of mefloquine alone and in combination with sulfadoxine-pyrimethamine in the prophylaxis of malaria. Trans R Soc Trop Med Hyg 1989; 83: 474-7. [PubMed: 2694482](Controlled trial of mefloquine alone vs mefloquine and sulfadoxine/pyrimethamine in 175 travelers; ALT or AST became abnormal in 18%, peak ALT 122 U/L with similar frequency in both groups; all resolving spontaneously).
- Lobel HO, Miani M, Eng T, Bernard KW, Hightower AW, Campbell CC. Long-term malaria prophylaxis with weekly mefloquine. Lancet 1993; 341: 848-51. [PubMed: 8096560](Study on 421 Peace Corps volunteers in West Africa on different antimalarial regimens including mefloquine, chloroquine and proguanil; no serious adverse events were reported, but ALT levels were not monitored; mefloquine decreased P. falciparum infection rate).
- Steffen R, Fuchs E, Schildknecht J, Naef U, Funk M, Schlagenhauf P, Phillips-Howard P, et al. Mefloquine compared with other malaria chemoprophylactic regimens in tourists visiting east Africa. Lancet 1993; 341: 1299-303. [PubMed: 8098447](Inflight questionnaires of returning travelers from East Africa between 1985 and 1991; side effects of mefloquine reported in 17-35% of subjects, higher with combinations; 4 deaths, evidently from malaria).
- Boudreau E, Schuster B, Sanchez J, Novakowski W, Johnson R, Redmond D, Hanson R, et al. Tolerability of prophylactic malaria regimens. Trop Med Parasitol 1993; 44: 257-65. [PubMed: 8256107](Controlled trial of mefloquine vs chloroquine in 359 US Marines for 12 weeks; no differences in ALT levels between groups; mefloquine caused mild psychological side effects and insomnia).
- Palmer KJ, Holliday SM, Brogden RN. Mefloquine. A review of its antimalarial activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1993; 45: 430-75. [PubMed: 7682911](Review on mefloquine which is used as prophylaxis and treatment of chloroquine resistant P. falciparum malaria; transient increases of ALT have been observed with prophylactic and therapeutic regimens; 4 cases of hepatitis have been reported to the manufacturer).
- Jaspers CAJJ, Hoperus Buma APCC, Van Thiel PPAM, Van Hulst RA, Kager PA. Tolerance of mefloquine chemoprophylaxis in Dutch military personnel. Am J Trop Med Hyg 1996; 55: 230-4. [PubMed: 8780466](Study of 73 volunteers on mefloquine for 25 weeks; slight increase in mean ALT levels but no value rose above normal).
- Barrett PJ, Emmins PD, Clarke PD, Bradley DJ. Comparison of adverse events associated with the use of mefloquine and combination of chloroquine and proguanil as antimalarial prophylaxis: a postal and telephone survey of travelers. BMJ 1996; 313: 525-8. [PMC free article: PMC2351944] [PubMed: 8789977](Mail questionnaire of 3851 British travelers taking mefloquine or chloroquine/proguanil for malaria prophylaxis; rates of side effects were similar [~41%], no mention of hepatic adverse events).
- Grieco A, Vecchio FM, Natale L, Gasbarrini G. Acute fatty liver after malaria prophylaxis with mefloquine. Lancet 1999; 353: 295-6. [PubMed: 9929030](46 year old developed nausea, weight loss and edema, found to have hepatomegaly and fatty liver [ALT 41 U/L, Alk P 161 U/L, no bilirubin values given], resolving over next month, but relationship to therapy unclear).
- Gotsman I, Azaz-Livshits T, Fridlender Z, Muszkat M, Ben-Chetrit E. Mefloquine-induced acute hepatitis. Pharmacotherapy 2000; 20: 1517-9. [PubMed: 11130224](68 year old man developed fatigue and nausea after 6 weeks of mefloquine prophylaxis [bilirubin 1.9 mg/dL, ALT 1277 U/L, LDH 7770 U/L, Alk P normal, INR 7.4], with rapid recovery on stopping; patient also had atrial flutter and heart failure and clinical presentation was more typical of ischemic hepatitis than drug induced liver injury).
- Overbosch D, Schilthuis H, Bienzle U, Behrens RH, Kain KC, Clarke PD, Toovey S, et al. Malarone International Study Team. Atovaquone-proguanil versus mefloquine for malaria prophylaxis in nonimmune travelers: results from a randomized, double-blind study. Clin Infect Dis 2001; 33: 1015-21. [PubMed: 11528574](Controlled trial of atovaquone-proguanil vs mefloquine for malaria prophylaxis in 483 travelers; similar efficacy [100%], but neuropsychiatric side effects were more common with mefloquine [29% vs 14%] including insomnia, anxiety, vivid dreams, dizziness and trouble concentrating; no mention of liver injury and ALT levels were not monitored).
- Croft AM, Herxheimer A. Adverse effects of the antimalaria drug, mefloquine: due to primary liver damage with secondary thyroid involvement? BMC Public Health 2002; 2: 6. [PMC free article: PMC101408] [PubMed: 11914150](Systematic review of 516 published case reports of adverse effects of mefloquine suggests that many such as malaise, fever, anorexia, headache, abdominal pain and nausea are due to “transient, anicteric chemical hepatitis,” although abnormal liver tests are found in only a proportion).
- Croft AM, Whitehouse DP, Cook GC, Beer MD. Safety evaluation of the drugs available to prevent malaria. Expert Opin Drug Saf 2002; 1: 19-27. [PubMed: 12904156](Review of adverse side effects of antimalarial agents, pointing out the need for careful prospective randomized controlled trials of prophylactic regimens).
- Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf 2004; 27: 25-61. [PubMed: 14720085](Review of the toxicities and side effects of antimalarials; mentions that mefloquine can cause transient ALT elevations and rarely hepatitis).
- Bruguera M, Herrera S. [Acute hepatitis associated with mefloquine therapy]. Gastroenterol Hepatol 2007; 30: 102-3. Spanish. [PubMed: 17335720](35 year old woman physician developed nausea and pruritus followed by jaundice 2 months after starting mefloquine prophylaxis [bilirubin 7.1 mg/dL, ALT 2411 U/L, Alk P 614 U/L], resolving 5 weeks after stopping: Case 1).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, one case was attributed to artesunate, but no other antimalarial agent was mentioned).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol 2010; 105: 2396-404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India; none were attributed to antimalarials).
- Tine RC, Faye B, Sylla K, Ndiaye JL, Ndiaye M, Sow D, Lo AC, et al. Efficacy and tolerability of a new formulation of artesunate-mefloquine for the treatment of uncomplicated malaria in adult in Senegal: open randomized trial. Malar J. 2012 Dec 12; 11: 416. [PMC free article: PMC3554515] [PubMed: 23234606](310 Senegalese patients with malaria were treated with artesunate and mefloquine vs artesunate and lumefantrine for 3 days; both regimens were highly effective and well tolerated, mean serum ALT levels did not change and no serious adverse events were reported).
- Rueangweerayut R, Phyo AP, Uthaisin C, Poravuth Y, Binh TQ, Tinto H, Pénali LK, et al; Pyronaridine–Artesunate Study Team. Pyronaridine-artesunate versus mefloquine plus artesunate for malaria. N Engl J Med 2012; 366: 1298-309. [PubMed: 22475593](Among 1271 patients in Asia and Africa treated for malaria with an artesunate combination regimen for 3 days, ALT elevations occurred in none of 423 mefloquine treated, but in 2.5% [21 of 848] of pyronaridine-artesunate treated subjects including 15 with ALT above 5 times ULN and 2 patients with jaundice, but with symptomatic hepatitis).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to an antimalarial agent).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. (In a. [PubMed: 23419359]population based study from Iceland, 96 cases of drug induced liver injury were identified over a 2 year period [2010 and 2011], but none were attributed to an antimalarial agent).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to an antimalarial agentl).
- Advice for travelers. Treat Guidel Med Lett 2015: 57 (1466): 52-8.(Concise guidelines on prevention of malaria in travelers indicates that mefloquine can be used as prophylaxis in chloroquine-reistant malaria areas of the world and is an appropriate prophylactic agent for patients who cannot tolerate chloroquine).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Efficacy and safety of atovaquone/proguanil compared with mefloquine for treatment of acute Plasmodium falciparum malaria in Thailand.[Am J Trop Med Hyg. 1999]Efficacy and safety of atovaquone/proguanil compared with mefloquine for treatment of acute Plasmodium falciparum malaria in Thailand.Looareesuwan S, Wilairatana P, Chalermarut K, Rattanapong Y, Canfield CJ, Hutchinson DB. Am J Trop Med Hyg. 1999 Apr; 60(4):526-32.
- Randomised trial of artesunate and mefloquine alone and in sequence for acute uncomplicated falciparum malaria.[Lancet. 1992]Randomised trial of artesunate and mefloquine alone and in sequence for acute uncomplicated falciparum malaria.Looareesuwan S, Viravan C, Vanijanonta S, Wilairatana P, Suntharasamai P, Charoenlarp P, Arnold K, Kyle D, Canfield C, Webster K. Lancet. 1992 Apr 4; 339(8797):821-4.
- Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study.[Lancet. 2000]Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study.Nosten F, van Vugt M, Price R, Luxemburger C, Thway KL, Brockman A, McGready R, ter Kuile F, Looareesuwan S, White NJ. Lancet. 2000 Jul 22; 356(9226):297-302.
- Review Clinical application of mefloquine pharmacokinetics in the treatment of P falciparum malaria.[Fundam Clin Pharmacol. 1994]Review Clinical application of mefloquine pharmacokinetics in the treatment of P falciparum malaria.Karbwang J, Na-Bangchang K. Fundam Clin Pharmacol. 1994; 8(6):491-502.
- Review Clinical pharmacokinetics of mefloquine.[Clin Pharmacokinet. 1990]Review Clinical pharmacokinetics of mefloquine.Karbwang J, White NJ. Clin Pharmacokinet. 1990 Oct; 19(4):264-79.
- Mefloquine - LiverToxMefloquine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...